A simple and “green” method for the synthesis of Au, Ag, and Au–Ag alloy nanoparticles
Poovathinthodiyil
Raveendran
*ab,
Jie
Fu
a and
Scott L.
Wallen
*a
aDepartment of Chemistry and NSF STC for Environmentally Responsible Solvents and Processes, The University of North Carolina, Chapel Hill, North Carolina -27599-3290, USA. E-mail: wallen@email.unc.edu
bNational Institute for Advanced Industrial Science and Technology, 4-2-1, Nigatake, Miyagino-ku Sendai 983-8551, Japan. E-mail: ravi@ni.aist.go.jp
First published on 30th November 2005
Abstract
Integration of “green chemistry” principles into nanotechnology is one of the key issues in nanoscience research today. In this work, we report an environmentally benign method for the preparation of Au, Ag, and Au–Ag nanoparticles in water, using glucose as the reducing agent and starch as the protecting agent. The alloy nanoparticles prepared in this way appears to be homogeneous and their sizes are well within the quantum size domain (<10 nm), where they are more amenable to size-dependent changes in electronic properties.
1. Introduction
Metal and alloy nanoparticles in the quantum-size domain have attracted much attention over the last decade with the possibilities of size dependent tuning of their electrical, optical and catalytic properties as well as the potential technological revolutions in the quantum-size domain.1–17 Alloy nanoparticles have received special attention due to the possibility of tuning the optical and electronic (and thus catalytic) properties over a broad range by simply varying the alloy composition. One of the key issues in the synthesis of alloy nanoparticles is the atomic scale phase separation resulting in core–shell nanoparticles.18–23 While there are several methods reported for the synthesis of metal and alloy nanoparticles, only some of these methods result in the formation of alloy nanoparticles below 10 nm size range, where their properties exhibit considerable size-dependent variations. With significant growth in the rather cross-disciplinary nanoscience research involving chemists, physicists, biologists and engineers, researchers have begun concerned about the need for developing more environmentally friendly and sustainable methods for the synthesis of nanomaterials. There is a current drive to integrate all the “green chemistry” approaches to design environmentally benign materials and processes.24–25 Such an approach will also be of advantage for the integration of metal and alloy quantum dots into biologically relevant systems. Recently, we had suggested an environmentally friendly method for the synthesis and stabilization of metal nanoparticles by suitable choice of materials and solvents.26 It was shown that by gently heating an aqueous solution of silver nitrate, soluble starch and glucose, one can synthesize “starched” silver nanoparticles in the 1–8 nm size range. While glucose serves as an environmentally benign reducing agent for the metal ions, starch provides stable surface passivation or protection to prevent the aggregation of these particles. Apart from being environmentally benign, there is a key advantage in using glucose as the reducing agent. It is a mild reagent (requires heat or a base catalyst for activation) and thereby it is possible to control the kinetics of the reduction process by varying the temperature or the pH of the solution. In the present work, we demonstrate the synthesis of starch-protected silver (Ag), gold (Au) and Ag–Au alloy nanoparticles.2. Results and discussion
The intra- and inter-molecular associations of the starch molecules facilitated by the extensive networks of hydrogen bonds provide nanoscopic solution domains for the growth of nanoparticles. The starch hydroxyl groups also help to passivate the surfaces of these particles, in the absence of which they will aggregate as a result of high surface energies. The production of such “starched” silver particles is very simple.26 By gentle heating of an aqueous silver nitrate solution containing soluble starch and D-glucose, we had obtained relatively mono-disperse silver nanoparticles. We extended this method for the production of silver nanoparticles by incorporating microwave heating. Aqueous starch dispersions containing Ag+ ions are prepared by adding 10 µl of a 0.1 M solution of AgNO3 (Sigma) and 25 µl of a 0.1 M solution of D-glucose (Fluka) to about 2 ml of 0.20%w aqueous solution of soluble starch (Sigma). This was then treated in a microwave oven for 60 s for the reduction of metal ions. In this case, the reduction takes place under boiling conditions.The advantage of using microwave radiation is that it provides uniform heating around the nanoparticles and can assist the digestive ripening of such particles without aggregation. The transmission electron microscope (TEM) image of the silver nanoparticles synthesized using this method is presented in Fig. 1. The corresponding UV-Visible absorption spectrum (λmax = 418 nm) and a histogram describing the dispersity in size distribution of the particles are also shown.
