Process intensification: oxidation of benzyl alcohol using a continuous isothermal reactor under microwave irradiation
R. J. J.
Jachuck
*a,
D. K.
Selvaraj
a and
R. S.
Varma
b
aProcess Intensification and Clean Technology (PICT) Group, Department of Chemical and Biomolecular Engineering, Clarkson University, Potsdam, NY – 13699, USA. E-mail: rjachuck@clarkson.edu
bNational Risk Management Research Laboratory, US Environmental Protection Agency, 26 West MLK Drive, MS 443, Cincinnati OH – 45268, USA
First published on 14th November 2005
Abstract
In the past two decades, several investigations have been carried out using microwave radiation for performing chemical transformations. These transformations have been largely performed in conventional batch reactors with limited mixing and heat transfer capabilities. The reactions were performed under adiabatic conditions where the enhancements in the reaction rate reported in these publications may be due to the rapid increase in the reaction temperature during the course of the reaction. The concept of process intensification has been used to develop a narrow channel reactor that is capable of carrying out reactions under isothermal conditions while being exposed to microwave irradiation. Oxidation of benzyl alcohol to benzaldehyde has been carried out using the above-mentioned isothermal micro reactor. Results and the findings of this investigation are discussed in this paper.
Introduction
Chemical reactions under microwave radiation have been studied with great interest since the sixties.1,2 Significant momentum in this field was generated after it was shown in 19863,4 that the rate of organic chemical synthesis of chemicals was much faster under microwave irradiation as compared to conventional methods. Since then, numerous researchers have studied and proved that various types of reactions experienced huge rate enhancements under the influence of a microwave field.5–11 There have been few instances where microwave enhanced reactions were carried out in continuous reactors.12,13 Virtually none of the reactions reported in published literature were carried out in a continuous isothermal reactor and hence there has been considerable debate over the true rate enhancement mechanism of microwave fields, as rapid increase in reaction temperature could result in significant rate enhancement.Benzaldehyde is one of the most industrially useful members of the aromatic aldehyde family. It is used as a raw material for a large number of products in organic synthesis, including perfumery, beverage and pharmaceutical industries.14 Microwave oxidation of benzyl alcohol into benzaldehyde using clayfen was studied by Varma and Dahiya15 and it was found that microwaves did play an influential role in increasing the rate of the reaction.
As part of this investigation, it was intended to design, fabricate and study the performance of a micro reactor capable of operating isothermally under a microwave field. The aim of the study was to investigate the oxidation of benzyl alcohol and obtain experimental data to prove conclusively that the rate enhancement observed by Varma and Dahiya was due to a true microwave influence, such as rotational movement of the bonds, and not just a temperature effect.
Experimental facility
Experiments were carried out using a 900 Watts Westpointe microwave operating at 2.45 GHz with an internal volume of 0.9 m3. The test facility shown in Fig. 1 consisted of the isothermal micro reactor, which was placed inside the microwave unit and was connected to inlet–outlet ports of the heat transfer side and reactant–product ports of the reaction side.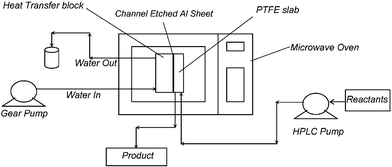 | ||
| Fig. 1 Schematic of the experimental setup. | ||
The reactor was designed and developed by the PICT group at Clarkson University. Fig. 2 shows that the micro reactor consisted of two main sections: an aluminium section and a Poly Tetra Fluoro Ethylene (PTFE) section, used for the heat transfer side and the reaction side respectively. The overall heat transfer coefficient of the reactor, U, was found to be about 2.5 kW m−2 K−1. The heat generated due to the exposure to a microwave (MW) environment on the reaction side was rapidly absorbed by the heat transfer fluid (H2O) on the heat transfer side. The materials used in the construction were carefully selected in order to allow near 100% transparency to microwave in the reaction zone and 0% transparency on the heat transfer side. The reactor had a volume of 2.7 × 10−7 m3 in the reaction zone and 6 × 10−6 m3 in the heat transfer zone.
