Test of a stand-off laser-induced breakdown spectroscopy sensor for the detection of explosive residues on solid surfaces
Cristina
López-Moreno
a,
Santiago
Palanco
a,
J.
Javier Laserna
*a,
Frank
DeLucia Jr
b,
Andrzej W.
Miziolek
b,
Jeremy
Rose
c,
Roy A.
Walters
c and
Andrew I.
Whitehouse
d
aDepartment of Analytical Chemistry, Faculty of Sciences, University of Málaga, E-29071, Spain. E-mail: laserna@uma.es
bUS Army Research Laboratory, AMSRD-ARL-WM-BD, Aberdeen Proving Ground, MD 21005-5069, USA. E-mail: miziolek@arl.army.mil
cOcean Optics, Inc., Winter Park, FL 32792-6819, USA. E-mail: royW@oceanoptics.com
dApplied Photonics Ltd, Unit 8, Carleton Business Park, Skipton, North Yorkshire, UK BD23 2DE. E-mail: andy.whitehouse@appliedphotonics.co.uk
First published on 9th November 2005
Abstract
The detection and characterization of energetic materials at distances up to 45 m using stand-off laser induced breakdown spectroscopy (LIBS) has been demonstrated. A field-portable open-path LIB spectrometer working under a coaxial configuration was used. A preliminary study allowed choosing a single-pulse laser source over a double-pulse system as the most suitable source for the stand-off analysis of organic samples. The C2 Swan system, as well as the hydrogen, oxygen and nitrogen emission intensity ratios were the necessary parameters to identify the analyte as an organic explosive, organic non-explosive and non-organic samples. O/N intensity ratios of 2.9 and 2.16 with relative standard deviations of 4.03% and 8.36% were obtained for 2,4-dinitrotoluene and aluminium samples, respectively. A field test with known samples and a blind test were carried out at a distance of 30 m from the sample. Identification of energetic compounds in such conditions resulted in 19 correct results out of 21 samples.
Introduction
Detection of energetic materials requires the development of new analytical techniques.1,2 A suitable analytical tool should be able to differentiate these compounds from innocuous substances with a reasonable level of confidence. Although several systems are commercially available, until now, no instrument can afford detectability for the wide range of energetic materials in any quantity and in any form without false alarms being detected. Radiation-based and vapor-based are the main techniques currently used in the detection of explosives. In the first group, neutron activation analysis can be highlighted. This technique is based on the measurement of the γ emission generated in a material in response to an activation by thermal neutrons. The intensity and the spatial distribution of the γ-photons are indicative of the presence of N in the material. Several of the most usual explosives have a high nitro group content. However, there are highly nitrogen-containing polymers such as melamine or the polyamides which often induce false positives. Among vapor-based techniques, it is worth mentioning ion mobility spectrometry owing to its high sensitivity to volatile compounds evaporating from most explosives. However, the biggest disadvantage of these vapor-based techniques is their limitation to organic and easily vaporizable compounds.1The techniques used up to now still require approaching the sample in order to perform the analysis which entails a risk for the operator. The fact that dogs remain the most effective explosive detectors nowadays is highly indicative that exploration of new analytical techniques is critical. In this sense, remote analytical techniques are the only ones that offer real-time results maintaining a security distance from the sample and avoiding risks for the operator. Laser-induced breakdown spectroscopy (LIBS) has been successfully used for the laboratory detection and identification of chemical and biological warfare agents, explosives and other hazards.3–5 LIBS possesses many desirable attributes for a fast field-portable sensor system. The portability6,7 and the remote capabilities8,9 of LIBS make this technique the most suitable for hazardous materials in the field. However, energetic materials are commonly organic compounds and LIBS is mainly an elemental technique owing to the high-energies related to the focused short laser pulses.
Identification of organic compounds with LIBS has been reported in several papers.10–20 Anzano et al.10 proved the feasibility of a data correlation method11 for the identification of organic polymers. More often, oxygen, nitrogen and hydrogen emission lines and a number of molecular bands are used for this task. The analysis of molecular bands12,13 is focused on the detection of CN molecular violet bands at 386.17 nm, 387.14 nm, and 388.34 nm and C2 carbon Swan bands at 516.52 nm. It is well known that the intensity of the Swan system is proportional to the concentration of the carbon dimmer in the excited state while the CN bands emission could be also due to the CN generation in the ambient air. Thus, only the measurement of the C2 bands is reliable for the analysis in the open atmosphere. Although the raw atomic emission has been used for the analysis of organics,12,14–16 the peak ratio method has been found to yield better results.17–21 The intensity ratio is related to the difference between the upper energy levels of the lines and also is proportional to the ratio of Boltzmann factors, becoming independent of the ablated mass.22 Thus, it is expected to yield a lower RSD than the single peak intensity alone, since there is a reduction in the flicker associated with the LIBS signal.
In the present work, peak ratio analysis in combination with analysis of molecular bands was used for the identification of energetic materials. A number of organics and explosive samples in the form of fingerprints or solution thin films evaporated on solid surfaces were analyzed. Although the energetic materials chosen are mainly of military use, the present work must be understood in the framework of an exploratory investigation on the capabilities of LIBS for the stand-off detection of explosive residues in the field prior to a deeper study with a wider range of materials and conditions. In this sense, a fingerprint (10–100 ng) deposited on a car body was considered as a satisfactory limit of detection for this stage of application of the technique.
Experimental
The remote LIBS system used has been described previously by Palanco et al.23Fig. 1 shows the experimental setup. A Quantel Briliant Q-switched Nd:YAG laser operating at 1064 nm and producing 350 mJ single pulses at 20 Hz was used. The beam focusing component must accomplish the task of focusing the laser energy to a sufficiently small spot in the remote sample surface in order to provide the necessary irradiance to produce a plasma without inducing undesired air breakdown on the sample proximity. Expanding the beam prior to focusing is essential in order to reduce the spot size focused at a distance, which in turn, will enable the necessary irradiance levels to induce plasma formation. Known as a Herschelian telescope, this design in Fig. 1 is common in collimation and target projection systems. The fact that light gathering does not employ refracting optics frees the return path from chromatic aberration. Plasma light enters a fiber optic cable with an aperture ratio close to the f/10 of the primary mirror. This fiber optic cable leads the plasma light to an Oriel MS125 crossed Czerny-Turner spectrometer—125 mm focal length, f/3.8, 600 line mm−1 grating, 25 μm slit-fitted with an Andor DH501-25F-03 intensified CCD detector (1024 × 128 pixel, 26 μm2 pixel, intensifier diameter 25 mm). | ||
| Fig. 1 Experimental setup. 1, Laser source. 2, Folding mirror. 3, Lenses. 4, Dichroic mirror. 5, Flat aluminium mirror. 6, Parabolic aluminium mirror. 7, Fiber optic cable. 8, Spectrograph. 9, iCCD. 10, Pulse and delay generator. 11, Power laser supply. 12, Computer. | ||
Results and discussion