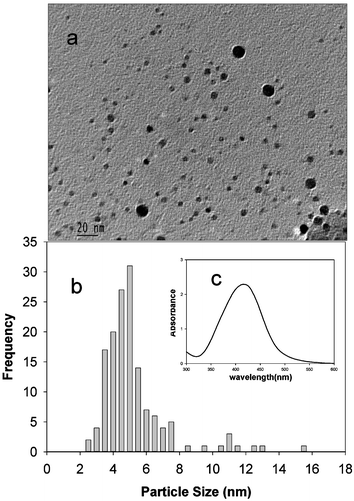 | ||
| Fig. 1 (a) Typical TEM image of the “starched” silver nanoparticles synthesized in microwave radiation (average particles size = 5.9 nm, δ = 2.0 nm); (b) particle size distribution; (c) UV-Visible absorption spectrum. | ||
It is seen that the size dispersity is close to that observed in our previous experiments26 in the absence of microwave radiation. The non-aggregation of the particles even in the presence of microwave heating further confirms that the “starched” silver nanoparticles prepared thus are quite stable. Since the synthesis utilizes only non-toxic materials and solvent, it is thought viable to readily integrate these particles into a variety of systems, especially those that are relevant to biological and biomedical applications.
While mild heating is sufficient for the glucose reduction of silver ions, the reduction of the Au3+ ions of AuCl4− takes place only in the presence of a base such as NaOH. We used chlorauric acid (HAuCl4·3H2O), soluble starch and anhydrous D-glucose as purchased. Gold nanoparticles are prepared by adding 40 µl of a 0.1 M solution of HAuCl4 and 60 µl of a 0.1 M solution of D-glucose to about 2 ml of 0.20%w aqueous solution of soluble starch followed by 15 µl 1M solution of NaOH. The solution remained colorless for the initial 30 min, following which it turned red slowly, indicating the formation of gold nanoparticles. The base facilitates the opening of the glucose ring by the abstraction of the α-proton of the sugar ring oxygen and the metal ions oxidize glucose to gluconic acid. The process is similar to the classical reaction of glucose with Tollen's reagent. The time evolution of the absorption spectra showing the growth of Au nanoparticles is shown in Fig. 2.
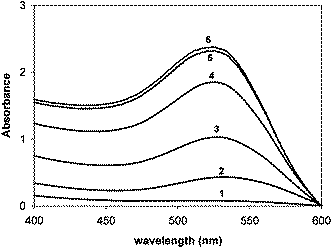 | ||
| Fig. 2 Time evolution of the surface plasmon absorption band indicating the continuous formation of the gold nanoparticles. The time intervals are: (1) 40 min; (2) 43 min; (3) 44 min; (4) 45 min; (5) 47 min; (6) 50 min. | ||
We monitored the growth of the gold nanoparticles at varying times (and the changing solution pH) using both the UV-Visible absorption spectroscopy and the TEM images of the dispersions sampled on TEM grids at each time. The intensity of the surface plasmon absorption increased with time indicating continued reduction of the metal ions. We did not observe any notable shift in the λmax with time indicating the size selectivity of the Au nanoparticles. The TEM images and the particle size distribution histograms corresponding to different reaction times are shown in Fig. 3.