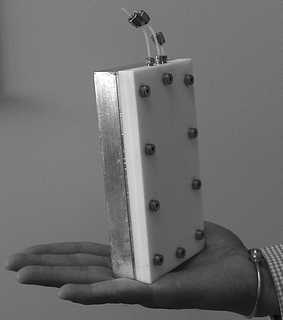 | ||
| Fig. 2 Isothermal continuous narrow channel reactor. | ||
The following is the chemical reaction studied under the influence of microwave irradiation:
The reactant was prepared at room temperature by dissolving solid Fe(NO3)3·9H2O (98+% ACS Reagent) in the benzyl alcohol solution (99+% ACS Reagent). The mixture was stirred thoroughly and filtered using a Fisherbrand® filter paper. Experiments were performed using the continuous isothermal reactor under the influence of microwave irradiation for a range of residence times (3–17 s) corresponding to different flow rates (1–5 ml min−1) and different microwave intensities (0 W to 39 W). The feed was pumped through the reactor using an HPLC pump (Model: LKB Bromma 2150) and the heat transfer fluid (water) was circulated through the heat transfer zone of the reactor at 120 ml min−1 by using a peristaltic pump (Model: Cole Parmer Instrument Co. 7520-01).
In order to benchmark the performance of the narrow channel reactor under the influence of microwaves, several batch experiments were also carried out without exposure to the microwaves. Limited tests were carried out using a PTFE capillary reactor under microwave irradiation to investigate the rate of reaction under adiabatic conditions. These experiments were carried out under non-isothermal conditions by passing the reactants continuously through the narrow channel PTFE tubing for similar residence times as in the isothermal continuous narrow channel reactor.
The inlet and the outlet temperatures of both the reaction and the heat transfer fluid were monitored continuously using a PICO temperature recorder. All the thermocouples were of K type with an error of ±0.3 °C. Analyses of the results were performed in a Mattson Galaxy Series FTIR-5000. Fig. 3 shows the distinct peaks of benzyl alcohol, in the region of 3100–3600 cm−1 and benzaldehyde in the region of 1670–1720 cm−1. Calibration was done using pure benzyl alcohol and benzaldehyde (99.5+% ACS Reagent) solutions. The peak height ratios obtained from the calibration data were used to find the conversion percentage to benzaldehyde from oxidation of benzyl alcohol. Benzyl alcohol and benzaldehyde peaks in the FTIR spectrum obtained from experimental samples were compared with the FTIR library from Mattson Instruments Inc. Peaks obtained from the samples matched accurately with the peaks of the library spectrum. The findings of the experimental investigations for the above conditions have been discussed in the following section.
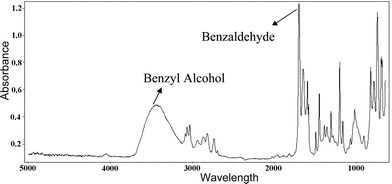 | ||
| Fig. 3 FTIR Graph showing the peaks of benzyl alcohol and benzaldehyde. | ||
Results and discussion
In this section, experimental results are presented and discussed critically in order to validate the initial aim of the investigation. Initially, tests were performed using a narrow channel reactor to ensure the following.(1) Negligible temperature differential (ΔT) between the inlet and the outlet temperature of water (on the heat transfer side), when flowing through the heat transfer side without any reactant flow and the microwave turned off. This was essential in order to prove that any ΔT seen in the inlet and outlet water temperature was due to the heat generated by the reaction when the reactant is exposed to a microwave environment. As can be seen in Table 1, ΔT = 0.2 °C, which is well within the experimental error range.
| Condition | Water inlet (T1)/°C | Water outlet (T2)/°C | (T1 − T2); ΔT | Reactant inlet (t1)/°C | Product outlet (t2)/°C | (t1 − t2); Δt | Conclusion |
|---|---|---|---|---|---|---|---|
| Microwave off | 21.9 | 22.1 | 0.2 | — | — | — | Negligible temperature rise due to friction |
| No reactant flow | |||||||
| Microwave on | 22.7 | 23.0 | 0.3 | — | — | — | Effective microwave shielding of the cooling compartment |
| No reactant flow | |||||||
| Microwave on | 22.7 | 25.9 | 3.2 | 21.7 | 21.9 | 0.2 | Water absorbing heat. Reaction under isothermal conditions. |
| Reactant flow |
(2) Under the influence of microwaves, with no reactant in the reaction zone, the ΔT in water stream (heat transfer side) remained negligible. This was important to prove that the microwave did not penetrate the heat transfer side but only penetrated the reaction side, as can be clearly seen in Table 1.
(3) The heat generated on the reaction side when the reactor system was exposed to microwave was completely absorbed by the water flowing on the heat transfer side. This test was essential in order to prove that during the course of the reaction there was negligible rise in the reaction temperature (<0.3 °C), ensuring the system was operating under isothermal conditions. As seen in Table 1, when the reaction was operated with both the reactant and the heat transfer fluid flow in their respective chambers coupled with microwave irradiation, a ΔT of 0.2 °C at the reaction zone and a ΔT of 3.2 °C at the heat transfer zone were measured. This clearly showed that the heat generated by the microwave irradiation and the heat of reaction was absorbed by the heat transfer fluid, thus providing isothermal characteristics to the reactor.
This set of experiments clearly proves and validates our claims that the reactor fabricated is, indeed, an isothermal one suitable for operation under the effect of microwave irradiation.
Influence of residence time
Having performed the initial experiments to establish the capability of the reactor for characterizing isothermal reactions, tests were carried out to study the influence of residence time on the conversion of benzyl alcohol to benzaldehyde under microwave irradiation. The residence time was varied by changing the flow rate from 1–5 ml min−1. The length of the reactor was kept constant. Since all the experiments were carried out under laminar flow conditions as shown in Table 2, it may be assumed that the transport mechanism (mixing) was purely diffusive and hence the approach of studying the residence time effect by changing the flow rate is valid for the conditions experimentally studied. The conversion of benzyl alcohol for various residence times is shown in Fig. 4. Every experimental data point used in Fig. 4, 5, 6, 8 and 9 is an average of data sets based on three experiments under identical conditions and each product sample was analyzed at least three times. The average deviation of the repeat experimental runs was always less than 5%.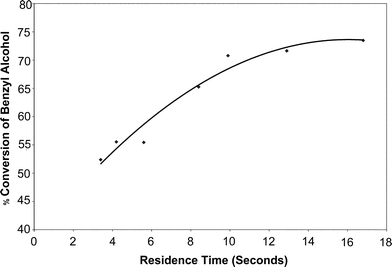 | ||
| Fig. 4 Conversion of benzyl alcohol as a function of residence time under isothermal conditions. | ||
| Flow rate/ml min−1 | Residence time/s | Re = DhVmρ/μ |
|---|---|---|
| D h = hydraulic diameter, m; Vm = velocity, m s−1; ρ = density, kg m−3; μ = viscosity, kg m−1 s−1. | ||
| 1 | 17 | 3.14 |
| 1.3 | 13 | 4.09 |
| 1.7 | 10 | 5.35 |
| 2 | 8.4 | 6.29 |
| 3 | 5.6 | 9.44 |
| 4 | 4.2 | 12.58 |
| 5 | 3.4 | 15.73 |
Comparison of adiabatic and isothermal conditions
Tests were also carried out to compare the conversion achieved under isothermal conditions with those under adiabatic conditions. This test was deemed essential in order to justify whether the rate enhancements predicted under microwave irradiation were purely temperature effect or whether it was a combined effect of temperature and bipolar movements. The adiabatic tests were carried out in a 57 cm long, 1/16″ OD, and 1/32″ ID PTFE capillary tube. It can be clearly seen in Fig. 5 that the conversion achieved under adiabatic conditions was higher than the conversion achieved under isothermal conditions, especially for higher residence times. For lower residence times, the conversion was similar for both adiabatic and isothermal conditions. Higher conversions were experienced under adiabatic conditions because the reaction was performed at approximately 10 °C higher temperature. A temperature difference of 10 degrees was recorded for the adiabatic tests as compared to a ΔT of 0.2 °C for isothermal experiments. Conversions for shorter residence times were similar for both isothermal and adiabatic runs because the temperature rise was very small for shorter residence times during the adiabatic tests.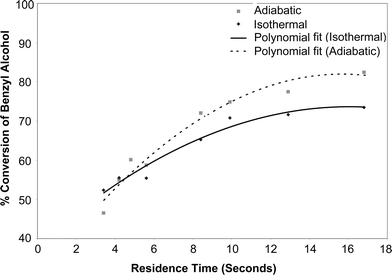 | ||
| Fig. 5 Comparison under adiabatic and isothermal conditions. | ||
Influence of microwave intensity
Experiments were also carried out to study the effect of the influence of the microwave intensity on the conversion of benzyl alcohol. This was done by changing the in-built power settings in the control panel of the microwave oven. Table 3 illustrates that the intensity of the microwave played a very important role in the conversion of benzyl alcohol to benzaldehyde. The actual power consumed by the reactants was calculated experimentally using the equation, Q = mCpΔT, where m = mass flow rate, kg s−1 and Cp = specific heat, J kg−1 °C−1. Fig. 6 shows a graphical representation of the change in conversion with changes in the microwave intensities. These studies were carried out for a reaction residence time of 17 seconds.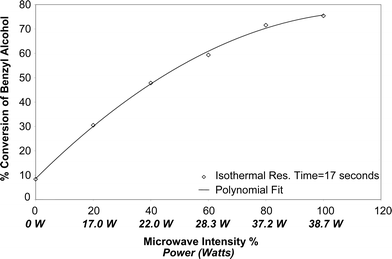 | ||
| Fig. 6 Influence of microwave intensity on benzyl alcohol conversion under isothermal conditions. | ||
| Residence time/s | Microwave intensity (%) | Conversion (%) | Power/W |
|---|---|---|---|
| 17 | 100 | 75.39 | 38.7 |
| 17 | 80 | 71.56 | 37.2 |
| 17 | 60 | 59.33 | 28.3 |
| 17 | 40 | 47.82 | 22.0 |
| 17 | 20 | 30.50 | 17.0 |
| 17 | 0 | 8.37 | 0 |
Comparison with batch experimental results
Experiments were also carried out to see the effectiveness of microwave radiation when compared to the traditional batch methods. Table 4 shows the various conditions and the conversion data. It can be seen that the conversion of benzyl alcohol to benzaldehyde was much faster under the influence of microwave irradiation than the traditional batch methods. Varma and Dahiya15 achieved 92% conversion in 15 seconds in a batch system. But the volume of the reactants that was used with a different catalyst, clayfen, was miniscule (about 0.175 ml). In this study, 96% conversion was achieved under adiabatic conditions for a residence time of 75 seconds (flow rate of 4 ml min−1: 5 ml benzyl alcohol converted in 75 seconds). Fig. 7 shows a graphical representation of the time required to convert 100 ml of benzyl alcohol to benzaldehyde. It can be seen that using a continuous microwave reactor, a significant saving in processing time can be achieved.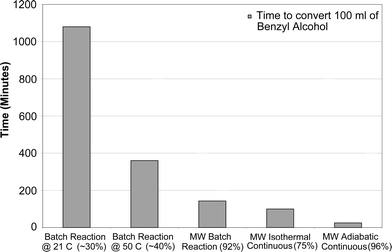 | ||
| Fig. 7 Equivalent time to convert 100 ml of benzyl alcohol using different methods. | ||
| Conditions | Residence time | Conversion (%) |
|---|---|---|
| Batch mixing at room temperature (21 °C) | 18 hours | ∼30 |
| Batch mixing at 50 °C | 6 hours | ∼40 |
| MW assisted isothermal continuous reaction using iron (III) nitrate | 17 seconds | 75 |
| MW assisted adiabatic continuous reaction using iron (III) nitrate | 75 seconds | 96 |
| MW assisted batch reaction using clayfen15 | 15 seconds | 92 |
Reaction kinetics
Calculations were performed to determine the rate of the reaction of the oxidation of benzyl alcohol into benzaldehyde for both isothermal and adiabatic conditions under the influence of microwave irradiation. Using the integrated rate law method, it was found that the reaction was second order with respect to benzyl alcohol for both cases. It can be seen from Fig. 8 and 9 that plots of 1/[benzyl alcohol] vs. time resulted in a linear fit.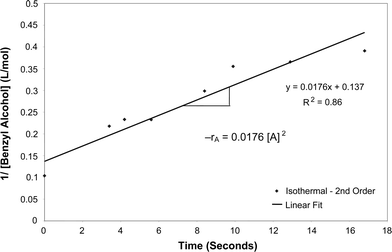 | ||
| Fig. 8 Determination of the order of reaction under isothermal conditions. | ||
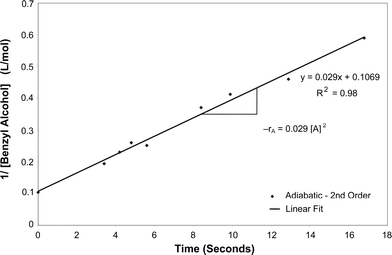 | ||
| Fig. 9 Determination of the order of reaction under adiabatic conditions. | ||
The rate constant, k, for the continuous isothermal oxidation of benzyl alcohol under the effect of microwave irradiation was determined to be 0.0176 l mol−1 s−1. The rate law for the isothermal reaction was found to be −rA = 0.0176[A]2, where A = benzyl alcohol. Similarly, the rate constant for the same reaction under adiabatic conditions was found to be 0.029 l mol−1 s−1. The rate law was found to be −rA = 0.029[A]2 for adiabatic oxidation of benzyl alcohol under the influence of microwave irradiation.
Conclusions
A compact continuous flow reactor based on narrow channel configuration was investigated to understand the influence of microwave irradiation of benzyl alcohol oxidation under isothermal conditions. The influences of microwave intensity and residence time were studied and it was seen that microwave truly does have a positive influence on the reaction rate. Having isolated the temperature effect, it was found that the microwave does invigorate the molecules to achieve higher reaction rate.The findings of this research have wide ranging implications in not just promoting the concept of green technology, but also addressing the potential commercial opportunity for using a microwave environment for producing chemical feedstock. This reactor in its current form is particularly suitable for high value, low throughput problems seeking high yield, selectivity and conversion. There are several reactions reported in literature which result in products with high selectivity5–11 and sometimes unique structures12 when microwave radiation is used to initiate the reaction. These reactions, we believe, can be carried out continuously in an isothermal narrow channel reactor.
Acknowledgements
The authors would like to acknowledge the contributions made by the members of the PICT lab at Clarkson University and New York State Office of Science, Technology and Academic Research (NYSTAR) for the financial support for this work.References
- N. F. Brockmeier, Chemical Reactions Induced in a Microwave Discharge, PhD Dissertation, Massachusetts Institute of Technology, 1966 Search PubMed.
- W. W. Cooper IV, The Oxidation of Hydrogen Chloride in a Microwave Discharge, PhD Dissertation, Massachusetts Institute of Technology, 1966 Search PubMed.
- R. Gedye, F. Smith, K. Westaway, H. Ali, L. Baldisera, L. Laberge and J. Rousell, The use of microwave ovens for rapid organic synthesis, Tetrahedron Lett., 1986, 27(3), 279–282 CrossRef CAS.
- R. J. Giguere, T. L. Bray, S. M. Duncan and G. Majetich, Application of commercial microwave ovens to organic synthesis, Tetrahedron Lett., 1986, 27(41), 4945–4948 CrossRef CAS.
- B. L. Hayes, Microwave Synthesis: Chemistry at the Speed of Light, CEM Publishing, Matthews, NC, USA, 2002 Search PubMed.
- R. S. Varma, Advances in Green Chemistry: Chemical Syntheses using Microwave Irradiation, Kavitha Printers, Bangalore, India, 2002 Search PubMed.
- Evalueserve, Special Report; Developments in Microwave Chemistry, Chem. World, 2005, 2(4) Search PubMed.
- C. O. Kappe, Synthetic methods: Controlled microwave heating in modern organic synthesis, Angew. Chem., Int. Ed., 2004, 43(46), 6250–6284 CrossRef CAS.
- M. Nüchter, B. Ondruschka, W. Bonrath and A. Gum, Microwave assisted synthesis—a critical technology overview, Green Chem., 2004, 6(3), 128–141 RSC.
- M. Nuechter, U. Mueller, B. Ondruschka, A. Tied and W. Lautenschlaeger, Microwave-assisted chemical reactions, Chem. Eng. Technol., 2003, 26(12), 1207–1216 CrossRef.
- A. Fini and A. Breccia, Chemistry by microwaves, Pure Appl. Chem., 1999, 71(4), 573–579 CrossRef CAS.
- R. Rodriguez-Clemente and J. Gomez-Morales, Microwave precipitation of CaCO3 from homogeneous solutions, J. Cryst. Growth, 1996, 169(2), 339–346 CrossRef CAS.
- P. He, S. J. Haswell and P. D. I. Fletcher, Microwave-assisted Suzuki reactions in a continuous flow capillary reactor, Appl. Catal., A: Gen., 2004, 274, 111–114 CrossRef CAS.
- J. A. B. Satrio and L. K. Doraiswamy, Production of benzaldehyde: a case study in a possible industrial application of phase-transfer catalysis, Chem. Eng. J., 2001, 82(1–3), 43–56 CrossRef CAS.
- R. S. Varma and R. Dahiya, Microwave-assisted oxidation of alcohols under solvent-free conditions using clayfen, Tetrahedron Lett., 1997, 38(12), 2043–2044 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |