The fractionation of valuable wax products from wheat straw using CO2
Fabien E. I.
Deswarte
a,
James H.
Clark
*a,
Jeffrey J. E.
Hardy
a and
Paul M.
Rose
b
aCentre for Clean Technology, Department of Chemistry, University of York, Heslington, York, UK YO10 5DD. E-mail: jhc1@york.ac.uk; Fax: +44 (0)1904 432705; Tel: +44 (0)1904 432559
bBotanix Ltd, Hop Pocket Lane, Paddock Wood, Kent, UK TN12 6DQ. E-mail: paul.rose@botanix.co.uk; Fax: +44 (0) 1892 836987; Tel: +44 (0) 1892 833415
First published on 24th November 2005
Abstract
Liquid and supercritical CO2 have been used for the first time to achieve direct isolation of valuable wax products from wheat straw (J. H. Clark, F. E. I. Deswarte and J. J. E. Hardy, PCT Pat. Appl., PCT/GB 0502337.9, 2005).1
Introduction
A biorefinery is a factory that processes crops to produce various refined specialised products in a similar way to which a petroleum refinery processes oil to make specialised chemical products.2 The ideal biorefinery of the future should be capable of taking low value local feedstocks, such as agricultural co-products, extracting the high value components and subsequently transforming the residues into ‘platform molecules’ or bioproducts, biofuel, and bioenergy.3 It is vital to the environmental sustainability of the biorefinery that low environmental impact technologies are used throughout the processing. We must learn from what are now considered to be unacceptable aspects of petrochemical production and avoid hazardous and environmentally harmful process auxiliaries such as toxic reagents and volatile organic solvents.4A particularly good example of an abundant and low value bio-feedstock in many countries is wheat straw. In the UK for example, 10 million tonnes of wheat straw are produced annually of which 4 million tonnes have no commercial market. The isolation, characterisation and use of the major components of wheat straw (cellulose, hemicellulose and lignin) has been extensively studied but little work has been done on the secondary metabolites.5–7
Wheat straw, like many other plants, is known to contain a significant quantity of wax (ca. 1% by weight);8 wax is normally made up of a mixture of primarily long chain fatty acids and fatty alcohols, sterols and alkanes. Natural waxes have a wide range of industrial uses in cosmetics, personal care products, polishes and coatings with a world market of tens of thousands of tonnes.
Plant waxes are traditionally extracted by volatile organic solvents including hexane, chloroform, dichloromethane and benzene.9 Apart from the environmental and toxicological problems of using such solvents, the extraction is unselective co-extracting a large number of unwanted compounds such as pigments, polar lipids, and free sugars.10 Alternative low environmental impact, efficient and non-toxic extraction methods are therefore highly desirable. Here we report for the first time, the selective extraction/fractionation of waxes from agro-residue wheat straw by liquid and supercritical CO2.