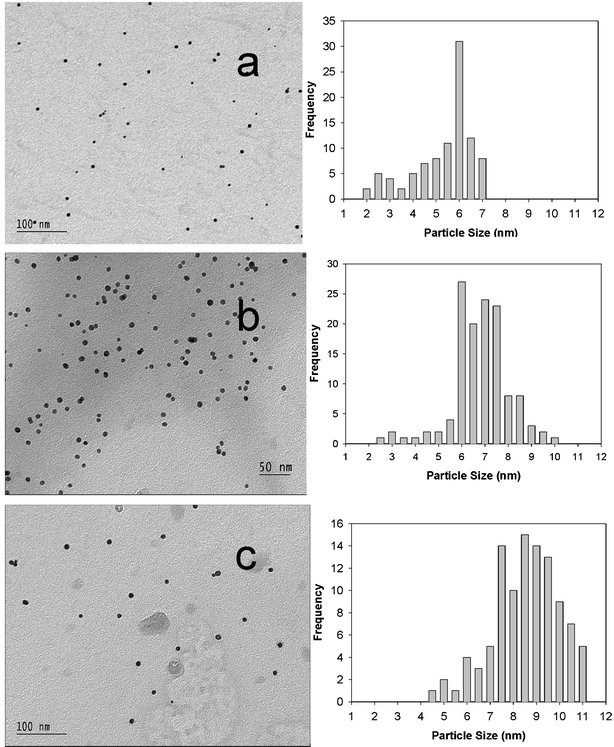 | ||
| Fig. 3 TEM images and the time evolution of the size distribution of the “starched” gold nanoparticles. The average particle sizes are: (a) 5.6 nm, σ = 1.37 nm, 40 min, pH = 10.17; (b) 6.70 nm, σ = 1.24 nm, 43 min, pH = 9.84; (c) 8.80 nm, σ = 1.39 nm, 49 min, pH = 9.44. | ||
The histograms clearly reveal an increase in the particle sizes with increasing time. The observed pattern indicates slow growth of Au nanoparticles in the starch solution template to the size limit of the template parameters. An analysis of the time evolution of the particle size distribution further suggests a clear size selectivity of the reaction and the preferred particle sizes seem to be around 6–10 nm.
Nanoparticles of bimetallic alloys are also of interest since it is possible to tune the optical and electronic properties as a function of the alloy composition and various approaches have been reported previously for the synthesis of Au–Ag alloy nanoparticles.18–23 We employed the sugar–starch combination scheme26 for the synthesis of Au–Ag alloy nanoparticles by the co-reduction of the silver and gold ions using glucose in the presence of alkali. Alloy nanoparticles with various Au/Ag mole ratios (0 ∶ 1, 0.25 ∶ 0.75, 0.5 ∶ 0.5, 0.75 ∶ 0.25, and 1 ∶ 0) are prepared by using pre-determined initial mole ratios of the gold and silver ions in the solution. The resulting nanoparticle dispersions exhibited different colors based on the initial compositions, indicating the formation of Au–Ag alloy nanoparticles (Fig. 4). While the dispersions containing pure silver and gold particles exhibited light yellow and purple colors respectively, the intermediate compositions resulted in colors that are varying between yellow and red as can be seen from the digital photographs in Fig. 4. The corresponding UV-Visible absorption spectra are also given in Fig. 4.
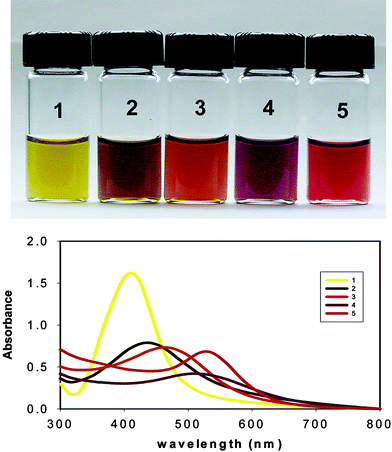 | ||
| Fig. 4 Photographs of the aqueous dispersions of starch stabilized Au, Ag and the various Au–Ag alloy compositions. The Au/Ag mole ratios are: (1) 0 ∶ 1; (2) 0.25 ∶ 0.75; (3) 0.5 ∶ 0.5; (4) 0.75 ∶ 0.25; (5) 1 ∶ 0. The corresponding surface–plasmon absorption bands are also shown. | ||
Some of the previous reports on alloy synthesis revealed a phase separation at the atomic scale leading to the formation of core–shell particles.27–30 However, several groups have reported the synthesis of homogeneous Au–Ag alloy nanoparticles.21–23,31–34 In order to examine whether the nanoparticles synthesized using the current methodology are alloys as against the core–shell particles, we have carried out the UV-Visible absorption spectroscopic studies. It is well known that the surface plasmon bands are characteristic for the metal and alloy nanoparticles.21–23,35–36 The core–shell nanoparticles will give rise to two surface plasmon absorption bands and the individual band intensities should depend on the initial composition of the metal ions. A similar situation will arise from a dispersion containing separate gold and silver nanoparticles instead of the homogeneous alloy particles.23
It is seen from Fig. 4 that only a single absorption band is obtained for each composition with the absorption maxima varying between those for pure gold and silver nanoparticle dispersions, supporting that only homogeneous alloy particles are formed. It is also expected that in an ideal alloy system the surface plasmon absorption maximum should be linearly related to the alloy compositions. The plot of the absorption maximum versus the initial Au/Ag mole fractions is presented in Fig. 5.
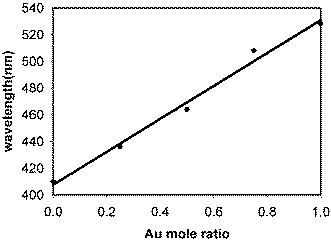 | ||
| Fig. 5 Plot of the plasmon absorption maximum (λmax) against the Au mole ratio for the various alloy compositions. | ||
It is observed that there is a linear relationship between the λmax values and the Au-mole ratio supporting that the particles are homogeneous alloys of Au and Ag. Thus, this approach is suitable for the synthesis of alloy nanoparticles.
In summary we have shown that it is possible to prepare starch stabilized aqueous dispersions of Au, Ag and Au–Ag alloy nanoparticles after being reduced from the corresponding metal ions by glucose. The metal and alloy nanoparticles prepared thus are highly stable and do not show any signs of aggregation even after storage for several months. Since the reagents used in the synthetic procedure are entirely non-toxic, the method can be readily integrated with biological applications. Since starch can easily form gel with water, we presume that it will help to synthesize nanogels for a wide range of biomedical applications. These aqueous nanofluids can also find several applications in heat transfer devices.
3. Experimental
1. Synthesis of silver nanoparticles
Aqueous starch dispersions containing Ag+ ions are prepared by adding 10 µl of a 0.1 M solution of AgNO3 (Sigma) and 25 µl of a 0.1 M solution of D-glucose (Fluka) to about 2 ml of 0.20%w aqueous solution of soluble starch (Sigma). This was then treated in a microwave oven for 60 s for the reduction of metal ions. In this case, the reduction takes place under hot conditions.2. Synthesis of gold nanoparticles
We used chlorauric acid (HAuCl4·3H2O), soluble starch and anhydrous D-glucose as purchased. Gold nanoparticles are prepared by adding 40 µl of a 0.1 M solution of HAuCl4 and 60 µl of a 0.1 M solution of D-glucose to about 2 ml of 0.20%w aqueous solution of soluble starch followed by 15 µl 1 M solution of NaOH. The solution remained colorless for the initial 30 min, following which it turned red slowly, indicating the formation of gold nanoparticles.3. Synthesis of alloy nanoparticles
Alloy nanoparticles with various Au/Ag mole ratios (0 ∶ 1, 0.25 ∶ 0.75, 0.5 ∶ 0.5, 0.75 ∶ 0.25, and 1 ∶ 0) are prepared using the same procedure for the gold nanoparticles, using pre-determined initial mole ratios of the gold and silver ions in the solution.Acknowledgements
We acknowledge partial financial support from the STC Program of the National Science Foundation under Agreement No. CHE-9876674. PR also thanks JSPS for a fellowship.References
- J. A. Creighton and D. G. Eadon, J. Chem. Soc., Faraday Trans., 1991, 87, 3881 RSC
.
- M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 7, 801 RSC
.
- T. S. Ahmadi, Z. L. Wang, T. C. Green, A. Henglein and M. A. El-Sayed, Science, 1996, 272, 1924 CrossRef CAS
.
- J. Belloni, Curr. Opin. Colloid Interface Sci., 1996, 1, 1996
.
- A. Ullman, Chem. Rev., 1996, 96, 1533 CrossRef CAS
.
- S. Link and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 8410 CrossRef CAS
.
- C. N. R. Rao, G. U. Kulkarni, P. J. Thomas and P. P. Edwards, Chem. Eur. J., 2002, 8, 28 CrossRef CAS
.
- R. Wang, J. Yang, Z. Zheng, M. D. Carducci, J. Jiao and S. Seraphin, Angew. Chem., Int. Ed., 2001, 40, 549 CrossRef CAS
.
- C. T. Campbell, S. C. Parker and D. E. Starr, Science, 2002, 298, 811 CrossRef CAS
.
- S-J Park, T. A. Taton and C. A. Mirkin, Science, 2002, 295, 1503 CrossRef CAS
.
- Y. Sun and Y. Xia, Science, 2002, 298, 2176 CrossRef CAS
.
- M. Valden, X. Lai and D. W. Goodman, Science, 1998, 281, 1647 CrossRef CAS
.
- K. Esumi, A. Suzuki, A. Yamahira and K. Torigoe, Langmuir, 2000, 16, 2604 CrossRef CAS
.
- R. G. Ispasoiu, L. Balogh, O. P. Varnavski, D. A. Tomalia and T. Goodson, J. Am. Chem. Soc., 2000, 122, 11005 CrossRef CAS
.
- M. Zhao, L. Sun and R. M. Crooks, J. Am. Chem. Soc., 1998, 120, 4877 CrossRef CAS
.
- R. S. Ingram, M. J. Hostetler and R. W. Murray, J. Am. Chem. Soc., 1997, 119, 9175 CrossRef CAS
.
- N. R. Jana and X. Peng, J. Am. Chem. Soc., 2003, 125, 14280 CrossRef
.
- G. C. Papavassiliou, J. Phys. F: Metal Phys., 1976, 6, L103 CrossRef CAS
.
- B. K. Teo, K. Keating and Y. H. Kao, J. Am. Chem. Soc., 1987, 109, 3494 CrossRef CAS
.
- N. Toshima, T. Yonezawa and K. Kushihashi, J. Chem. Soc., Faraday Trans., 1993, 89, 2537 RSC
.
- S. Link, Z. L. Wang and M. A. El-Sayed, J. Phys. Chem. B, 1999, 103, 3529 CrossRef CAS
.
- Y. H. Chen and C. S. Yeh, Chem. Commun., 2001, 371 RSC
.
- M. P. Mallin and C. J. Murphy, Nano Lett., 2002, 2, 1235 CrossRef CAS
.
-
P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford Oniversity Press Inc., New York, 1998 Search PubMed
.
-
A. S. Matlack, Introduction to Green Chemistry, Marcel Decker Inc., New York, 2001 Search PubMed
.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940 CrossRef CAS
.
- C. S. Ah, S. D. Hong and D. J. Jang, J. Phys. Chem. B, 2001, 105, 7871 CrossRef CAS
.
- K. Mallik, M. Mandal, N. Pradhan and T. Pal, Nano Lett., 2001, 1, 319 CrossRef CAS
.
- S. Mandal, P. R. Selvakannan, R. Pasricha and M. Sastry, J. Am. Chem. Soc., 2003, 125, 8440 CrossRef CAS
.
- L. Lu, H. Wang, Y. Zhou, S. Xi, H. Zhang, J. Hu and B. Zhao, Chem. Commun., 2002, 144 RSC
.
- L. M. Liz-Marzan and A. Philipse, J. Phys. Chem., 1995, 99, 15120 CrossRef CAS
.
- N. Kometani, M. Tsubonishi, M. Fujita, K. Asami and Y. Yonezawa, Langmuir, 2001, 17, 578 CrossRef CAS
.
- D. Chen, J. Mater. Chem., 2002, 12, 1557 RSC
.
- I. Lee, S. Han and K. Kim, Chem. Commun., 2001, 1782 RSC
.
- J. Sinzig, U. Radtke, M. Quinten and U. Kreibig, Z. Phys. D, 1993, 26, 242 CrossRef CAS
.
- M. Moskovitz, I. Srnova-Sloufova and B. Vlckova, J. Chem. Phys., 2002, 116, 10435 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2006 |