Characterization of organic compounds using LIBS requires careful data processing due to the similarity in composition of organic compounds which results in the LIB spectra of these compounds sharing the same emission lines (C, H, O and N). Fig. 2 shows a single shot spectrum acquired at 45 m from the sample, the solid residue of a 2,4-dinitrotoluene (DNT) acetone solution evaporated on an aluminium plate. The spectrum illustrates the great surface sensitivity of the technique with almost no traces of aluminium emission found in the spectra as second orders of the intense resonant Al(I) emission between 308.2 nm and 309.3 nm. As expected, the C2(0,0) 516 nm Swan band is present along with the second order of the CN(0,0) 388 nm band, although the latter was not used owing to K spectral interferences. The delay time was set to 0.4 μs and the integration gate was set to 10 μs. It was found experimentally that these settings were optimum for the observation at 45 m of the atomic nitrogen emission between 742.4 nm and 746.8 nm and the atomic oxygen emission between 777.2 nm and 777.5 nm. However, it must be stated that the observed oxygen and nitrogen emissions are a result from both DNT and air contents in these elements. | ||
| Fig. 2 Single shot LIB spectra of a DNT sample at a 45 m distance, 1 μs acquisition delay and 10 μs integration time. | ||
Fig. 3 compares the O/N ratios obtained for the DTN sample and for an aluminium foil. The insets show the spectra of the DNT (top) and of the aluminium foil (bottom). Oxygen and nitrogen emission can be noticed in both spectra, the lines present in the spectrum of the aluminium sample are due to air only. The points plotted in the figure correspond to single laser shots on fresh positions of each sample. The average O/N intensity ratio in 10 of the individual aluminium spectra was 2.16 with an RSD of 4.03% whilst the average O/N intensity ratio for the DNT sample was 2.9 with an RSD of 8.36%. The higher O/N ratio shown by the DNT sample can be due only to its additional content in these elements as compared to Al. According to Yinon,24 a measurement of the oxygen and nitrogen densities, to an uncertainty of 20%, gives a unique separation of explosives from other compounds. This statement covers the majority of the high-energy explosives. However, there are very few non-explosives compounds—such as melanine, polyurethane and solid nylon—that have high atomic densities of nitrogen and could give a false positive response.
 | ||
| Fig. 3 O(I) 777.2 nm to N(I) 746.8 nm peak intensity ratio for 10 single shots spectra at a 45 m distance from an aluminium foil (■) and a DNT solution deposited on the same aluminum foil (○). The inset show DNT (top) and aluminium (bottom) spectra. | ||
The presence of the C2(0,0) 516 nm Swan band—proportional to the concentration of the carbon dimer in the excited state—in the DNT spectrum was used to discriminate organic samples from inorganic ones. In combination with the peak ratio analysis this spectral feature was used for the detection of a number of explosive samples.
Field tests
The suitability of stand-off LIBS for the analysis of energetic compounds was tested in the field. Fig. 4 shows a snapshot of the stand-off LIBS instrument. For this experiment, a number of samples were deposited on the door of a vehicle located at a 30 m distance from the instrument. The samples were circled with a marker pen to delimit the area to be sampled. A test was performed by printing—or dropping when using solutions—fifteen known samples on the car door. The energetic compounds and potential interferences used were TNT (fingerprint), RDX (fingerprint), COMP B (fingerprint), TNT (100 ppm solution), PETN (100 ppm solution), acetone (one drop), C4 (fingerprint), grease (fingerprint), diesel (fingerprint), oil (fingerprint), beef grease (fingerprint), cheese (fingerprint), tape (residue) and ink. The structure and molecular formula of the energetic compounds is shown in Table 1. Additionally, six unknown samples were placed on the car in order to perform a blind test. The sampling procedure consisted of shooting twelve laser pulses in fresh positions inside the circles where a fingerprint or a solution had been deposited. Both to increase the surface sensitivity and to avoid damages to the vehicle surface, the irradiance was lowered by defocusing the laser beam. Fig. 5 and 6 illustrate the spectra corresponding to four of the known samples and the six samples in the blind test, respectively. Preliminary data processing was carried out by analyzing these spectra according to the following criteria which allowed the discrimination between “organic non-explosive”, “organic explosive” and “non-organic” samples: (a) Any organic compound is detected by the presence of C2 Swan bands in the 510 nm region, H alpha at 656.2 nm and O at 777.2 nm. (b) Explosives are differentiated from other organics by the presence of N between 742 nm and 746 nm as well as by a lower intensity of the C2 bands when compared to the O, N and H emission. (c) A single positive result out of the twelve spectra acquired for each of the six unknown samples is enough to mark the sample as positive. | ||
| Fig. 4 Snapshot of the system during a field test of the stand-off LIBS sensor featuring C. López-Moreno. | ||
 | ||
| Fig. 5 Single shot stand-off LIBS spectra of four of the known samples at a 30 m distance. (a) 100 ppm TNT solution, (b) C4 fingerprint, (c) car paint, (d) oil fingerprint. | ||
 | ||
| Fig. 6 Single shot stand-off LIBS spectra of the blind-test samples at a 30 m distance. (a) Acetone, (b) human fingerprint, (c) 100 ppm TNT solution, (d) TNT fingerprint, (e) no sample and (f) C4 fingerprint. | ||
| Explosive | Structure | Molecular formula |
|---|---|---|
| TNT |

|
C7H5N3O6 |
| RDX |

|
C3H6N6O6 |
| C4 |
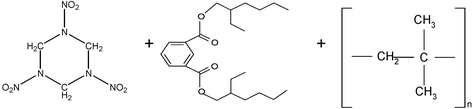
|
C3H6N6O6 + C24H38O4 + [C4H8]n |
| Comp B |

|
C3H6N6O6 + C7H5N3O6 |
| PETN |

|
C5H8O12 |
As can be seen in Fig. 5, C2 bands are present in all samples, being indicative of organic components present. Also, the O(I) 777.2 nm and the N(I) 746.8 nm peaks are present to a higher extent in the explosive samples. The presence of the H alpha emission at 656.2 nm in the energetic materials can be seen, this peak being lower in intensity for the innocuous samples. In Fig. 6, the spectra corresponding to the blind test are illustrated showing C2 Swan bands in all the samples but sample E, which corresponds to a blank—i.e. no sample inside the marked circle. The N and H peaks can be appreciated in spectra C, D and F, all of them corresponding to organic explosive samples. Also, the intensity of the O peak is higher in these spectra than in the other cases.
A more thorough data processing was performed by developing an algorithm on the basis of the results obtained in the test with known samples. Fig. 7 shows the algorithm flowchart which, although not statistically validated, showed its strength at discriminating the nature of the different samples. Despite being a potential source of environmental interferences, it was empirically found that using Na(I) 589 nm and K(I) 766.5 nm lines improved the detection efficiency of the algorithm. All the spectra acquired were interrogated by using this flowchart. Although the test with known samples was the basis for the algorithm, the results for this test are also reported in Table 2, the blind test results being summarized in Table 3. Still, a false positive and a false negative corresponding to the tape residue and the PETN drop, respectively, were found out of the 15 samples in the known test. For the blind test, all of the 6 samples were correctly identified using this data processing algorithm. The high success rate achieved with the first visual processing of the spectra and later by using the algorithm shows the great potential of this technique as a fast tool for the identification of energetic materials at remote distances.
 | ||
| Fig. 7 Representative decision making strategy for stand-off LIB spectral analysis of energetic materials. | ||
| Actual | Presence C2, H, N | O/H2 ratio | O/H ratio | O/K ratio | O/N ratio | Na/C2 ratio | Flow chart result | Correct |
|---|---|---|---|---|---|---|---|---|
| TNT fingerprint | Yes | 0.75 | 1.07 | 1.46 | — | 0.52 | Explosive | ✓ |
| [Yes] | [Yes] | [Yes] | [Yes] | |||||
| Diesel fuel fingerprint | Yes | 5.07 | 0.42 | — | 3.77 | — | Non-explosive | ✓ |
| [No] | [No] | [No] | ||||||
| RDX 100 ppm | Yes | 0.60 | 0.72 | 1.40 | — | 0.52 | Explosive | ✓ |
| [Yes] | [Yes] | [Yes] | [Yes] | |||||
| Acetone | No | — | — | — | — | — | Non-explosive | ✓ |
| C4 fingerprint | Yes | 1.82 | 0.75 | 1.90 | — | 0.89 | Explosive | ✓ |
| [Yes] | [Yes] | [Yes] | [Yes] | |||||
| Tape residue | Yes | 1.13 | 1 | 1.19 | — | 0.84 | Explosive | X false positive |
| [Yes[ | [Yes[ | [Yes[ | [Yes[ | |||||
| TNT 100 ppm | Yes | 1.60 | 0.72 | 1.21 | — | 1.62 | Explosive | ✓ |
| [Yes] | [Yes] | [Yes] | [Yes] | |||||
| Beef fingerprint | Yes | 3.40 | 0.55 | — | 2.55 | — | Non-explosive | ✓ |
| [No] | [No] | [No] | ||||||
| CompB 100 ppm | Yes | 0.71 | 1.14 | 1.36 | — | 0.69 | Explosive | ✓ |
| [Yes] | [Yes] | [Yes] | [Yes] | |||||
| Cheese fingerprint | Yes | 1.24 | 0.67 | 0.63 | 5.26 | 3.11 | Non-explosive | ✓ |
| [Yes] | [No] | [No] | [Yes] | [No] | ||||
| PETN 100 ppm | No | — | — | — | — | — | Non-explosive | X false negative |
| Grease fingerprint | Yes | 4.82 | 0.48 | — | 2.35 | — | Non-explosive | ✓ |
| [No] | [No] | [No] | ||||||
| Paint | Yes | 0.73 | 1.55 | — | 3.39 | — | Non-explosive | ✓ |
| [Yes] | [No] | [No] | ||||||
| Oil fingerprint | Yes | 1.48 | 0.46 | — | 3.35 | — | Non-explosive | ✓ |
| [yes] | [No] | [No] | ||||||
| Ink | Yes | 2.65 | 0.60 | — | 2.65 | — | Non-explosive | ✓ |
| [Yes] | [No] | [No] |
| Actual | Presence C2, H, N, O | H/C2 ratio (0.5–3.0) | O/H ratio (0.7–1.4) | O/K ratio >1 | O/N ratio >4 | Na/C2 ratio < 2 | Flow chart result | Correct |
|---|---|---|---|---|---|---|---|---|
| Acetone | Yes | 4.29 | 0.82 | 1.42 | 4.23 | 2.19 | Non-explosive | ✓ |
| Human fingerprint | Yes | 1.15 | 1.26 | 0.49 | 6.14 | 4.10 | Non-explosive | ✓ |
| TNT solution | Yes | 3.50 | 0.73 | 1.14 | 4.49 | 2.41 | Explosive | ✓ |
| TNT fingerprint | Yes | 1.71 | 1.08 | 1.31 | 7.02 | 1.19 | Explosive | ✓ |
| No sample | No | 0.68 | 2.17 | 0.32 | 1.46 | 7.84 | Non-explosive | ✓ |
| C4 fingerprint | Yes | 2.52 | 0.73 | 2.20 | 4.27 | 0.99 | Explosive | ✓ |
Conclusions
LIBS has been demonstrated as a fast and reliable technique for stand-off identification of energetic materials. The use of a single pulse was proven to be suitable for the analysis of organic compounds by using low delay times in the detection. A visual examination of the spectra was enough to distinguish between organic and non-organic samples, using the presence of carbon Swan bands in the spectrum. A more exhaustive identification was carried out by using an algorithm based in the peak intensity ratios that allowed a classification of the samples as inorganic, “organic non-explosive” and “organic explosive” compounds. A 30 m distance known-samples test was performed, reaching 13 successes out of 15 samples. Also, a blind-test was carried out, reaching 6 successes out of 6 samples.The authors consider that the detection of energetic materials in 1 fingerprint or one 100 ppm solution drop containing about 5 μg of explosive is a satisfactory limit of detection for this application. Possibly, the technique could measure lower amount of samples, but in real cases, detecting this level of explosives at a 30 m distance is more than enough.
References
- P. Kolla, Anal. Chem., 1995, 67, 184A–187A CAS.
- J. Köhler and R. Meyers, Explosives, VCH, Weinheim, Germany, 1993 Search PubMed.
- A. C. Samuels, F. C. DeLucia Jr, K. L. McNesby and A. Miziolek, Appl. Opt., 2003, 42, 6205–6209 CrossRef CAS.
- S. Morel, N. Leone, P. Adam and J. Amoroux, Appl. Opt., 2003, 42, 6184–6191 CrossRef CAS.
- F. C. DeLucia Jr, R. S. Harmon, K. L. Mcnesby, R. J. Winkel Jr and A. Miziolek, Appl. Opt., 2003, 42, 6148–6152 CrossRef.
- S. Palanco, A. Alises, J. Cuñat, J. Baena and J. J. Laserna, J. Anal. At. Spectrom., 2003, 18, 933–938 RSC.
- S. Palanco and J. J. Laserna, Rev. Sci. Instrum., 2004, 75, 2068–2075 CrossRef CAS.
- J. Cuñat, S. Palanco, F. Carrasco, M. D. Simón and J. J. Laserna, J. Anal. At. Spectrom., 2005, 20, 295–300 RSC.
- C. López-Moreno, S. Palanco and J. J. Laserna, J. Anal. At. Spectrom., 2004, 19, 1479–1484 RSC.
- J. M. Anzano, I. B. Gornushkin, B. W. Smith and J. D. Winefordner, Poly. Eng. Sci., 2000, 40, 2423–2429 Search PubMed.
- I. B. Gornushkin, B. W. Smith, H. Nasajpour and J. D. Winefordner, Anal. Chem., 1999, 71, 5157–5164 CrossRef CAS.
- L. St-Onge, R. Sing, S. Béchard and M. Sabsabi, Appl. Phys., 1999, 69, 913–916 Search PubMed.
- A. Portnov, S. Rosenwaks and I. Bar, Appl. Opt., 2003, 42, 2835–2842 CrossRef CAS.
- D. R. Anderson, C. W. McLeod and T. A. Smith, J. Anal. At. Spectrom., 1994, 9, 67–72 RSC.
- A. R. Boyain-Goitia, D. C. S. Beddows, B. C. Griffiths and H. H. Telle, Appl. Opt., 2003, 42, 6119–6132 CrossRef CAS.
- J. D. Hybl, G. A. Lithgow and S. G. Buckley, Appl. Spectrosc., 2003, 57, 1207–1215 CrossRef CAS.
- R. Sattmann, I. Mönch, H. Krause, R. Noll, S. Couris, A. Hatziapostolou, A. Mavromanolakis, C. Fotakis, E. Larrauri and R. Miguel, Appl. Spectrosc., 1998, 52, 456–461 CAS.
- M. Tran, Q. Sun, B. W. Smith and J. D. Winefordner, J. Anal. At. Spectrom., 2001, 16, 628–632 RSC.
- L. St-Onge, E. Kwong, M. Sabsabi and E. B. Vadas, Spectrochim. Acta, Part B, 2002, 57, 1131–1140 CrossRef.
- F. Ferioli, P. V. Puzinauskas and S. G. Buckley, Appl. Spectrosc., 2003, 57, 1183–1189 CrossRef CAS.
- S. Kaski, H. Häkkänen and J. Korppi-Tommola, J. Anal. At. Spectrom., 2004, 19, 474–478 RSC.
- C. Aragon, J. A. Aguilera and J. Campos, Appl. Spectrosc., 1993, 47, 606–608 CAS.
- S. Palanco, C. López-Moreno and J. J. Laserna, Spectrochim. Acta, Part B Search PubMed , submitted.
- J. Yinon, Forensic and Environmental Detection of Explosives, John Wiley & Sons Inc, New York, 1999 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
