Benign approaches for the synthesis of bis-imine Schiff bases
Tania R.
van den Ancker
*a,
Gareth W. V.
Cave
b and
Colin L.
Raston
c
aDepartment of Biological and Physical Sciences, University of Southern Queensland, Toowoomba, QLD 4350, Australia. E-mail: ancker@usq.edu.au; Fax: +61 7 4631 1530; Tel: +61 7 4631 2363
bSchool of Biomedical and Natural Sciences, Nottingham Trent University, Nottingham, NS11 8NS, UK. E-mail: gareth.cave@ntu.ac.uk; Fax: +44 (0)115 848 6636; Tel: +44 (0)115 848 3242
cSchool of Biomedical, Biomolecular and Chemical Sciences, University of Western Australia, Crawley, WA 6009, Australia. E-mail: clraston@chem.uwa.edu.au; Fax: +61 8 9380 1005; Tel: +61 8 9380 3045
First published on 17th November 2005
Abstract
Pure bis-imine Schiff bases are readily accessible in high yield, typically >95%, when aliphatic diamine/aldehyde condensation reactions are carried out under solvent-free conditions or in poly(propyleneglycol) (PPG) as a recyclable reaction medium, with negligible waste.
Introduction
Schiff base compounds are widely studied and used, attracting much attention in both organic synthesis and metal ion complexation.1,2 The traditional syntheses of such compounds are still commonplace alongside modern synthetic protocols.2 Typically, the synthesis employs the use of a high boiling point volatile organic solvent such as toluene, followed by extensive recrystallisation and/or chromatography. In embracing the principles of green chemistry,3 herein we report two general approaches to the synthesis of Schiff base imines, (i) the use of solvent-free or the so-called solventless conditions, and (ii) the use of an alternative and recyclable reaction medium, poly(propyleneglycol) (PPG). The high yields and overall low waste generation of these approaches give them attractive green chemistry metrics, along with remarkable versatility.Solvent-free reaction protocols used in the synthesis of condensation reactions such as aldol and Michael reactions are fast becoming the best synthetic approaches.3,4 New reactors designed specifically for such processes, both in the research laboratory and in industrial process intensification, has led to a realisation of the synthetic potential of solvent-free protocols that afford near quantitative yields with little or no waste.5 Such reactors also deal with heat transfer for highly exothermic reactions.
Ionic liquids, which have negligible vapour pressure, have become commonplace in research laboratories as viable alternative reaction media to volatile organic solvents.6,7 Despite claims that these expensive reaction media can be readily recycled, the process can seldom repeated in the same ionic liquid more than a dozen times before the overall efficiency starts to decline.7 Moreover, there are concerns about the toxicity of ionic liquids.7
Results and discussion
We have previously reported a facile “green” synthesis of resorcinol[4]arene and pyrogallol[4]arene utilising a solvent-free approach and the use of PPG as an alternative reaction medium.8 The fact that PPG can be readily prepared from biomass, and is extensively utilised in both the food and pharmaceutical industries, led us to explore its potential as an inexpensive and toxicologically tested reaction medium for other condensation reactions. To date there have been few studies on the use of PPG as a reaction medium,9 in contrast to the more hydrophilic poly(ethyleneglycol) (PEG).10Herein, two benign, simple and versatile routes to bis-imine Schiff bases in good yield have been demonstrated. The study investigates the synthesis and characterisation of thirteen bis-imine products (two of which are previously unpublished) by (i) a solvent-free method and (ii) using PPG as a benign reaction medium.
An overview of the synthetic details is summarised in Scheme 1. Typically in the solvent-free preparation of 1–13 (each entry number for Table 1 corresponds to the number of the compound), the bis-imine Schiff base product is formed in near-quantitative yield by low intensity grinding of the diamine with two molar equivalents of the aldehyde using a pestle and mortar over a period of ca. 10 min, associated with constant agitation of the mixture. During the reaction a eutectic melt forms followed by formation of the product as a white or coloured solid. As the product solidifies from the reaction mixture, care needs to be taken to prevent it coating small quantities of the starting materials.
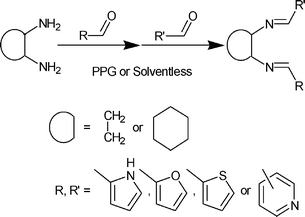 | ||
| Scheme 1 Synthetic route to 1,2-bis-imines. | ||
Analysis of the bulk product by 1H and 13C NMR show only the pure products (typically >90%) and the unreacted starting materials. Potential limitations in the solvent-free method may be overcome using ultra high intensity grinding in a ball mill – we predict that the implementation of a spinning disk/cylinder reactor will also lead to improved conversion without additional purification steps.
The second method implemented in this study utilises PPG as a benign alternative solvent system and is designed to circumvent additional purification steps. Typically, the diamine and two molar equivalents of the aldehyde were heated in a minimal volume of PPG and stirred until the reaction was complete, as determined by TLC. The pure product precipitates out of the reaction medium on cooling and can be collected by vacuum filtration. PPG with Mn = 425, 725, 1000 and 2000 all yielded the pure product in similar yield; however, PPG with Mn = 425 was chosen due to its lower viscosity and subsequent ease of filtration. Traces of PPG can be removed from the product by washing with water. Any unreacted starting materials can be reclaimed from the bulk product by filtration of the pure product from PPG and subsequent removal of the starting materials from the PPG by vacuum distillation.
Mass spectrometry of the PPG following filtration of the pure product shows that a trace quantity of the product remains in the PPG (typically <3% by NMR). This can be removed by the addition of water, leading to the precipitation of the product from the PPG and water. The water is then removed from the PPG under vacuum before recycling.
In a batch process the PPG was recycled without removing the trace quantities of product, leading to a saturated PPG sample (typically containing ∼5% product). In this study, the PPG was recycled five times as a batch process for representative samples. The PPG can be recycled in this way without any noticeable drop in overall yield. Typically, the reclaimed PPG reaction medium turned from a colourless solution to a transparent brown solution following the cycle. This decolourisation is attributed to the thermal degradation of the product and PPG over time. Analysis of the recycled PPG by MS and NMR techniques showed only PPG and product.
In quantitatively exploring this benign approach to the synthesis of Schiff base imines, two racemic aliphatic diamines, ethylene-1,2-diamine and cyclohexane-1,2-diamine, were treated with a variety of aromatic aldehydes, including heterocycles, the results of which are summarised in Table 1.
Cyclohexane-1,2-diamine was treated with equimolar quantities of 2-pyridine carboxaldehyde and 4-pyridine carboxaldehyde using both the solvent-free and PPG methods. In the solvent-free approach, 2- and 4-pyridine carboxaldehyde were added to the amine at the same time, and the mixture agitated (ca. 10 min). In the PPG reaction, the amine was added to PPG at 60 °C and stirred (ca. 2 min), 4-pyridine carboxaldehyde was added, and the stirring continued (ca. 2 min). 2-Pyridine carboxaldehyde was then added and the resulting mixture stirred overnight. Analysis of the crude reaction mixtures by NMR and MS showed a statistical (1 ∶ 2 ∶ 1) distribution of the three bis-imines.
Crystals of 7, 8 and 13 were isolated from the bulk PPG reactions and analysed by single crystal X-ray diffraction. The molecular structures of 7, 8 and 13 are shown in Fig. 1. All the products in this study are racemic, and the molecular structures in Fig. 1 are those of an isolated single crystal and do not represent the bulk material. They nevertheless reveal the relative orientation of the heteroatoms of the aromatic rings and imine N-centres.
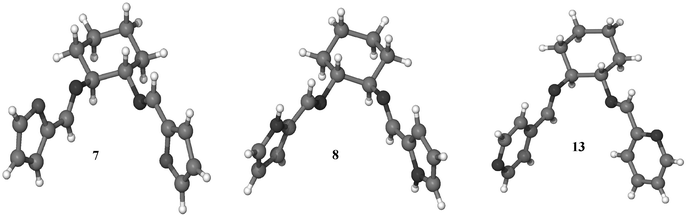 | ||
| Fig. 1 Molecular structures of (a) N,N′-bis(furan-2-ylmethylene)cyclohexane-1,2-bis-imine, 7; (b) N,N′-bis(1H-pyrrol-2-ylmethylene) cyclohexane-1,2-bis-imine, 8; (c) N-(pyridin-2-ylmethylene)-N′-(pyridin-4-ylmethylene)cyclohexane-1,2-bis-imine, 13. | ||
Conclusions
We have demonstrated that solvent-free conditions and the use of PPG as an alternative reaction media, are suitable methods for the synthesis of Schiff base bis-imines. During this feasibility study, we restricted the starting materials to inexpensive racemic amines; future experiments will look towards synthesising novel chiral analogues and their metal ion binding properties. We are currently investigating modern spinning disk/cylinder and micro-reactor technologies as potential process intensification techniques for downstream implementation of this new methodology.Experimental
Synthesis
General experimental procedures:Method 1: In a typical solventless experiment the aldehyde was added to the amine and the mixture agitated (ca. 1 min), affording an oil (ranging from cream to dark brown in colour). On further grinding (ca. 5 min) the solid bis-imine product was formed. If required, analytically pure product can be obtained by recrystallisation (from methanol or PPG).
Method 2: In a typical PPG experiment the amine was added to the PPG and stirred (ca. 2 min). The aldehyde was subsequently added and heated to between 60 and 90 °C (1 to 24 h). The reaction was cooled and the solid collected and washed with water. The precipitation of the product from the PPG can be facilitated by the addition of water to the reaction mixture. (In general the pure product crystallised out from the mother liquor as colourless crystals suitable for structural elucidation using single crystal diffraction data.) Any water in the PPG medium can be removed under vacuum at 100 °C, and the PPG recycled over 5 times with no reduction in the yield.
Compounds 1–13 were prepared from the appropriate aldehyde and diamine using the above procedures. The analyses (mp, NMR and/or MS) of compounds 1–11 were consistent with literature values.11
Compound 12: yellow crystalline solid, yield 90%. δH (250 MHz, CDCl3,) 8.71 (d, 2H), 8.52 (dd, 2H), 8.20 (s, 2H), 7.93 (ddd, 2H), 7.23 (d, 2H), 3.42 (m, 2H), 1.85 (br, 8H).
Crystallographic data
N,N′-Bis(furan-2-ylmethylene)cyclohexane-1,2-bis-imine, 7, C16H18N2O2. Crystals suitable for structural analysis were grown from PPG. A colourless prism (0.33 × 0.29 × 0.10 mm3) was mounted with oil on a thin quartz fibre. The molecular structure was largely unexceptional with an absence of both directional H-bonding and π-stacking of the aromatics. Monoclinic, space group P21/n, a = 9.4647(2), b = 9.9534(2), c = 15.7079(4) Å, β = 98.5090(10)°, V = 1463.49(6) Å3, Dc = 1.227 g cm−3 for Z = 4. Least-squares refinement based on 11417 reflections with Inet > 4σ(Inet) (out of 3316 unique reflections) led to final value of R1 = 0.0467.N,N′-Bis(1H-pyrrol-2-ylmethylene)cyclohexane-1,2-bis-imine, 8, C16H20N4. Crystals suitable for structural analysis were grown from PPG. A colourless prism (0.31 × 0.15 × 0.14 mm3) was mounted with oil on a thin quartz fibre. Monoclinic, space group C2/c, a = 18.5478(4), b = 8.9189(2), c = 9.2148(2) Å, β = 101.3880(10)°, V = 1494.36(6) Å3, Dc = 1.193 g cm−3 for Z = 4. Least-squares refinement based on 3200 reflections with Inet > 4σ(Inet) (out of 1807 unique reflections) led to final value of R1 = 0.0371.
N-(Pyridin-2-ylmethylene)-N′-(pyridin-4-ylmethylene)cyclohexane-1,2-bis-imine, 13, C18H20N4. Crystals suitable for structural analysis were grown from PPG. A colourless plate (0.34 × 0.27 × 0.10 mm3) was mounted with oil on a thin quartz fibre. The molecular structure was largely unexceptional with an absence of both directional H-bonding and π-stacking of the aromatics. Monoclinic, space group C2/c, a = 35.5824(14), b = 5.7010(3), c = 16.8318(9) Å, β = 112.752(2)°, V = 3148.7(3) Å3, Dc = 1.234 g cm−3 for Z = 8. Least-squares refinement based on 11262 reflections with Inet > 4σ(Inet) (out of 3804 unique reflections) led to final value of R1 = 0.0665.
The intensity data was collected at 150(2) K using a Enraf–Nonius KappaCCD diffractometer and Mo-Kα radiation (λ = 0.71073 Å). The structures were solved by direct methods using SHELXS and refined using SHELXL software.12 CCDC reference numbers 280847, 280848 and 280849 for 7, 8 and 13 respectively. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b513289d.
Acknowledgements
We thank the Australian Research Council, EPSRC, Nottingham Trent University and University of Southern Queensland for the support of this work. T. A. thanks the RSC Journals Grants for International Authors.References
- R. W. Layer, Chem. Rev., 1963, 63, 489 CrossRef CAS; V. Alexander, Chem. Rev., 1995, 95, 273 CrossRef CAS; F. Toda and M. Ochi, Enantiomer, 1996, 1, 85 Search PubMed; F. Toda and M. Imai, J. Chem. Soc., Perkin Trans. 1, 1994, 2673 RSC; T. Wagner-Jaurgg, Synthesis, 1976, 349 CrossRef CAS; N. S. Isaacs, Chem. Soc. Rev., 1976, 5, 181 RSC; J.-L. Renaud, C. Brueau and B. Demerseman, Synlett, 2003, 408 CrossRef CAS; S. Roland and P. Mangeney, Eur. J. Org. Chem., 2000, 4, 611 CrossRef; D. P. Lydon, G. W. V. Cave and J. P. Rourke, J. Mater. Chem., 1997, 7, 403 RSC; G. W. V. Cave, D. P. Lydon and J. P. Rourke, J. Organomet. Chem., 1998, 555, 81 CrossRef CAS; D. J. Saccomando, C. Black, G. W. V. Cave, D. P. Lydon and J. P. Rourke, J. Organomet. Chem., 2000, 601, 305 CrossRef CAS.
- W. H. Correa and J. L. Scott, Molecules, 2004, 9, 513 Search PubMed; J. Schmeyers, F. Toda, J. Boy and G. Kaupp, J. Chem. Soc., Perkin Trans. 2, 1998, 989 RSC; W. H. Correa, S. Papadopoulos, P. Radnidge, B. A. Roberts and J. L. Scott, Green Chem., 2002, 4, 245 RSC; K. Tanaka and R. Shiraishi, Green Chem., 2000, 2, 272 RSC.
- P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford Science Publications, New York, 1998 Search PubMed; P. Anastas and T. Williamson, Green Chemistry, Frontiers in Benign Chemical Synthesis and Processes, Oxford Science Publications, New York, 1998 Search PubMed; K. Tanaka, Solvent-free Organic Synthesis, Wiley-VCH, Weinheim, 2003 Search PubMed.
- G. W. V. Cave and C. L. Raston, J. Chem. Educ., 2005, 82, 468 CrossRef CAS; G. W. V. Cave and C. L. Raston, Tetrahedron Lett., 2005, 46, 2361 CrossRef CAS; C. L. Raston and G. W. V. Cave, Chem.—Eur. J., 2004, 10, 279 CrossRef CAS; G. W. V. Cave and C. L. Raston, J. Supramol. Chem., 2002, 2, 317 Search PubMed; G. W. V. Cave and C. L. Raston, Chem. Commun., 2000, 2199 RSC; G. W. V. Cave and C. L. Raston, J. Chem. Soc., Perkin Trans. 1, 2001, 3258 RSC; G. W. V. Cave, C. L. Raston and J. L. Scott, Chem. Commun., 2001, 2159 RSC.
- J. R. Burns and R. Jachuck, Appl. Therm. Eng., 2001, 21, 495 CrossRef CAS; K.V.K. Boodhoo, W. A. E. Dunk, M. S. Jassim and R. Jachuck, J. Appl. Polym. Sci., 2004, 91, 2079 CrossRef CAS.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792 CrossRef; J. D. Holbery and K. R. Seddon, J. Chem. Soc., Dalton Trans., 1999, 2133 RSC; T. Welton, Chem. Rev., 1999, 99, 2701 CrossRef; J. L. Scott, D. R. MacFarlane, C. L. Raston and C. M. Teoh, Green Chem., 2000, 2, 123 RSC; R. Mestres, Green Chem., 2004, 6, 583 RSC; D. B. Zhao, Z. F. Fei, C. A. Ohlin, G. Laurenczy and P. J. Dyson, Chem. Commun., 2004, 2500 RSC; R. Vijayaraghavan, M. Surianarayanan and D. R. MacFarlane, Angew. Chem., Int. Ed., 2004, 43, 5363 CrossRef CAS; M. Antonietti, D. B. Kuang, B. Smarsly and Z. Yong, Angew. Chem., Int. Ed., 2004, 43, 4988 CrossRef CAS.
- P. Wasserscheid, R. van Hal and A. Bosmann, Green Chem., 2002, 4, 400 RSC; R. P. Swatloski, J. D. Holbrey and R. D. Rogers, Green Chem., 2003, 5, 361 RSC; F. Stock, J. Hoffmann, J. Ranke, R. Störmann, B. Ondruschka and B. Jastorff, Green Chem., 2004, 6, 286 RSC.
- J. Antesberger, G. W. V. Cave, M. C. Ferrarelli, M. W. Heaven, C. L. Raston and J. L. Atwood, Chem. Commun., 2005, 892 RSC; B. A. Roberts, G. W. V. Cave, C. L. Raston and J. L. Scott, Green Chem., 2001, 3, 280 RSC; G. W. V. Cave, M. C. Ferrarelli and J. L. Atwood, Chem. Commun., 2005, 2787 RSC.
- N. F. Leininger, R. Clonta, J. L. Gainer and D. J. Kirwan, Chem. Eng. Commun., 2003, 190, 431 Search PubMed; G. W. V. Cave, M. J. Hardie, B. A. Roberts and C. L. Raston, Eur. J. Org. Chem., 2001, 3227 CrossRef CAS.
- J. Chen, S. K. Spear, J. G. Huddleston and R. D. Rogers, Green Chem., 2005, 64 RSC; C. B. Smith, C. L. Raston and A. N. Sobolev, Green Chem., 2005, 7, 650 RSC.
- M. H. Fonsesca, E. Eibler, M. Zabel and B. König, Inorg. Chim. Acta, 2003, 352, 136 CrossRef; W. S. Emerson and T. M. Patrick, Jr., J. Org. Chem., 1949, 14, 790 CrossRef CAS; J. Weber, Inorg. Chem., 1967, 6, 258 CrossRef CAS; G. K. Patra and I. Goldberg, Eur. J. Inorg. Chem., 2003, 969 CrossRef CAS; C. R. Baar, M. C. Jennings and R. J. Puddephatt, Organometallics, 1999, 18, 4373 CrossRef CAS; D. H. Busch and J. C. Blair, J. Am. Chem. Soc., 1956, 78, 1137 CrossRef CAS; W.-Y. Sun, B.-L. Fei, T. Okamura, W.-X. Tang and N. Ueyama, Eur. J. Inorg. Chem., 2001, 1855 CrossRef CAS; X.-M. Ouyang, B.-L. Fei, T. Okamura, H.-W. Bu, W.-Y. Sun, W.-X. Tang and N. Ueyama, Eur. J. Inorg. Chem., 2003, 618 CrossRef CAS.
- G. M. Sheldrick, SHELXS-97, Program for solution of crystal structures, University of Göttingen, Germany, 1997 Search PubMed; G. M. Sheldrick, SHELXL-97, Program for refinement of crystal structures, University of Göttingen, Germany, 1997 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |