A cyclic voltammetric technique for the detection of micro-regions of bmimPF6/Tween 20/H2O microemulsions and their performance characterization by UV-Vis spectroscopy
Yan'an
Gao
ab,
Na
Li
a,
Liqiang
Zheng
*a,
Xueyan
Zhao
a,
Shaohua
Zhang
a,
Buxing
Han
*b,
Wanguo
Hou
a and
Ganzuo
Li
a
aKey Laboratory of Colloid and Interface Chemistry (Shandong University), Ministry of Education, Jinan 250100, China
bCenter for Molecular Science, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, China
First published on 16th November 2005
Abstract
As green solvents, ionic liquids (ILs) can be substitutes for traditional organic solvents, and form useful microemulsions with water. Microemulsions consisting of the IL, 1-butyl-3-methylimidazolium hexafluorophosphate (bmimPF6), the non-ionic surfactant Tween 20 and water were formed at 30.0 °C, and the phase behavior of the ternary system was investigated. Three regions of the microemulsions: water-in-bmimPF6 (W/IL), bicontinuous, and bmimPF6-in-water (IL/W) were identified by cyclic voltammetry using potassium ferrocyanide K4Fe(CN)6 as an electroactive probe. The polarity of the microemulsion environment was investigated by UV-Visible spectroscopy using methyl orange as a probe. Use of the ionic compound K3Fe(CN)6 in UV-Visible measurements revealed that the bmimPF6/Tween 20/H2O microemulsions could solubilize salt species into the microemulsion droplets. Moreover, the solubilization of riboflavin in the microemulsions was also shown by UV-Visible spectra. These results show that these IL-based microemulsions have potential in the production of metal nanomaterials, in biological extractions or as solvents for enzymatic reactions.
Introduction
Ionic liquids (ILs) are receiving much attention as a class of solvents, because of their special physical and chemical properties, such as low volatility, non-flammability and high thermal stability.1–5 They are also regarded as environmentally benign solvents for chemical reactions, separations, electrochemical applications and, more recently, for syntheses of biopolymers, molecular self-assembly and interfacial syntheses.1,6,7 As potential substitutes for traditional organic solvents, they afford significant environmental benefits and can contribute to green chemistry techniques.The structure of microemulsions is a field of much current interest.8–11 In order to study ionic liquid microemulsions, it is necessary to investigate the microemulsion structure and structural transitions. Although conductivity is frequently used to investigate structure and structural changes in microemulsions on the basis of percolation theory, the microstructure of the ionic liquid microemulsions cannot be differentiated through traditional conductivity measurements because ionic liquids are essentially molten salts. Several measurements of the diffusion coefficients of micelles or microemulsions have been performed using Fourier transform pulsed-gradient spin-echo NMR12–15 and dynamic light scattering,16–18 which provide sensitive tests of structural models of micelles or microemulsions. Also, electrochemical cyclic voltammetry has been successfully used to obtain information about the microstructure of micelles or microemulsions.10,19–35 Changes in the microstructure were identified by using electrochemical probes such as ferrocene or its derivatives, ferricyanide, or methyl viologen. This detection was actually accomplished by determining diffusion coefficients of the probes which made it possible to investigate different microenvironments, in that the electrochemical reversibility of the probes was affected by the structure of the microemulsions and appeared to reflect the ease of mobility across interphases.10 Chokshi and Shah28,29 concluded that the diffusion coefficients probed by electrochemical probes and cyclic voltammetric measurements could also be considered self-diffusion coefficients in microemulsion systems.
As is well known, solubilization of a solute and chemical reactivity are dependent on the micropolarity of dispersed droplets in reverse microemulsions. The local environment within a microemulsion droplet may be characterized by UV-Vis measurements with solvatochromic probes. The solvatochromic probes selected should be anchored to the polar core of the aggregates, to satisfy the procedural requirement of being soluble in the local environment media. Furthermore, this probe must be sensitive to the polarity of its environment and reflect the polarity through a shift of absorption maximum. Methyl orange has been successfully used to detect the microenvironment in ammonium carboxylate perfluoro polyether (PFPE) surfactant reverse microemulsions in CO2,36 and TX-100 reverse micelles in cyclohexane.37 Recently, our group showed that a water domain exists in Dynol-604-based water-in-CO2 microemulsions.38 A low polarity environment in fluorous surfactant F-53B based water-in-CO2 microemulsions has also been found.39
UV-Vis measurements are frequently used to characterize the solubilization of metal salts or biochemical compounds. Aqueous acidified K2Cr2O7 has been found to be completely soluble in water-in-CO2 microemulsion by UV-Vis spectroscopy.36 Han and co-workers employed UV-Vis spectroscopy to monitor the extraction of trypsin solubilized in AOT/decane/water reverse micelles into compressed CO2.40
Recently, microemulsions have been used in the preparation and processing of various materials.41–46 In the production of metal or semiconductor nanomaterials, water-in-oil microemulsions act as aqueous micro-reactors to dissolve metal salts. One major limitation to the broader use of ILs is their inability to dissolve a number of chemicals including some hydrophilic substances. In order to extend IL-based microemulsions to the field of nanomaterials, it is necessary to investigate the solubilization of metal salts in ionic liquid microemulsions. Similarly, the solubilization of biological molecules in microemulsions is essential to biological applications such as enzyme-catalyzed reactions and extraction. To overcome these limitations, microemulsions consisting of 1-butyl-3-methylimidazolium hexafluorophosphate (bmimPF6), water and TX-100 have been prepared in which water is dispersed in a continuous ionic liquid phase,47 thus allowing hydrophilic substances to be solubilized in the microscopic water droplets. In this work, microemulsions consisting of bmimPF6, the non-ionic surfactant Tween 20 and water were formed at 30.0 °C, and the phase behavior of the system was investigated. Cyclic voltammetry provided direct insight into the microstructure and structural changes of our ionic liquid microemulsions, and methyl orange was also used to probe the polarity environment of the ionic liquid microemulsions by UV-Vis spectroscopy. The ionic complex K3Fe(CN)6 and riboflavin were chosen as the test salt and biological molecule, respectively. The experimental results showed that water domains with the approximate properties of bulk water exist in the microemulsions, thus allowing solubilization of ionic and biological compounds.
Experimental
Materials
The non-ionic surfactant Tween 20 and biochemical reagent riboflavin were purchased from Sigma and used as received. Potassium ferrocyanide, K4Fe(CN)6, used as an electrochemical probe, was purchased from Shanghai Experimental Reagent Co., Ltd. Potassium ferricyanide K3Fe(CN)6 was obtained from Fisher. Methyl orange was supplied by Beijing Chemical Reagent Co. The ionic liquid bmimPF6 used in our experiments was synthesized according to the method reported previously.48 Water used was deionized and doubly distilled.Apparatus and procedure
For cyclic voltammetry, an electrochemical analyzer, CHI832A (CH Instruments, Austin, TX) was used. The electrochemical measurements were conducted using a three-electrode configuration, which consisted of a glassy carbon working electrode, a Ag/AgCl reference electrode, and a platinum flake counter-electrode. The spacing between adjacent electrodes was set at 2.0 cm. All potentials quoted are with respect to the Ag/AgCl reference. Before each measurement, the working electrode was polished using 0.05 µm aluminum oxide slurries and then washed carefully with distilled water. After polishing, the electrode was ultrasonicated in distilled water for about 5 min immediately before use. The working electrode areas were determined using cyclic voltammetry experiments on a reversible system (4 mM K3Fe(CN)6 in 1 M KCl), by use of the diffusion coefficient D = 6.3 × 10−10 m2 s−1.20 The electrode area was 4.15 × 10−6 m2. The potential was scanned between 0.5 and −0.5 V, and the sweep rate range was 20–100 mV s−1. The experiments were carried out under a nitrogen atmosphere to avoid the effect of oxygen. All experiments were carried out at 30 ± 0.5 °C.The UV-Vis spectra were performed on a computer-controlled UV-Vis spectrometer (TU-1201, Beijing Instrument Company). The path length of the quartz cell used in this experiment was 1 cm. Appropriate amounts of substances were uniformly mixed in advance and then added to the quartz cell. The experiment was carried out at 30.0 °C.
Results and discussion
The electrochemical behavior of the K4Fe(CN)6 probe in the ionic liquid microemulsion
In cyclic voltammetry, the peak current ip for a redox-active reversible system is given by the Randles–Sevcik equation:28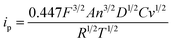 | (1) |
It follows from eqn (1) that ipwill increase linearly with v1/2 for a given electrode and a constant electroactive probe concentration. Diffusion coefficients can be obtained from a linear regression of the slope of ip versus v1/2 using the known surface area of the electrode. With microemulsion systems involving an electrochemical probe completely solubilized in the microemulsion, the diffusion coefficient D in eqn (1) corresponds to the microemulsion diffusion coefficient, since the probe diffuses with the microemulsion droplets.19 The cyclic voltammetry experiments were carried out at various scan rates in microemulsion environments in order to verify the diffusion-controlled nature of the process. Electrochemical charge transfer must be diffusion-controlled in order to study the microstructure of microemulsions by cyclic voltammetry.28Fig. 1 shows typical plots of ip versus v1/2 for 3 microemulsion systems, and the plots are straight lines passing through the origin. This fact indicates that the electron transport for the [Fe(CN)6]3−/[Fe(CN)6]4− electrode reactions in the microemulsion medium is diffusion-controlled.19,23,24 Similar results were observed for all the other microemulsion systems we made. Thus, potassium ferricyanide, K4Fe(CN)6, is a suitable electrochemical probe to study the microstructures and structural transitions of these microemulsions.
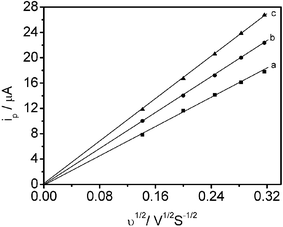 | ||
| Fig. 1 Scan rate dependence of peak current in ionic liquid microemulsions: (a) 66.9% Tween 20 + 11.8% bmimPF6 + 21.3% H2O; (b) 54.1% Tween 20 + 9.6% bmimPF6 + 36.3% H2O; (c) 46.7% Tween 20 + 8.2% bmimPF6 + 45.1% H2O. 0.037 g K4Fe(CN)6 was added as electroactive probe for all systems. | ||
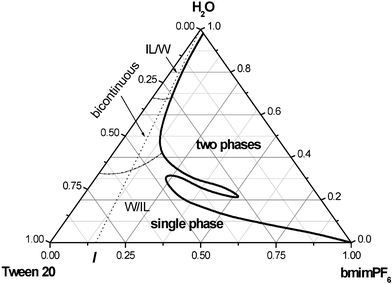
Phase behavior of the Tween 20/bmimPF6/H2O three-component system
The test microemulsions consisted of hydrophobic ionic liquid bmimPF6, surfactant Tween 20 and water. In preparation of the microemulsions, each component was added by weight and the solution mechanically stirred until clear and homogeneous. The aqueous solution of probe was freshly made and used immediately. The phase behavior of the Tween 20/bmimPF6/water three-component microemulsions at 30.0 °C is shown in Fig. 2. The phase diagram was constructed by titration with water as follows. Firstly, the required masses of Tween 20 and bmimPF6 were mixed. For each titration, the bmimPF6-to-Tween 20 weight ratio (I) was fixed. The phase boundaries were determined by observing the transition from turbidity to transparency or from transparency to turbidity. In Fig. 2, the region marked “single phase” was transparent and the region marked “two phases” was turbid. By repeating the experiment for other I values, the phase diagram was established.The diffusion behavior of the probe in the microemulsion
Test microemulsions were prepared using the ratios indicated by the dashed line in the phase diagram (Fig. 2): bmimPF6-to-Tween 20 weight ratios, I = 0.05, 0.11, 0.18, 0.25 and 0.33 were chosen. This range of compositions corresponds to a relatively low IL content and a wide range of water content. The compositions of the microemulsion were changed by adding water. All results are, therefore, reported as a function of water content. The content of each component in the solutions is derived from precise weight measurements. No phase separation was found in any of the microemulsions investigated after storage at room temperature for two months. It is obvious from Fig. 2 that the compositions of the microemulsions changed in a systematic fashion to span the range of possible structures from microdroplets to bicontinuous structures, because a single microemulsion region can always be observed along the dashed line from relatively high bmimPF6 content to relatively high water content. The single phase region is suitable for studying the microstructure and structural transition of the microemulsions.10,19,20 The electroactive probe concentration had little effect on the diffusion coefficients D, since the shape and size of the microemulsions were not disturbed by the presence of the electroactive probe over a large concentration range.33 This is confirmed by the fact that the electron transport of [Fe(CN)6]3−/[Fe(CN)6]4− electrode reactions was diffusion-controlled. Therefore, our experiments were carried out with a fixed initial K4Fe(CN)6 quantity and the concentration of the probe changed by the addition of water.As an example, the changes in the diffusion coefficient of the probe, estimated from the Randles–Sevcik equation with I = 0.18 as a function of added water in microemulsions, are illustrated in Fig. 3. It can be seen that the diffusion coefficients of K4Fe(CN)6 increase with increasing water content in the whole single phase microemulsion region. When the water content was less than about 34%, the diffusion coefficients increased gradually, because at low water content, a water-in-bmimPF6 microemulsion formed. K4Fe(CN)6 is expected to probe the water environment preferentially due to its limited solubility in bmimPF6. Therefore, the diffusion coefficient of K4Fe(CN)6 should correspond to the diffusion coefficient of water-in-bmimPF6 microemulsion droplets. Thus the value of the diffusion coefficient of the probe is relatively small.
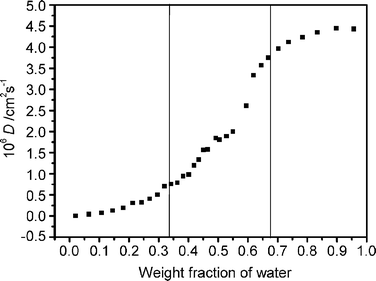 | ||
| Fig. 3 The diffusion coefficient of K4Fe(CN)6 as a function of water content with bmimPF6-to-TX100 weight ratio I = 0.18 and initial K4Fe(CN)6 quantity = 0.037 g. | ||
Lindman et al.49 also discovered that molecules confined in a closed domain will give rise to low self-diffusion coefficients. The diffusion coefficients increase with the increasing water content, but as long as the diffusion of probe is confined in microemulsion droplets, the curve increases gently. Therefore, the gradual change indicates that the microenvironment of the microemulsions is unchanged. A similar trend was also observed for this microemulsion system when the water content was above 67%. In the latter case, the ionic liquid microdroplets were dispersed in a continuous water medium. The diffusion coefficient of the probe may correspond to the continuous water medium and show high mobility, and thus a high diffusion coefficient. The gradual increase of the diffusion coefficients derives from the fact that the aqueous microenvironment is not sensitive to water content in continuous water phase microemulsions. However, a relatively dramatic change in probe diffusion coefficients with water content is observed when the water content is in the range 34–67%, which indicates that the environment of the microemulsions is different from the droplet structure of the microemulsions. According to the principle of distinguishing sub-regions in microemulsions using apparent diffusion coefficients,19,20,49 these results suggest that when the water content is 34–67%, a bicontinuous microstructure, in which water and immiscible bmimPF6 are both local continuous phases, is formed. Lindman et al.49 stated that molecules in the continuous medium will be characterized by rapid diffusion. Thus a continuous structure is expected to give a higher self-diffusion coefficient than a droplet structure.25 Therefore, we can recognize the structure and structural transition of the microemulsions by comparison of the diffusion coefficients of the electrochemical probe, and deduce the existence of three types of microstructure of IL microemulsions including W/IL, bicontinuous and IL/W, differentiated by different I values. The structural transition points at different I values are listed in Table 1, and the differentiated microemulsion domains are shown in Fig. 2.
Microenvironment of the ionic liquid microemulsions
Methyl orange is a sensitive solvatochromic probe of aqueous and microemulsion environments. As the water content in microemulsions increases, the visible absorption maximum λmax of methyl orange shifts to longer wavelengths (red shift), indicating greater polarity.39Fig. 4 shows the UV-Vis absorbance spectra of different amounts of 9.6 × 10−5 M aqueous methyl orange solution added to the microemulsion. As expected, the absorption maximum λmax of methyl orange increases. The λmax of methyl orange in the microemulsions is in the range 425.8–433.8 nm, while the λmax of methyl orange is 464 nm in pure water and 417 nm in ethanol. These results suggest that the polarity of the environment of the solubilized methyl orange is intermediate between that of bulk water and ethanol; that is, the polarity of the water domains in water-in-bmimPF6 reverse microemulsions is lower than that of bulk water, which has also been observed for other reverse microemulsions.50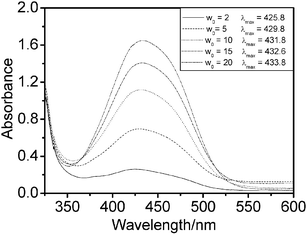 | ||
| Fig. 4 Absorbance of methyl orange in the ionic liquid microemulsion at I = 4.00 as a function of added aqueous methyl orange solution at 30.0 °C. | ||
The solubilities of salt and biochemical species
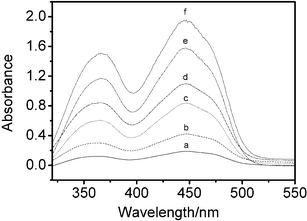
The use of conventional water-in-oil reverse microemulsions as micro-reactors is well established. Several different processes have been investigated including enzymatic reactions51–54 and synthesis of metallic and semiconductor nanomaterials.41–46 One of the interests in this study is to investigate the possibility of dissolution of ionic metal compounds and biochemical reagents in ionic liquid microemulsions. These compounds can only dissolve if the solvating properties of the medium approach those of pure water. K3Fe(CN)6 was chosen mainly due to the fact that K3Fe(CN)6 is insoluble in bmimPF6 and Tween 20, even after prolonged ultrasonication of the mixture. However, after adding water, the microemulsion system takes on a distinct yellow-green color, indicating that K3Fe(CN)6 is solubilized in micro-aqueous regions. The UV-Vis spectra (Fig. 5) reveal that the spectroscopic features are similar to those in conventional aqueous solution. The effect of different concentrations of K3Fe(CN)6 in water-in-bmimPF6 reverse microemulsions on the absorption spectra was also studied. A series of aqueous K3Fe(CN)6/Tween 20/bmimPF6 microemulsions were prepared with a fixed bmimPF6-to-Tween 20 weight ratio, I = 4.00, and a fixed water content (ratio of water to surfactant, W0 = 10), but with different K3Fe(CN)6 concentrations. As shown in Fig. 6, the observed absorbance of K3Fe(CN)6 at the maximum wavelength increases linearly with K3Fe(CN)6 concentration. The result is in good agreement with the Lambert–Beer plot.55 Thus, UV-Vis spectroscopy confirms the solubilization of K3Fe(CN)6 in the ionic liquid microemulsions. Considering that K3Fe(CN)6 is a highly ionic compound and requires a strong aqueous environment to dissolve,56 it is likely that the microemulsion system will dissolve other metal ions.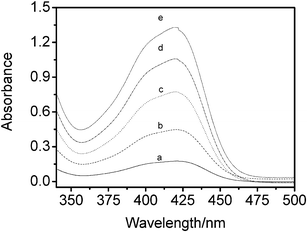 | ||
| Fig. 5 Absorption spectra of K3Fe(CN)6 in water-in-bmimPF6 reverse microemulsions at 30.0 °C. a: 0.17 mM; b: 0.40 mM; c: 0.72 mM; d: 1.00 mM; e: 1.22 mM. | ||
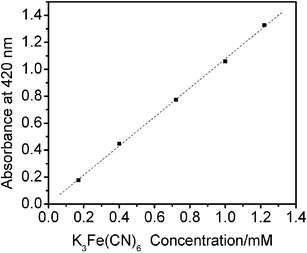 | ||
| Fig. 6 The maximum absorbance intensity of aqueous K3Fe(CN)6 solution in ionic liquid microemulsions with different K3Fe(CN)6 concentrations. | ||
In biochemistry, a number of intractable questions focus on the separation of biological molecules. The use of microemulsion droplets to extract biological molecules and support biological reactions has been investigated.52–54 We investigated the solubilization of biochemical reagents in the bmimPF6/Tween 20/water IL microemulsion. Riboflavin, a yellow-orange compound, was used as a molecular spectroscopic probe. Riboflavin does not dissolve in pure bmimPF6, however, if water-in-IL reverse microemulsions are present, the solvent takes on a yellow-orange transparent phase, indicating that the microemulsion droplets can dissolve riboflavin.
A saturated aqueous riboflavin solution (about 3 × 10−4 M at 30.0 °C) was used to adjust the ratio of water to surfactant, W0. Fig. 7 shows the UV-Vis spectra for riboflavin in microemulsions with different W0 at I = 4.00. Over the wavelength range from 320 to 550 nm, there are two absorbance peaks at 363 ± 2 and 445 ± 1 nm, while the UV-Vis spectrum of riboflavin in pure water has λmax at 373 ± 2 and 444 ± 2 nm.56 The aqueous environment in the ionic liquid microemulsions is not exactly the same as in bulk water. The riboflavin absorbance increased with the addition of saturated riboflavin solution, but the plot of maximum absorbance values versus riboflavin concentration was not linear (Fig. 8). The maximum absorbances are distinctly lower (relative to the dashed line) for the microemulsions with low water content, such as W0 = 2 and 5. The reason is that the solubilized water in these microemulsion systems exhibits behavior markedly different from that of bulk water, and the bulk water content is too small to dissolve riboflavin in a microemulsion with a small W0 value. It is likely that in these samples a portion of the riboflavin was still in the solid particle form, dispersed in these microemulsion droplets. Even so, this class of novel microemulsions has the potential to dissolve biological molecules.
 | ||
| Fig. 7 Absorption spectra of riboflavin in water-in-IL microemulsions at I = 4.00 with different W0 values at 30.0 °C. a: W0 = 2; b: W0 = 5; c: W0 = 8; d: W0 = 10; e: W0 = 16 and f: W0 = 20. | ||
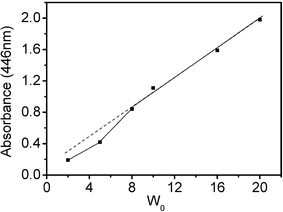 | ||
| Fig. 8 The maximum absorbance intensity of aqueous riboflavin solution in IL microemulsions with different W0 values (the components of the samples are the same as those in Fig. 7). | ||
Conclusion
In summary, microemulsions consisting of bmimPF6, the surfactant Tween 20 and water were prepared and the phase behavior of the ternary system was investigated. The water-in-bmimPF6 (W/IL), bicontinuous, and bmimPF6-in-water (IL/W) micro-regions of the ionic liquid microemulsions were successfully identified by a simple cyclic voltammetry method using potassium ferrocyanide K4Fe(CN)6 as the electroactive probe. The UV-Vis absorption spectra of methyl orange indicated that the polarity of the water domains in water-in-bmimPF6 reverse microemulsions was lower than that of bulk water. The experimental results revealed that the water domains were sufficiently well formed to support the solubility of ionic species in the water-in-bmimPF6 microemulsions, and spectroscopic data indicated that the ionic species was in aqueous solution. We have also demonstrated that a biological molecule, riboflavin, may be solubilized in the ionic liquid microemulsion droplets. These results are essential to the use of water-in-bmimPF6 microemulsions as solvent systems in the preparation and processing of semiconductors and nanomaterials, in enzyme-catalyzed reactions and in extraction applications.Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (20133030, 50472069) and the Ministry of Science and Technology for financial support (2003CCA02900). They would also like to thank Dr Pamela Holt, Shandong University, for helpful discussions in the preparation of the manuscript.References
- T. Welton, Room-temperature ionic liquids. solvents for synthesis and catalysis, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS.
- S. G. Kazarian, B. J. Briscoe and T. Welton, Combining ionic liquids and supercritical fluids: in situ ATR-IR study of CO2 dissolved in two ionic liquids at high pressures, Chem. Commun., 2000, 2047–2048 RSC.
- H. X. Gao, J. C. Li, B. X. Han, W. N. Chen, J. L. Zhang, R. Zhang and D. D. Yan, Microemulsions with ionic liquid polar domains, Phys. Chem. Chem. Phys., 2004, 6, 2914–2916 RSC.
- J. L. Anderson, J. Ding, T. Welton and D. W. Armstrong, Characterizing ionic liquids on the basis of multiple solvation interactions, J. Am. Chem. Soc., 2002, 124, 14247–14254 CrossRef CAS.
- J. L. Anderson, V. Pino, E. C. Hagberg, V. V. Sheares and D. W. Armstrong, Surfactant solvation effects and micelle formation in ionic liquids, Chem. Commun., 2003, 2444–2445 RSC.
- B. P. Binks, A. K. F. Dyab and P. D. I. Fletcher, Novel emulsions of ionic liquids stabilised solely by silica nanoparticles, Chem. Commun., 2003, 2540–2541 RSC.
- T. Nakashima and N. Kimizukua, Interfacial synthesis of hollow TiO2 microspheres in ionic liquids, J. Am. Chem. Soc., 2003, 125, 6386–6387 CrossRef CAS.
- P. G. de Gennes and C. Taupin, Microemulsions and the flexibility of oil/water interfaces, J. Phys. Chem., 1982, 86, 2294–2304 CrossRef CAS.
- E. W. Kaler, K. E. Bennett, H. T. Davies and L. E. Scriven, Toward understanding microemulsion microstructure: A small-angle X-ray scattering study, J. Chem. Phys., 1983, 79, 5673–5684 CrossRef CAS.
- R. A. Mackay, S. A. Myers, L. Bodalbhai and A. Brajter-Toth, Microemulsion structure and its effect on electrochemical reactions, Anal. Chem., 1990, 62, 1084–1090 CrossRef CAS.
- A. Ceglie, K. P. Das and B. Lindman, Microemulsion structure in four-component systems for different surfactants, Colloids Surf., 1987, 28, 29–40 CrossRef CAS.
- C. H. Chew, L. M. Gan, L. H. Ong and K. Zhang, Bicontinuous structures of polymerized microemulsions: 1H NMR self-diffusion and conductivity studies, Langmuir, 1997, 13, 2917–2921 CrossRef CAS.
- A. Spernath, A. Yaghmur, A. Aserin, R. E. Hoffman and N. Garti, Self-diffusion nuclear magnetic resonance, microstructure transitions, and solubilization capacity of phytosterols and cholesterol in Winsor IV food-grade microemulsions, J. Agric. Food Chem., 2003, 51, 2359–2364 CrossRef CAS.
- H. Kataoka, T. Eguchi, H. Masui, K. Miyakubo, H. Nakayama and N. Nakamura, Scaling relation between electrical conductivity percolation and water diffusion coefficient in sodium bis(2-ethylhexyl) sulfosuccinate-based microemulsion, J. Phys. Chem. B, 2003, 107, 12542–12548 CrossRef CAS.
- E. Johannessen, H. Walderhaug and B. Balinov, Aqueous microemulsions of a fluorinated surfactant and oil studied by PFG-NMR: transformation from threadlike to spherical micelles, Langmuir, 2004, 20, 336–341 CrossRef CAS.
- C. A. T. Laia, P. Lopez-Cornejo, S. M. B. Costa, J. d'Oliveira and J. M. G. Martinho, Dynamic light scattering study of AOT microemulsions with nonaqueous polar additives in an oil continuous phase, Langmuir, 1998, 14, 3531–3537 CrossRef CAS.
- C. A. T. Laia, W. Brown, M. Almgren and S. M. B. Costa, Light scattering study of water-in-oil AOT microemulsions with poly(oxy)ethylene, Langmuir, 2000, 16, 465–470 CrossRef CAS.
- M. M. Velazquez, M. Valero and F. Ortega, Light scattering and electrical conductivity studies of the aerosol OT toluene water-in-oil microemulsions, J. Phys. Chem. B, 2001, 105, 10163–10168 CrossRef CAS.
- C. Mo, 1.5-Order differential electroanalysis on Triton X-100 microemulsion, Langmuir, 2002, 18, 4047–4053 CrossRef CAS.
- C. Mo, M. H. Zhong and Q. Zhong, Investigation of structure and structural transition in microemulsion systems of sodium dodecyl sulfonate + n-heptane + n-butanol + water by cyclic voltammetric and electrical conductivity measurements, J. Electroanal. Chem., 2000, 493, 100–107 CrossRef CAS.
- J. F. Rusling, C. N. Shi and T. F. Kumosinski, Diffusion of micelle-bound molecules to electrodes in solutions of ionic surfactants, Anal. Chem., 1988, 60, 1260–1267 CrossRef CAS.
- B. Geetha and A. B. Mandal, Self-diffusion studies on ω-methoxy polyethylene glycol macromonomer micelles by using cyclic voltammetric and fourier transform pulsed gradient spin-echo nuclear magnetic resonance techniques, Langmuir, 1995, 11, 1464–1467 CrossRef CAS.
- A. B. Mandal, Self-diffusion studies on various micelles using ferrocene as electrochemical probe, Langmuir, 1993, 9, 1932–1933 CrossRef CAS.
- A. B. Mandal and B. U. Nair, Cyclic voltammetric technique for the determination of the critical micelle concentration of surfactants, self-diffusion coefficient of micelles, and partition coefficient of an electrochemical probe, J. Phys. Chem., 1991, 95, 9008–9013 CrossRef CAS.
- S. A. Myers, R. A. Mackay and A. Brajter-Toth, Solution microstructure and electrochemical reactivity. Effect of probe partitioning on electrochemical formal potentials in microheterogeneous solutions, Anal. Chem., 1993, 65, 3447–3453 CrossRef CAS.
- C. Mo, The structural conversion in microemulsion systems of C12H25SO3Na–C4H9OH–C7H16–H2O, Chin. Chem. Lett., 2000, 11, 271–274 CAS.
- A. B. Mandal, B. U. Nair and D. Ramaswamy, Determination of the critical micelle concentration of surfactants and the partition coefficient of an electrochemical probe by using cyclic voltammetry, Langmuir, 1988, 4, 736–739 CrossRef CAS.
- K. Chokshi, S. Qutubuddin and A. Hussam, Electrochemical investigation of microemulsions, J. Colloid Interface Sci., 1989, 129, 315–326 CrossRef CAS.
- D. M. Shah, K. M. Davies and A. Hussam, Electrochemical investigation of amphiphilic cobalt(III) complexes and ferrocene derivatives in sodium dodecyl sulfate microemulsions, Langmuir, 1997, 13, 4729–4736 CrossRef CAS.
- X. L. Zu and J. F. Rusling, Amphiphilic ferrocene alcohols as electroactive probes in micellar solutions and microemulsions, Langmuir, 1997, 13, 3693–3699 CrossRef CAS.
- J. Santhanalakshmi and G. Vijayalakshmi, Microstructure and diffusion properties of dodecane ionic microemulsions containing cobalticenium ion as the electrochemical probe, Langmuir, 1997, 13, 3915–3920 CrossRef CAS.
- J. F. Rusling and G. N. Kamau, Electrocatalytic reactions in organized assemblies. Part II. Electrocatalytic reduction of allyl chloride by tris(2,2′-biypridyl)cobalt(II) in micelles of dodecyclsulfate, J. Electroanal. Chem., 1985, 187, 355–359 CrossRef CAS.
- D. M. Shah, K. M. Davies and A. Hussam, Electrochemical investigation of amphiphilic cobalt(III) complexes and ferrocene derivatives in sodium dodecyl sulfate microemulsions, Langmuir, 1997, 13, 4729–4736 CrossRef CAS.
- G. Gounili, J. M. Bobbitt and J. F. Rusling, Electron transfer between amphiphilic ferrocenes and electrodes in a bicontinuous microemulsion, Langmuir, 1996, 11, 2800–2805.
- R. A. Mackay, S. A. Myers, L. Bodalbhai and A. Brajter-Toth, Microemulsion structure and its effect on electrochemical reactions, Anal. Chem., 1990, 62, 1084–1090 CrossRef CAS.
- M. J. Clarke, K. L. Harrison, K. P. Johnston and S. M. Howdle, Water in supercritical carbon dioxide microemulsions: spectroscopic investigation of a new environment for aqueous inorganic chemistry, J. Am. Chem. Soc., 1997, 119, 6399–6406 CrossRef CAS.
- D. M. Zhu and Z. A. Schelly, Investigation of the microenvironment in Triton X-100 reverse micelles in cyclohexane, using methyl orange as a probe, Langmuir, 1992, 8, 48–50 CrossRef CAS.
- J. Liu, B. Han, G. Li, X. Zhang, J. He and Z. Liu, Investigation of nonionic surfactant Dynol-604 based reverse microemulsions formed in supercritical carbon dioxide, Langmuir, 2001, 17, 8040–8043 CrossRef CAS.
- Y. N. Gao, W. Z. Wu, B. X. Han, G. Z. Li, J. W. Chen and W. G. Hou, Water-in-CO2 microemulsions with a simple fluorosurfactant, Fluid Phase Equilib., 2004, 226, 301–305 CrossRef CAS.
- J. Chen, J. Zhang, D. Liu, Z. Liu, B. Han and G. Yang, Investigation on the precipitation, microenvironment and recovery of protein in CO2-expanded reverse micellar solution, Colloids Surf., B, 2004, 33, 33–37 CrossRef CAS.
- H. N. Ghosh and S. Adhikari, Trap state emission from TiO2 nanoparticles in microemulsion solutions, Langmuir, 2001, 17, 4129–4130 CrossRef CAS.
- M. Summers, J. Eastoe and S. Davis, Formation of BaSO4 nanoparticles in microemulsions with polymerized surfactant shells, Langmuir, 2002, 18, 5023–5026 CrossRef CAS.
- Z. L. Yin, Y. Sakamoto, J. L. Yu, S. X. Sun, O. Terasaki and R. R. Xu, Microemulsion-based synthesis of titanium phosphate nanotubes via amine extraction system, J. Am. Chem. Soc., 2004, 126, 8882–8883 CrossRef CAS.
- R. B. Khomane, A. Manna, A. B. Mandale and B. D. Kulkarni, Synthesis and characterization of dodecanethiol-capped cadmium sulfide nanoparticles in a Winsor II microemulsion of diethyl ether/AOT/water, Langmuir, 2002, 18, 8237–8240 CrossRef CAS.
- S. Modes and P. Lianos, Luminescence probe study of the conditions affecting colloidal semiconductor growth in reverse micelles and water-in-oil microemulsions, J. Phys. Chem., 1989, 93, 5854–5859 CrossRef CAS.
- J. L. Zhang, M. Xiao, Z. M. Liu, B. X. Han, T. Jiang, J. He and G. Y. Yang, Preparation of ZnS/CdS composite nanoparticles by coprecipitation from reverse micelles using CO2 as antisolvent, J. Colloid Interface Sci., 2004, 273, 160–164 CrossRef CAS.
- Y. A. Gao, S. B. Han, B. X. Han, G. Z. Li, D. Shen, Z. H. Li, J. M. Du, W. G. Hou and G. Y. Zhang, TX-100/water/1-butyl-3-methylimidazolium hexafluorophosphate microemulsions, Langmuir, 2005, 21, 5681–5684 CrossRef CAS.
- J. Dupont, C. S. Consorti, P. A. Z. Suarez and R. F. Souza, Preparation of 1-butyl-3-methylimidazolium-based room temperature ionic liquids, Org. Synth., 1999, 79, 236–240.
- P. Guering and B. Lindman, Droplet and bicontinuous structures in microemulsions from multicomponent self-diffusion measurements, Langmuir, 1985, 1, 464–468 CrossRef CAS.
- M. B. Lay, C. J. Drummond, P. J. Thistlethwaite and F. Grieser, ET(30) as a probe for the interfacial microenvironment of water-in-oil microemulsions, J. Colloid Interface Sci., 1989, 128, 602–604 CAS.
- Y. L. Khmelnitsky, A. V. Hock, C. Veeger and A. J. W. G. Visser, Detergentless microemulsions as media for enzymatic reactions: spectroscopic and ultracentrifugation studies, J. Phys. Chem., 1989, 93, 872–878 CrossRef CAS.
- P. Plucinsk and W. Nistsch, Kinetics of interfacial phenylalanine solubilization in a liquid/liquid microemulsion system, J. Phys. Chem., 1993, 97, 8983–8998 CrossRef.
- D. K. Lavallee, E. Huggins and S. Lee, Kinetics and mechanism of the hydrolysis of chlorophyll a in ternary solvent microemulsion media, Inorg. Chem., 1982, 21, 1552–1553 CrossRef CAS.
- M. Varshney, T. E. Morey, D. O. Shah, J. A. Flint, B. M. Moudgil, C. N. Seubert and D. M. Dennis, Pluronic microemulsions as nanoreservoirs for extraction of bupivacaine from normal saline, J. Am. Chem. Soc., 2004, 126, 5108–5112 CrossRef CAS.
- M. J. Meziani and Y. P. Sun, Spectrophotometry study of aqueous salt solution in carbon dioxide microemulsions, Langmuir, 2002, 18, 3787–3791 CrossRef CAS.
- B. H. Hutton, J. M. Perera, F. Grieser and G. W. Stevens, AOT reverse microemulsions in scCO2 – a further investigation, Colloids Surf., A, 2001, 189, 177–181 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |