Lipase-catalyzed glycerolysis of fats and oils in ionic liquids: a further study on the reaction system
Zheng
Guo
and
Xuebing
Xu
*
BioCentrum-DTU, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark. E-mail: xx@biocentrum.dtu.dk; Fax: +45 45884922; Tel: +45 45252773
First published on 23rd November 2005
Abstract
Candida antarctica lipase B-catalyzed glycerolysis of sunflower oil in a tetraammonium-based ionic liquid (IL) was studied to elucidate its distinct characteristics and to evaluate the contributions of important parameters. Mass transfer limitations and occurring partial phase separation were found to have a profound effect on the lower initial rate and the occurrence of the induction period. The investigation on the rheological behavior of the IL and its mixture with substrates showed that the plot of the viscosity of pure IL against temperature was better fitted with the Vogel–Tammann–Fulcher (VTF) equation, and the viscosity of the mixture is strongly agitation-dependent. A comparable diffusion time constant of the oil molecule in the IL to that of the reaction shows that the glycerolysis in the IL is controlled both diffusionally and kinetically, as experimentally verified by agitation effect and enzyme loading study. Interestingly, increasing water activity resulted in a decreasing initial reaction rate and a prolonged induction period, which possibly resulted from an elevated solvation barrier and the phase separation at higher water content. Studies on thermodynamics of glycerolysis show that there is a bigger energy barrier for the IL system, about 1.5 times that of the solvent-free or 3 times that of the tert-butyl alcohol system. Kinetic studies also show that IL system has the biggest Vmax and Km among the three tested systems, indicating, respectively, its high productivity, and low substrate affinity to enzyme due to mass transfer limitation.
Introduction
Increasing interests in ionic liquids (ILs) stem primarily from the progressive concerns for environmental problems.1–4 A contradictory challenge facing chemists is to establish efficient as well as environmentally benign processing technology comparing to current processing approaches. Ionic liquids appear to be a possible solution to this objective. As molten salts, ILs not only possess negligible volatile pressure but also act much like common solvents, dissolving both polar and nonpolar compounds and even performing much better than commonly used solvents in many cases.5–8 Most importantly, as a reaction medium for biocatalysis, ILs have other important advantages beyond green technology. The advantages include adjustable solubility properties, protective effect or increased stability for enzymes, positive effects on the specificity of enzymes or on the shift of reaction equilibrium, as well as recoverability and recyclability.9–11 A key feature of ILs is that their properties can be modified by altering the nature of the cations, anions and substituents. This makes ILs real engineered solvents, which could provide new opportunities for many biocatalytic processes and create novel possibilities which are impossible in traditional solvents. These have been well documented by a number of excellent reviews and books.12–15Lipase-catalyzed lipid modification constitutes an important aspect with industrial potentials in biocatalysis area. Enzymatic lipid processing has been extensively studied employing common solvents or in solvent-free systems.16–18 Many enzymatic processes have exhibited comparable advantages to chemical methods and some of them have been even used in industrial production.19–21 However, few protocols with practical interests and industrial potentials have been reported so far by employing ILs as reaction media.22–25 In practice, there remain big challenges in this area, especially for enzymatic production of partial glycerides.26,27
Recently, for the first time, we have demonstrated a novel protocol for monoglyceride production employing an amphiphilic tetraammonium-based ionic liquid as a reaction medium to create a compatible system for glycerol and oils.28 This approach achieved higher conversion of triglycerides and higher yields of desired products. The universal validity of the method has also been verified by being applied to different commercial oils and fats. However, interesting observations such as lower initial reaction rate, occurrence of the induction period and bulky substrate-tolerating capacity of the IL remain to be explored. The unique properties of cocosalkyl pentaethoxymethyl ammonium methosulfate (CPMA·MS), the interactions between water and the IL molecule or substrates, and their contributions to mass transfer limitations await further elucidation. Thus, in this paper, we investigated the rheological behaviors of CPMA·MS and its mixtures with substrates as well as their effects on mass transfer and reaction performance. Effects of some important parameters were evaluated. The relationship of water content and water activity in the IL was examined and its influence on the reaction rate was also discussed. Kinetic and thermodynamic studies on the glycerolysis of sunflower oil in the IL were conducted and compared with the reaction in solvent-free and tert-butyl alcohol systems.
Results and discussion
Characteristics of the glycerolysis in CPMA·MS and important factors
Fig. 1 depicts a typical time course of the glycerolysis of sunflower oil in CPMA·MS. As also demonstrated in the previous work,28 the reaction shows a higher yield of monoglyceride, a temperature-dependent induction period, a jump after the induction period, and a longer equilibrium time resulting from the lower initial reaction rate. Besides the above concerns, many other questions regarding the glycerolysis of triglycerides in the IL need to be addressed, such as: is this a kinetically controlled enzymatic reaction or a mass-transfer-controlled one? Besides temperature, are there any other contributing factors to the unique behavior of the reaction, and if so, to what extent?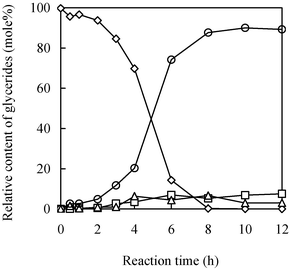 | ||
| Fig. 1 Time course of the glycerolysis of sunflower oil in CPMA·MS. The reaction was conducted at 45 °C with magnetic agitation at 600 rpm. (◇), (△), (○) and (□) represent tri-, di-, mono- glycerides and fatty acid. The concentration of sunflower oil, glycerol and Novozym 435 is 0.188 mol L−1, 0.94 mol L−1 and 37.6 g L−1, respectively. | ||
One of the crucial significances of the IL is that it can provide an environment where oil and glycerol can interact efficiently. In reality, this capacity is not without limitation and can be lost when too high a substrate concentration is applied. A partial phase separation occurs at higher substrate concentration as shown in the study. Therefore, it is important to understand what has happened in such a system. In order to examine the presence of external mass resistance, the effect of agitation speed on the reaction rate was investigated in the range of 100–800 rpm (Fig. 2). It was observed that the conversion of triglyceride increased with the agitation rate until after 600 rpm. This observation indicated the existence of the external mass transfer limitation, which probably resulted from partial phase separation and high viscosity of the IL (a detail discussion on the effect of the viscosity see the following section). Mass transfer between two phases directly relates to the interfacial area, which is dependent on the shear rates and the two-phase volume ratio.29 An increase in speed of agitation will lead to the increasing dispersion of triglyceride into the IL phase and consequently a more efficient interaction with glycerol and the immobilized lipase, which resided in the IL phase. Overall, this will yield an increasing reaction rate. The result shows that a proper agitation rate in this reaction system is necessary to eliminate the external mass transfer limitation.
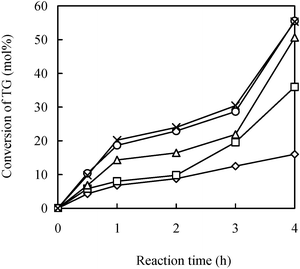 | ||
| Fig. 2 Effect of agitation speeds. Reaction conditions: 50 °C, the concentration of substrates and enzyme is the same as in Fig. 1. (◇), (□), (△), (○) and (×) represent 100, 200, 400, 600 and 800 rpm, respectively. | ||
If no mass transfer limitations are present, the dependence of reaction rate upon the enzyme concentration should be linear; this is partially supported by performing the reaction at different enzyme loadings (Fig. 3A). The initial rate shows an approximately linear increase against the enzyme concentration at the range of 10.8 to 32 g L−1. The increase slows down when enzyme loading is over 32 g L−1 (Fig. 3B). At the same time the enzyme efficiency also obtains a relatively higher value at this lipase concentration. Thus from the commercial consideration, 30–40 g L−1 should be a recommended enzyme dosage.
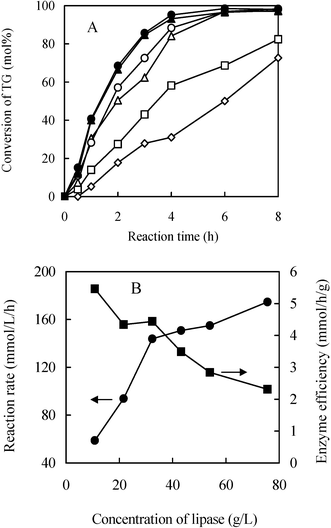 | ||
| Fig. 3 Effects of enzyme loading on reaction rate (A) and enzyme efficiency (B). Reaction conditions: magnetic agitation, 300 rpm; 55 °C; concentration of sunflower oil, 0.432 and glycerol 2.16 mol L−1; concentration of Novozym 435, 10.8 (◇), 21.6 (□), 32.4 (△), 43.2 (○), 54.0 (▲) and 75.6 (●) g L−1. | ||
On the other hand, the linearity alone in Fig. 3 does not rule out the possibility of mass transfer limitations, especially internal diffusion, on the reaction rate. The presence of mass transfer limitations has received further support from the linear dependence of glycerolysis rates upon sunflower oil concentrations (Fig. 4). It is known that a mass-transfer-controlled reaction produces a linear rate dependency upon substrate concentrations. A very good linear correlation between the concentration of sunflower oil and reaction rate at the range of 0.19–0.5 mol L−1 was observed. As discussed previously, CPMA·MS has a specific substrate-buffering capacity,28 where the reaction will exhibit a different behavior beyond this capacity. At 0.55 mol L−1 in this study, the volume of substrates is about twice the IL volume. Obviously, the reaction behavior at this concentration or higher significantly differs from a typical glycerolysis of triglyceride in CPMA·MS. Most likely this constitutes the leading cause for the decrease of reaction rate with the further increase of concentration of sunflower oil.