Experimental
Isolation of wheat straw waxes by organic solvents
Ground consort variety wheat straw (30 g) was Soxhlet extracted with 900 cm3 of solvent for 5 h. The recovered extracts were filtered, concentrated to dryness by rotary evaporation, then kept under vacuum in a dessicator overnight before weighing (crude yield). Crude extracts were then eluted over silica gel in a column with a 85∶15 v∶v mixture of hexane/diethylether to give the wax fraction (wax yield). The experiment was repeated with yields within ±6% of the original.Variation in crude and pure wax yields with wheat varieties, botanical components and age (hexane Soxhlet extraction)
The above experiment was repeated using Sabre, Maris Widgeon, IMP, Xi19, Wallace and Squarehead's Master wheat varieties grown under controlled field conditions. Similarly, we also studied the effect of the botanical component by separating the wheat straw into leaves, node and internodes (Consort variety) before carrying out extractions and separations on each component as described above. Crude and pure wax yields were reproducible between experiments on the same variety to ±6%. Finally, year to year variations were investigated by carrying out extractions of Sabre wheat straw harvested in 2003 and 2005 in North Yorkshire, UK.Isolation of wheat straw waxes using supercritical carbon dioxide and with a modifier
Ground wheat straw (ca. 200 g of Sabre variety with a water content of 9.7 w/w%) was exhaustively extracted by supercritical carbon dioxide at different pressures and temperatures with a constant supercritical fluid extraction (SFE) flow rate of 5kg h−1 for 420 min. The crude extracts (wax and water) were dried by evaporation and then left under vacuum in a dessicator overnight before weighing. The effect of a modifier (5–10 v∶v% ethanol and acetone) was also tested under the same conditions.Results and discussion
We now report the first example of the selective extraction of valuable wax products from wheat straw utilising CO2 as solvent. The products isolable from wheat straw were determined by Soxhlet extraction, with organic solvents of various polarities, in order to set a comparison basis for the CO2 study. Fig. 1 shows the selectivity achieved using hexane, toluene, methyltetrahydrofuran (methyl-THF), acetone and ethanol. Crude yields varied between 1.1 and 3.1% (EtOH extract) depending on the solvent used.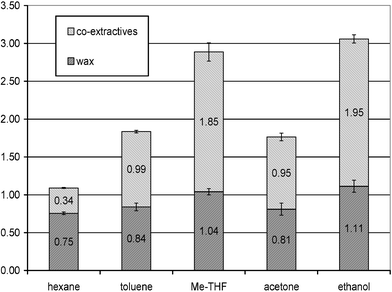 | ||
| Fig. 1 Purified wheat straw wax as a percentage of total wax extract from Soxhlet extraction with hexane, toluene, methyl-tetrahydrofuran (THF), acetone and ethanol. DM : dry matter. | ||
In the most part, the organic solvents provided a complete but unselective extraction of wheat straw wax (<50% of wax present in total extract); hexane proved to be the most selective solvent giving a 70% weight yield of wax compared to total extract.
We also studied the effect of changing the variety of the wheat (see Experimental) on the crude and wax yields using hexane as a solvent. Similarly, great variations in wax recoveries were observed through different varieties (from 0.5 up to 1.0% of dry matter for Sabre) highlighting the potential of increasing wax yield through breeding. In addition, it was demonstrated that wax yields were greatly dependant on the botanical component (experimental) with the leaves showing the highest yield (up to 1.6%) and the internodes the lowest (ca. 0.4%), consistent with previous observations.11 However no appreciable difference in the crude yield was observed from the same variety in different years (1.26 ± 0.04% for 2003 compared to 1.31 ± 0.01% for 2005; see experimental).
Supercritical and liquid CO2 have been shown to be excellent solvents for waxes from several material sources including cosmetic products and sheeps wool.12–13 The properties of CO2 mean that it is a tuneable system and when the system pressure is released it leaves a product with no solvent residue which is desirable in many product end-use industries such as cosmetics. Here CO2 was used as solvent under different conditions of temperature and pressure. The flow rate (5kg h−1) and particle size range (0.5–5 mm) were kept constant throughout the study. Unlike all the organic solvents tested in this study the extraction proved to be completely selective to the desired waxes over a range of conditions.
Experimental design (22 full factorial)14 was applied to determine the optimal temperature and pressure conditions for a maximum yield of the desired waxes (minimum temperature 40 °C and maximum pressure 300 bar corresponding to the maximum CO2 density and therefore solvent strength; Fig. 2). An experiment under parameters at the centre of the matrix (55 °C/200 bar) was conducted to estimate the experimental error and minimise the risk of missing a non-linear relationship in the middle of the domain. This gave a wax yield (0.36%) in agreement with the predicted value (0.35%). In addition, a dynamic study was carried out to determine the optimum extraction time for a maximum wax yield. It was determined that 99.9% of the total extractable wax could be recovered after ca. 100 min while 99.0% could be isolated after less than 70 min.
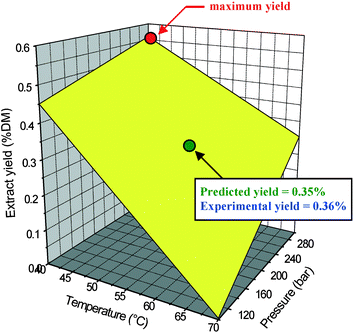 | ||
| Fig. 2 1st order factorial design to optimise extraction of pure waxes from wheat straw (DM: dry matter). | ||
Water is also extracted by the supercritical CO2 in quantities between 3.4–6.3% by weight (dependent on the CO2 pressure and temperature) and unlike the wax products, reaches a maximum at the highest temperature and pressure used (70 °C/300 bar).
The use of a modifier (ethanol and acetone in the range 5–10 v/v%) was also tested. This led to an unselective extraction similar to that obtained using the organic solvents and with total (crude) yields varying with the temperature and pressure.
Most significantly, we have demonstrated that by adjusting the supercritical/liquid CO2 conditions, the waxes can be fractionated into more valuable products. For example, at relatively low pressures, the extract contains a high proportion of alkanes (useful as insect semiochemicals) whereas at higher pressures the extracts contain a high proportion of fatty alcohols (used as cholesterol reducing agents). The waxes and wax fractions were characterised by high temperature GC and GCMS, examples of which are shown in Fig. 3.
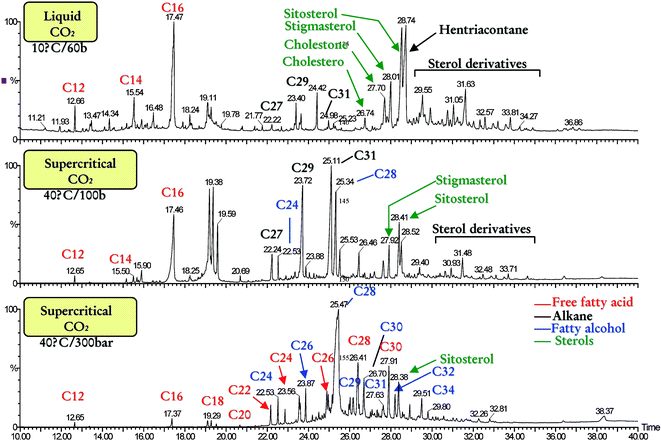 | ||
| Fig. 3 HT-GC/MS chromatograms of fractionated waxes obtained using CO2. Capillary column (DB17-HT, 30m) temperature programmed from 50 °C (1 min) to 350 °C (20 min) at 10 °C min−1. The identity of major compounds is shown on the chromatograms. | ||
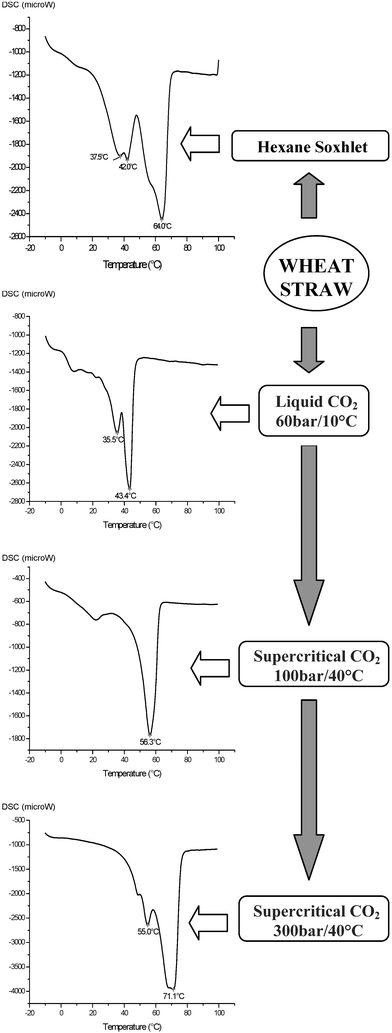
As mixtures of compounds, waxes are often characterised by their melting points; this physical measurement is a useful indicator for application value in areas such as cosmetics. The thermograms of different wax fractions obtained under different CO2 conditions, as measured by Differential Scanning Calorimetry (DSC), complemented the GC/GCMS data in terms of peak complexity and softening temperatures. Thus, while a hexane extract showed multiple peaks ranging from 37–65 °C, the CO2-extracts showed peaks over narrower temperature ranges. With CO2 at 40 °C and 100 bar, a single, albeit broad, peak was observed at <60 °C whereas higher temperature peaks were observed for wax fractions obtained at higher pressures. Interestingly, the DSC for the wax extract using liquid CO2 showed two peaks both at much lower temperatures than the DSCs for the wax extracts using hexane and supercritical CO2. Examples of these thermograms are shown in Fig. 4.
Conclusions
Greater utilisation of natural, renewable resources is vital for an economically viable and environmentally sound society. We have demonstrated a low environmental impact technology for the direct production of valuable consumer products from low or zero value wheat co-products, a sustainable and widely available resource. As part of a successful industrial collaboration with Botanix Ltd, we have scaled up the extractions to >75 kg and the products obtained are being tested in the preparation of formulations, which will be carried out in extended collaboration with industry. Not all the botanical components of the crop are equally valuable so wheat straw may be selectively harvested/chopped. This would have the added advantage of reduced transportation and storage costs. A single-pass multi-component harvesting technology that simultaneously and selectively harvests grain and the desired plant component has been developed in the USA to facilitate this.11 In addition, waxes and/or high-value wax fractions could be further enhanced through wheat selection programmes. Finally, an extension to Green Chemistry methodology to include simple and low environmental impact chemical modifications of the wax products so as to improve properties and add value is also planned.It has to be noted that the same methodology could be applied to other crops or any other process by-products and wastes as the first step of a single integrated facility, commonly named the biorefinery.
Acknowledgements
We thank the University of York and CSL for supporting this project, Botanix Ltd for the use of their pilot plant, and other members of the York Green Chemistry Group for their intellectual input.References
- J. H. Clark, F. E. I. Deswarte and J. J. E. Hardy, PCT Pat. Appl., PCT/GB 0502337.9, 2005 Search PubMed.
- E. Audsley and J. E. Annetts, Agric. Syst., 2003, 76, 39–59 CrossRef.
- Renewable Bioresources, ed. C. V. Stevens and R. Verhé, Wiley, Chichester, 2004 Search PubMed.
- Handbook of Green Chemistry and Technology, ed. J. H. Clark and D. J. Macquarrie, Blackwell, Oxford, 2002 Search PubMed.
- R. C. Sun, J. M. Lawther and W. B. Banks, Carbohydr. Polym., 1996, 29, 325–331 CrossRef CAS.
- R. Sun, J. M. Lawther and W. B. Banks, Ind. Crops Prod., 1996, 5, 291–300 CrossRef.
- X. F. Sun, R. C. Sun, P. Fawler and M. S. Baird, Carbohydr. Polym., 2004, 55, 379–391 CrossRef CAS.
- Waxes: Chemistry, Molecular Biology and Functions, ed. R. J. Hamilton, The Oily Press, Dundee, 1995 Search PubMed.
- A. P. Tulloch, Chemistry of Waxes of Higher Plant, in Chemistry and Biochemistry of Natural Waxes, Elsevier, Amsterdam, 1976 Search PubMed.
- W. W. Christie, Lipid Analysis, The Oily Press, Bridgwater, 3rd edn, 2003 Search PubMed.
- D. N. Thompson, P. G. Shaw and J. A. Lacey, Appl. Biochem. Biotech., 2003, 105–108, 205–218 CrossRef CAS.
- J. Li, Chemom. Intell. Lab. Syst., 1999, 45, 385–395 CrossRef CAS.
- R. Alzaga, E. Pascual, P. Erra and J. M. Bayona, Anal. Chim. Acta, 1999, 381, 39–48 CrossRef CAS.
- M. Kane, J. R. Dean and S. M. Hitchen, Anal. Chim. Acta, 1993, 271, 83–90 CrossRef.
| This journal is © The Royal Society of Chemistry 2006 |