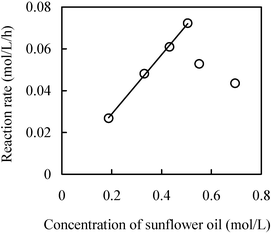 | ||
| Fig. 4 Effect of the concentration of sunflower oil. Reaction conditions: IL, 2 mL; Novozym 435, 100 mg; Stirring rate, 600 rpm; 70 °C; and mole ratio of glycerol : sunflower oil, 5 : 1. | ||
The glycerolysis of triglycerides is a two-step reversible reaction, in which excess glycerol could suppress the reversion of the first step and promote the further glycerolysis of the generated diglycerides, giving higher yield of MG. Fig. 5 reveals that there is no significant difference for the conversion of triglyceride from the stoichiometric ratio (2 ∶ 1) to excess of glycerol. However the yield of the desired product increases with the glycerol excess, which we quantify as the glycerolysis degree (GD). To reflect the change of both conversion of triglycerides and MG yield, a new term, the glycerolysis degree, has been introduced in this study to indicate the performance and progress of the reaction. Fig. 5 shows that glycerolysis degree is the conversion of TG plus half of the yield of MG when the yield of MG is lower, and a doubled value of MG yield when the yield of MG is higher. Glycerolysis degree is a combined index to synchronously denote the conversion of TG and yield of MG for the glycerolysis of triglycerides. The results also suggested that employing excess glycerol (more than 5 : 1) is still necessary to achieve a higher yield of MG even though the IL could induce the shift of equilibrium to the formation of monoglyceride by reducing the activity coefficients of partial glycerides.28
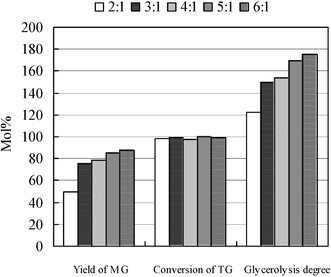 | ||
| Fig. 5 Effect of glycerol dosage. Reaction conditions: 2 mL IL, 0.5 mmol sunflower oil, 50 °C, 500 rpm. The mole ratio of glycerol/sunflower oil varied from 2 to 6. | ||
Rheological behavior of CPMA·MS and its effects on mass transfer
CPMA·MS is a highly viscous ionic liquid with the melting point at 5–10 °C. The plot of the viscosity of CAMA·MS against temperature is shown in Fig. 6. Generally ILs have a viscosity essentially governed by van der Waals interaction and H-bonding, which is of two or more orders of magnitude greater than normal solvents. The massive occurrence of long alkyl chain and functional groups will significantly increase the viscosity of ILs.30–32 In CPMA·MS, there are a C14 alkyl group and two polyethoxyl substituents, either of which could contribute to the increase of viscosity.32 However, the viscosity of the IL could be dramatically decreased by increasing temperature as indicated in Fig. 6A, which is important for its usage as a reaction medium. The change of viscosity of CPMA·MS seems well following Arrhenius model (eqn (1)), but is better described by the Vogel–Tammann–Fulcher (VTF) model33,34 (eqn (2)), though the difference is not so significant (Fig. 6B).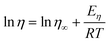 | (1) |
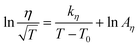 | (2) |
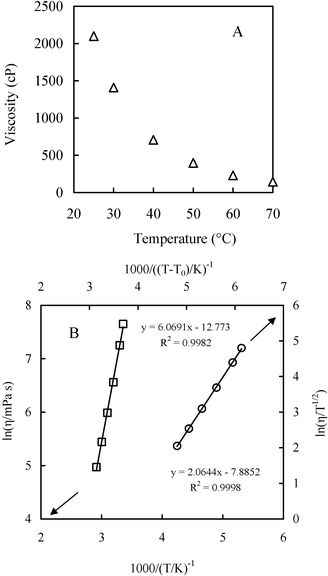 | ||
| Fig. 6 Temperature dependency of the viscosity of CPMA·MS (A) and fitting plot of Arrhenius (□) and VFT (○) model (B). | ||
Steady shear flow curves across a wide range of shear rates can provide important information regarding the ways in which the internal structure of the sample changes with the applied shear rate. The rheological behaviors of pure CPMA·MS and its mixtures with sunflower oil and glycerol have been shown in Fig. 7. Apparently the pure IL does not behave perfectly as a Newtonian liquid. The viscosity of CPMA·MS slightly increases with the increasing shear rate, in another word, exhibiting shear-thickening behavior.34 On the other hand, the viscosity of the mixtures of the IL and substrates significantly decreases with increasing rates and shear time, in which the behaviors are somehow similar to a thixotropic liquid.36 For a typical thixotropic liquid, the systems usually pack with loosely asymmetrical particles (network structure). There exists an equilibrium viscosity value at any valid shear rates and temperature. The time to attain equilibrium strongly depends on shear rates. Such phenomena have been observed for the IL–sunflower oil–glycerol system in this study. In general, there are two contrary actions, breakdown and recovery of the network structure during shearing processes of a thixotropic liquid. Likewise, there also occur two opposite tendencies for the partially miscible IL–sunflower oil system, that is, the potential of oil molecules being dispersed into the interspaces of the IL molecules to disorganize the intrinsic compact structure of the system and the tendency to recover partial phase separation. Besides the shearing action, the hydrophobic interaction between fatty acid chains of oil and the alkyl group in CPMA·MS will promote the former process and help keep the dispersion state, which will lead to a dramatic decrease in the apparent viscosity of the mixtures. The repulsion action between the hydrophobic oil molecules and the hydrophilic group in the IL molecule and its ionic nature, on the other hand, will drive the system to recover its separated state, in which viscosity will be dominated by the IL with higher viscosity. The result suggests that vigorous agitation will not only eliminate external mass transfer limitation but also significantly reduce the viscosity of the reaction mixture, so as to facilitate the diffusion of substrates in the porous support particles of lipase.
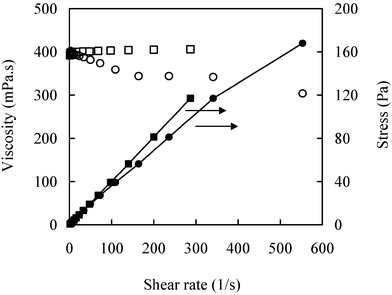 | ||
| Fig. 7 Rheological behavior of the IL (□ and ■) and its mixture with substrates (○ and ●). The measurement is at 50 °C. | ||
Novozym 435 is a commercial enzyme in which Candida antarctica lipase B is immobilized on macroporous polyacrylate resin beads. There is no doubt that only the substrates being diffused into the particles and reacted with the lipase anchored there can result in the occurrence of reaction. Even though the external mass transfer limitation could be eliminated by agitation as mentioned above, the diffusion rate inside particles will be a decisive step for the reaction due to high viscosity of reaction medium. To address this question, it is useful to compare the two time constants for reaction (tr) and diffusion (td), which are defined as follows, respectively:
| tr = c0/rc,0 | (3) |
| td = D/(kSL)2 | (4) |
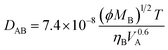 | (5) |
It was observed that, at 50 °C and a concentration of oil of 0.188 mol L−1, the initial reaction rate was 5.46 × 10−6 mol L−1 s−1. Therefore, tr should be 3.44 × 104 s according to eqn (3). The diffusion coefficient calculated by eqn (5) is 4.66 × 10−8 cm2 s−1 (sunflower oil is treated as trilinoleoyl glycerol). The diffusion time could be accordingly estimated as 1.756 × 104 s by eqn (4). At 35 °C, the calculated value of td is 4.51 × 104 s. The same order of magnitude for td and tr suggests that the effects of mass transfer on the glycerolysis of sunflower oil in CPMA·MS catalyzed by Novozym 435 should be seriously taken into account, especially when the reaction is performed at lower temperatures. In fact, the oil molecules might exist in clusters instead of singly; therefore the above calculation would only give a rough estimate and would not be an accurate description for the diffusion of oil molecules in the IL,39 that is to say that the diffusion of oil in the IL may be slower than the above-calculated rates. This implies that mass transfer is a serious problem for the efficiency of the reaction.
Since the reaction in the IL is controlled by both mass transfer and intrinsic reaction kinetics and the viscosity of medium strongly depends on reaction temperature, it will be useful to have a quantitative evaluation on the contribution of mass transfer to the change of reaction rates. A similar data processing as above demonstrated that the glycerolysis of sunflower oil in tert-butyl alcohol is a kinetically controlled reaction (data not shown), so the effect of mass transfer in tert-butyl alcohol system could be neglected in the kinetic study. From 35 to 55 °C, the reaction rate increases by only 2.2 times for tert-butyl alcohol system, instead of 11.5 times for the reaction in the IL system. Assuming the glycerolysis in the two systems possesses similar kinetic behavior; this significant difference should be mainly resulted from the marked decrease of the IL viscosity (from 1024 to 308 cP).
Water activity of the IL reaction system and its influence
The importance of hydration regarding enzyme activity in organic solvents and ionic liquids has been well-known.40,41 Enzyme activity, specificity, and hydrolytic activity are more or less dependent on the thermodynamic water activity (aw).40Fig. 8 shows the changes of water activities of CPMA·MS and its mixtures with sunflower oil and glycerol as a function of water contents. A linear correlation of water activity and water content for pure IL was observed. It is notable that the water activity still remains at a lower level even when the water content is over 5%, which significantly differs from other types of ILs.41 This phenomenon could be ascribed to the occurrence of polyethoxyl moiety in CPMA·MS, which causes the IL to have a strong association with water molecules as well as to restrict their freedom. Within the range of water content up to 2%, the water activity of the mixtures of IL and substrates also shows a similar linear increase with the increasing water content. The evident deviation after the water content at 2% might arise from the partial separation. During the measurement of water activity, it was observed that the phase separation became faster when more water was added to the sample. This is probably because more association of water molecules with the IL molecules enhances the repulsion between the IL molecules and oil molecules. This consequently promotes phase separation.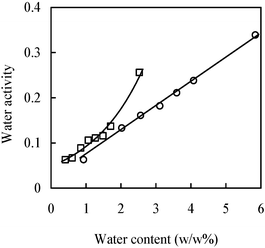 | ||
| Fig. 8 A dependent relationship of the water activity of the IL (○) or its mixture with substrates (□) with the corresponding water content. The mixture consists of 1 mmol IL, 1 mmol sunflower oil, 5 mmol glycerol and variable water. All measurements are performed at 40 °C. | ||
The real interest of investigation in water activity lies in the acquirement of the effects of aw on reaction performance. We conducted the glycerolysis of sunflower oil at varied aw (Fig. 9A). Interestingly, inconsistent with normal observations, the initial rates, denoted by the conversion of TG, increase with the decrease of aw. Similar observation for the transesterification in [bmim][PF6] (1-butyl-3-methylimidazolium hexafluorophosphate) and hexane employing Candida antarctica lipase B was also reported.41,42 One possible explanation lies in a possible transport limitation of the hydrophobic substrate from the solvent medium through the water layer surrounding the enzyme at higher water activities. As also observed in experiments, phase separation was intensified with increase of water activity, which could result in stronger obstacle for oil molecules to diffuse into ILs. This has affected the length of induction periods, which becomes longer with the increase of aw (Fig. 9A). With these considerations, we might propose a conceivable explanation for the occurrence of the induction period and a jumping increase after the induction period for the glycerolysis of triglyceride in this IL system as shown in Fig. 1. The mass transfer limitation and phase separation are likely the dominating reasons for the occurrence of the induction period. The steep increase of the reaction rate after the induction stage suggests that a critical phase transfer limitation point and a self-accelerating (self-affecting) phenomenon could have occurred. It is assumed that the yielded MG and DG promote phase homogenization, resulting in a significant diffusion enhancement and spectacular increase of rate.
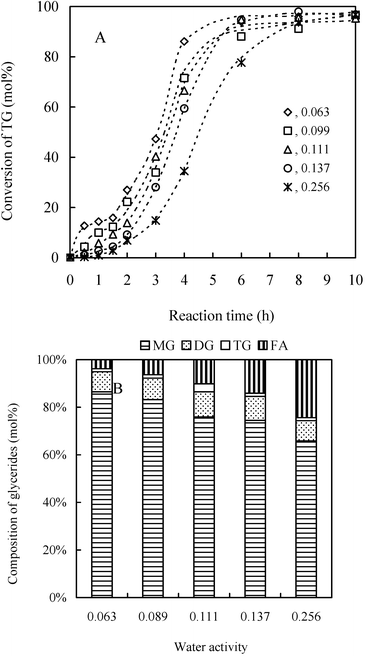 | ||
| Fig. 9 Progress curves of the conversion of TG for the glycerolysis in the IL with different aw (A); Effects of water activities on the composition of glycerides for the reaction in the IL at 10 h (B). The reaction system has the same components as in Fig. 8 other than 100 mg enzyme. The reaction is performed at 60 °C with magnetic stirring at 400 rpm. | ||
Another negative effect of higher water activity for the glycerolysis in the IL is that more hydrolytic products were produced (Fig. 9B). This result indicated that even though the IL molecules had the capacity to reduce the number of free water molecules, a steady increase of hydrolytic products against the increasing water activity is unavoidable due to the competition between hydrolysis and glycerolysis.
Kinetic properties of the glycerolysis in IL system
Differing from typical lipases,43Candida antarctica lipase B does not display any interfacial activation and usually adopts an “open” conformation which makes the active site accessible to solvents.44 It is well known that substrate specificity and catalytic efficiency depend upon the ability of the enzyme to utilize the free energy binding with the substrates.45 This binding energy reflects the difference between binding energies of the substrate-enzyme and the substrate-solvent interactions.46 Kinetic parameters for the description of enzyme behaviors, therefore, depend strongly on the solvent medium. In order to rationalize the lipase performance in the IL system and the effects of the IL, we have studied the kinetic behaviors of Novozym 435-catalyzed glycerolysis in the IL system and compared their performances with those in tert-butyl alcohol and solvent-free systems (Table 1).| E a/kJ mol−1 | Denoted by conversion of TG | Denoted by GD | V Gmax/VCmax | |||
|---|---|---|---|---|---|---|
| V Cmax/mol L−1 h−1 (g enzyme)−1 | K Cm/mM | V Gmax/mol L−1 h−1 (g enzyme)−1 | K Gm/mM | |||
| All kinetic assays were done at 60 °C with agitation speed 600 rpm and an excessive and constant concentration of glycerol. For the solvent-free, tert-butyl alcohol–IL system, the variation of concentration of sunflower oil is in the ranges of 0.32–0.76, 0.04–0.35 and 0.18–0.55 mol L−1, respectively. Ea was measured ata 35–65 °C,b 25–55 °C andc 35–65 °C. | ||||||
| Solvent-free | a65.37 | 0.86 | 445 | 0.98 | 419 | 1.14 |
| tert-Butyl alcohol | b33.20 | 1.14 | 147 | 1.83 | 124 | 1.60 |
| Ionic liquid | c101.04 | 1.39 | 848 | 2.47 | 830 | 1.78 |
The effect of temperature on the reaction activity of Novozym 435 in the IL system shows a spectacular increase of initial rates against temperature, yielding an enhanced activation energy estimated by Arrhenius equation. The Ea of the reaction in the IL system is about three times the value in tert-butyl alcohol and even considerably higher than the Ea for the solvent-free system. This striking difference is more likely to arise from the dramatic decrease of the IL viscosity with the increasing temperature and the resulting mass transfer enhancement, which has been proven to be one of the decisive factors for the glycerolysis in the IL as repeatedly mentioned above. The result also suggested that, in order to achieve an optimal reaction rate for the IL system, it is necessary to conduct the reaction at a higher temperature to overcome the elevated energy barrier.
To properly evaluate the selectivity of reaction systems, we studied the kinetic properties denoted by both conversion of TG and glycerolysis degree based on the Michaelis–Menten model (Table 1). The apparent value of Km in the IL system is markedly bigger than those in tert-butyl alcohol and solvent-free systems, which shows a good agreement with the change of Ea. However, this does not consequently indicate any change of the affinity of lipase to substrates;28 instead we believe that phase separation and mass transfer limitations cause the higher Km in the solvent-free and IL systems. Interestingly the maximum reaction rate in the IL system is much higher than those in the other two systems even though the IL has the biggest Km and a lower initial reaction rate. This indicates that the IL system has a higher productivity, which is consistent with our previous observation.28 The reason is that the IL is better able to accommodate the resulting MG molecules (the IL molecule has more H-bonding sites) than tert-butyl alcohol, so is still able to shift the equilibrium at higher substrate concentration. It is noteworthy that, in accordance with the change of GD values while the conversion of TG is close to 100%, the value of VGmax/VCmax is approximately equal to the doubled yield percentage of MG (Table 1). This might imply the indexation value of GD for reaction selectivity. In the solvent-free system, the relative yield of MG is very low, though VCmax is theoretically high (probably resulting from the high substrate concentration).
Conclusions
The primary motivation for this study is to explore the intrinsic reasons for the unique behavior of the glycerolysis of triglycerides in the IL system and to give a proper evaluation of the effects of the important parameters. It turns out that multiple factors result in the lower initial rate and the occurrence of induction period. Both the internal mass transfer limitation resulting from the high viscosity of the IL and the partial phase separation are found to play a crucial role. Therefore any measures to reduce the system viscosity or to eliminate the diffusion limitation such as by increasing temperature and improving mixing effect will significantly increase reaction rates and reduce the induction periods. Our observation also shows that the rheological behavior of CPMA·MS is slightly shear-thickening and could be more accurately described by VFT model, while the mixtures of the IL and substrate exhibit as a thixotropic liquid, suggesting that agitation not only facilitates diminishing the external mass transfer but also reduces the system viscosity to improve the internal mass transfer. The increase of water activity in the IL system leads to the decrease of reaction rate. Notably high Km and Vmax for the glycerolysis in the IL system indicate the special thermodynamic affinity property of the IL system and its higher productivity.This work involves quantitative description of the mass transfer in the practical IL system with high viscosity. These efforts are believed to be useful for a better understanding of how the IL sustains a complicated reactive environment and produces such unique reaction behaviors. The study will provide a sound basis for reaction optimization and further screening of ILs.
Experimental
Materials
Novozym 435 (Candida antarctica lipase B immobilized on macroporous polyacrylate resin beads) was provided by Novozymes A/S (Bagsvaerd, Denmark). Glycerol (minimum 99%) was purchased from Sigma-Aldrich Co. (St. Louis, USA). Sunflower oil was obtained from Aarhus United (Aarhus, Denmark). Cocosalkyl pentaethoxymethyl ammonium methosulfate (CPMA·MS) was procured from Solvent Innovation Gmbh (Köln, Germany) and of 98% minimum purity. Other chemicals and reagents were all of analytical grade and used as received.Activity evaluation of glycerolysis and definition of glycerolysis degree
Different from hydrolytic activity of lipases, the definition of the glycerolysis activity of lipases is always a disputing topic, since glycerolysis can occur between TG and glycerol, between DG and glycerol, and between TG and MG. Noureddini and Harmeier47 mentioned this problem and proposed a new definition of glycerolysis activity unit (GU) based on the consumption of both TG and glycerol. However, the method needs the accurate measurement of the consumption of glycerol, which is usually complicated. Theoretically the glycerolysis activity of lipases should include the formation of 1 mol DG and 1 mol MG from 1 mol TG as well as the formation of 2 mol MG from another 1 mol glycerol. This means that the glycerolysis activity can be expressed as the formation of DG (1 mol DG consumes 1 mol TG) and MG (3 mol MG consumes 2 mol glycerol), in which the amounts are obtained from the normalization of only glycerides and free fatty acids based on area percentages by TLC-FID analysis (All glycerides give similar response factors and the error is within ±5%). The glycerolysis degree (GD), is calculated as follows: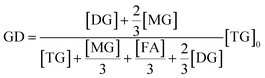 | (6) |
Parameter studies and kinetic measurement
Typical experimental procedure for glycerolysis of sunflower oil in IL: 2 mmol sunflower oil and 5 × 2 mmol glycerol were mixed with 2 mL (2.2 g) cocosalkyl pentaethoxymethyl ammonium methosulfate (CPMA·MS) in a 25 mL jacketed vial by magnetic stirring. The reaction was initiated by the addition of 100 mg Novozym 435 and conducted at the desired temperature controlled by circulated water. The evolution of the reaction was monitored by sample withdrawal and TLC-FID analysis after dissolving the sample in chloroform–methanol (2 ∶ 1 v/v) solution. All reactions were performed in duplicates. The developing solvents for TLC-FID consist of n-hexane, diethyl ether and acetic acid (35 ∶ 35 ∶ 1 v/v/v). The area percentage of glyceride and free fatty acid bands was used for the calculation of conversions of oil and yields of monoglycerides.48Important parameters influencing mass transfer and reaction, such as agitation rate, enzyme dosage, and substrate ratio, were investigated using the above experimental procedure with specific adjustment of parameters to meet the designed reactions.
The thermodynamic properties were acquired by investigating the plot of reaction rate against temperature and fitting the data to the Arrhenius equation, in which a 5-fold molar excess of glycerol to sunflower oil is applied to all reaction systems and the reaction rate indicates the molar conversion of TG.
Glycerolysis of sunflower oil is a two-substrate and two-step reaction. To simplify the kinetic model, an excess of glycerol was used for all systems and the change of its concentration during the measurement of reaction rate was neglected. Conversion of TG and glycerolysis degree were used to determine the maximum reaction rate and Michaelis–Menten constants.
Viscosity measurement and size assay of the immobilized particles
Absolute viscosity (η) of the pure IL and its mixture with substrates were measured with a Stresstech Rheometer (Rheologica Instruments AB, Lund, Sweden). Shear flow curves were obtained using a coaxial cylindrical geometry at shear rate ranging from 0 to 500 s−1. The measuring temperature was controlled by circulated water with an accuracy of 0.1 K. For all measurements, a 5 min preshear with constant rate of 200 s−1 was applied to the samples. The viscosities of the samples were first determined as a function of temperature during a heating cycle from 298 to 303 K. Afterwards the measurement was restarted after the solution was cooled to 298 K. All measurements were performed in duplicates.The particle size distribution of Novozym 435 was measured by a Masterizer 2000 particle size analyzer (Malvern Instrument, UK). The dry Novozym 435 has a mean volume weighted diameter of 0.513 mm. A slight diameter expansion to constant was observed during the reaction and storage in solvent. The recovered Novozym 435 from reaction was washed with 70% ethanol and the diameter was measured to give a mean value of 0.572 mm, identical with those stored in 70% ethanol for overnight.
Water content adjustment and aw measurement
The received CPMA·MS was first dried by vacuum of 1 mmHg at 50 °C for 8 h and its water content was measured with Karl Fischer titration. Then a series of mixtures with substrates in specific water content were prepared by adding a certain amount of water. The resulting samples were preequilibrated for 24 h with magnetic stirring in a sealed vial before being subjected to aw measurement. The water activity (aw) of the IL with specific water content was measured with an Aqualab Water Activity Meter (Decagon Devices, Washington, DC) at the set temperature. The profiles of aw for the IL and the mixture of the IL and substrates as a function of water content was accordingly established. The mixture of the IL and substrates with varied aw was finally used for glycerolysis studies to examine the effects of aw on the reaction.Acknowledgements
We thank Nancy Kjøbæk (BioCentrum-DTU) for her kind assistance for the viscosity measurement. Financial supports from Danish Technological Research Council (STVF) and Center for Advanced Food Studies (LMC) are acknowledged.References
- J. M. DeSimone, Science, 2002, 297, 799–803 CrossRef CAS.
- G. J. Lye, P. A. Dalby and J. M. Woodley, Org. Process Res. Devel., 2002, 6, 434–440 Search PubMed.
- M. M. Kirchhoff, Environ. Sci. Technol., 2003, 37, 5349–5353 CrossRef CAS.
- R. A. Sheldon, R. M. Lau, M. J. Sorgerdrager, F. van Rantwijk and K. R. Seddon, Green Chem., 2002, 4, 147–151 RSC.
- J. F. Brennecke and E. J. Maginn, AIChE J., 2001, 47, 2384–2389 CAS.
- J. S. Wilkes, J. Mol. Catal. A: Chem., 2004, 214, 11–17 CrossRef CAS.
- J. L. Anderson, J. Ding, T. Welton and D. W. Armstrong, J. Am. Chem. Soc., 2002, 124, 14247–14254 CrossRef CAS.
- J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667–3692 CrossRef CAS.
- S. Park and R. J. Kazlauskas, Curr. Opin. Biotechnol., 2003, 14, 432–437 CrossRef CAS.
- T. Itoh, E. Akasaki, K. Kudo and S. Shirakami, Chem. Lett., 2001, 262–263 CrossRef CAS.
- N. Jain, A. Kumar, S. Chauhan and S. M. S. Chauhan, Tetrahedron, 2005, 61, 1015–1060 CrossRef CAS.
- U. Kragl, M. Eckstein and N. Kaftzik, Curr. Opin. Biotechnol., 2002, 13, 565–570 CrossRef CAS.
- Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2003 Search PubMed.
- Ionic Liquids as Green Solvents, ed. R. D. Rogers and K. R. Seddon, American Chemical Society, Washington, DC, 2002 Search PubMed.
- F. Van Rantwijk, R. M. Lau and R. A. Sheldon, Trends Biotechnol., 2003, 21, 131–138 CrossRef CAS.
- R. D. Schmid and R. Verger, Angew. Chem., Int. Ed., 1998, 37, 1608–1633 CrossRef.
- Z. Guo, A. F. Vikbjerg and X. Xu, Biotechnol. Adv., 2005, 23, 203–259 CrossRef CAS.
- X. Xu, Eur. J. Lipid Sci. Technol., 2000, 102, 287–303 CrossRef CAS.
- V. M. Balcao and F. X. Malcata, Biotechnol. Adv., 1998, 16, 309–341 CrossRef CAS.
- T. Yamane, J. Am. Oil Chem. Soc., 1987, 64, 1657–1662 CrossRef CAS.
- M. T. Patel, R. Nagarajan and A. Kilara, Chem. Eng. Commun., 1996, 152–153, 365–404 Search PubMed.
- P. Lozano, T. de Diego, D. Carrié, M. Vaultier and J. L. Iborra, Chem. Commun., 2002, 692–693 RSC.
- M. T. Reetz, W. Wiesenhöfer, G. Franciò and W. Leitner, Chem. Commun., 2002, 992–993 RSC.
- R. M. Lau, F. van Rantwijik, K. R. Seddon and R. A. Sheldon, Org. Lett., 2000, 26, 4189–4191 CrossRef.
- S. Park and R. J. Kazlauskas, J. Org. Chem., 2001, 66, 8395–8401 CrossRef CAS.
- J. C. Bellot, L. Choisnard, E. Castillo and A. Marty, Enzyme Microb. Technol., 2001, 28, 362–369 CrossRef CAS.
- U. T. Bornscheuer, Enzyme Microb. Technol., 1995, 17, 578–586 CrossRef CAS.
- Z. Guo and X. Xu, Org. Biomol. Chem., 2005, 3, 2615–2619 RSC.
- G. D. Yadav and K. M. Devi, Biochem. Eng. J., 2002, 10, 93–101 CrossRef CAS.
- P. Bonhôte, A.-P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Grätzel, Inorg. Chem., 1996, 35, 1168–1178 CrossRef CAS.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Green Chem., 2001, 3, 156–164 RSC.
- O. O. Okoturo and T. J. VanderNoot, J. Electroanal. Chem., 2004, 568, 167–181 CrossRef CAS.
- J. R. Sanders, E. D. Ward and C. L. Hussey, J. Electrochem. Soc., 1986, 133, 325–330 CAS.
- A. Chagnes, A. Tougui, B. Carré, N. Ranganathan and D. Lemordant, J. Solution Chem., 2004, 33, 247–255 CrossRef CAS.
- F. W. Billmeyer, textbook of polymer science, John Wiley and Sons Inc., New York, 3rd edn, 1984 Search PubMed.
- M.-H. Oh, J.-H. So and S.-M. Yang, J. Colloid Interface Sci., 1999, 216, 320–328 CrossRef CAS.
- Perry's Chemical Engineers' Handbook, ed. R. H. Perry and D. W. Green, McGraw-Hill, New York, 6th edn, 1984, pp. 3–287 Search PubMed.
- H. A. Every, A. G. Bishop, D. R. MacFarlane, G. Orädd and M. Forsyth, Phys. Chem. Chem. Phys., 2004, 6, 1758–1765 RSC.
- E. L. Cussler, AIChE J., 1980, 26, 43–51 CrossRef CAS.
- P. J. Halling, Enzyme Microb. Technol., 1994, 16, 178–206 CrossRef CAS.
- J. A. Berberich, J. L. Kaar and A. J. Russell, Biotechnol. Prog., 2003, 19, 1029–1032 CrossRef CAS.
- F. Chamouleau, D. Coulon, M. Girardin and M. Ghoul, J. Mol. Catal. B, 2001, 11, 949–954 CrossRef CAS.
- M. Martinelle, M. Holmquist and K. Hult, Biochim. Biophys. Acta, 1995, 1258, 272–276 CrossRef CAS.
- J. Uppenberg, M. T. Hansen, S. Patkar and T. A. Jones, Structure, 1994, 2, 293–308 CAS.
- C. R. Wescott and A. M. klibanov, J. Am. Chem. Soc., 1993, 115, 1629–1631 CrossRef CAS.
- K. Ryu and J. S. Dordick, Biochemistry, 1992, 31, 2588–2598 CrossRef CAS.
- H. Noureddini and S. E. Harmeier, J. Am. Oil Chem. Soc., 1998, 75, 1359–1365 CrossRef CAS.
- T. Tatara, T. Fujii, T. Kawase and M. Minagawa, Lipids, 1983, 732–736 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
