Gold nanoparticle-catalysed [3 + 2]dipolar cycloaddition of 1,6-allenynebenzaldehydes: construction of polycyclic ring systems
Arun Kumar
Gupta
a,
Chul Yun
Rhim
a,
Chang Ho
Oh
*a,
R. S.
Mane
b and
Sung-Hwan
Han
*b
aGOS Lab, Department of Chemistry, Hanyang University, Seoul, 133 791, South Korea
bInorganic Nanomaterials Lab, Department of Chemistry, Hanyang University, Seoul, South Korea
First published on 24th November 2005
Abstract
We have isolated spherical-shaped monodispersed 12–14 nm range gold nanoparticles, when o-1,6-allenylbenzaldehyde underwent a novel mode of tandem cycloaddition and cyclization using AuCl3 precatalyst. This cyclization can be found in the construction of many polycyclic natural product skeletons.
Recently it has been found that anhydrous AuCl3 catalyzes the formation of C–C and C–O bonds, behaving as an effective Lewis-acid catalytic system;1 such as selective cross cycloisomerization/dimerization of propargyl or alkynyl ketones, benzoannulation between o-alkynylbenzaldehydes and alkynes or cyclizations of alkynyl furan.1a Due to the excellent alkynophilicity of gold; particular attention has been paid to the gold-based alkyne activation as an attractive strategy for developing new and efficient catalytic cyclizations. Yamamoto and co-workers recently reported a novel gold-catalyzed benzoannulation of o-alkynylbenzaldehydes with alkynes involving [4 + 2] cycloaddition of a Au-pyrylium intermediate with dienophiles such as alkynes and enol ethers.2 They also reported that ynals bearing a pendant alkyne group underwent [4 + 2] benzannulations intramolecularly.3 Recently we reported that enynes bearing an aldehyde group underwent unprecedented Rh-catalyzed cyclization which could involve [3 + 2] cycloaddition of a Rh-carbenoid dipolar carbonyl ylide intermediate.4 However, there were no reports on cycloaddition of such complexes to pendent allene group either inter or intramolecularly.
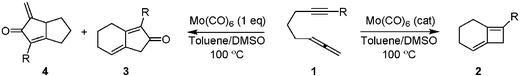
Unique reactivity of two orthogonal π-bonds present in allenynes have made them to be of great interest in the field of organometallics5 as well as for the construction of natural product 6 frameworks. Very recently, Qilong and Hammond reported that the formation of [2 + 2] cycloaddition product 2 from molybdenum catalyzed reactions of 1,7-allenynes 1, where the metal complex was used catalytically.7 A similar cycloaddition reaction under microwave irradiation was reported by Brummond and Chen,8 and their reports on a stoichiometric Pauson–Khand reaction (PKR) with a similar substrate 1 using the same catalyst, gave 3 and 4 as products (Scheme 1).9
 | ||
| Scheme 1 | ||
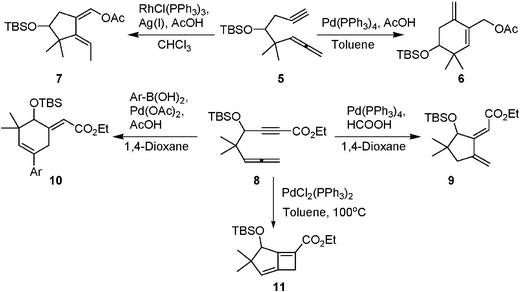
We have also showed that allenynes 5 have different cyclization modes with different palladium catalysts. Cycloreduction occurred at the triple bond to give an alkenylpalladium species that underwent carbo-palladation to give six-membered cycles 6, whereas rhodium catalyzed cyclizations of those allenynes 5 gave five membered rings 7 chemoselectively. In the case of 1,6-allenynes 8, these gave five membered ring cycles 9 upon cycloreduction, whereas six-membered ring systems 10 were obtained on arylative cyclization under palladium catalysis (Scheme 2).10
 | ||
| Scheme 2 | ||
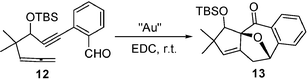
In continuation of our research interest in allenynes11 and also in pursuit of previous investigations, a closer outlook prompted us to secure cycloisomerisation involving Huisen-type cycloaddition of Au-pyrylium intermediates with dipolarophiles.12 Here we wish to report a highly economical as well as an environmentally benign methodology for intramolecular tandem [3 + 2] cycloaddition and cyclization of o-1,6-allenynebenzaldehydes under gold (III) chloride catalysis at room temperature. Also, the catalyst gold (III) chloride was recovered as monodispersed spherical nanoparticles and reused for the same reaction. At first, we have taken 3 mmol% gold (III) chloride and 1,6-allenynebenzaldehyde 12 (1 mmol) in a 10 mL test tube containing 2 mL of 1,2-dicholoroethane. Here we have found that compound 12 underwent a novel mode of cycloaddition–cyclization,13 with the formation of gold spherical, monodispersed nanoparticles. The structure of product 13 was confirmed by IR, H1 NMR, CMR, MS and HRMS.14 The gold nanoparticles’ structure, surface properties and particle size were characterized by XRD, SEM and finally confirmed with TEM.
In our experiments, we could isolate the product 13 exclusively, which is similar to our Rh-catalyzed reactions of enynals. Au-catalysed reactions of allenyne might also occur via 1,3-dipolar cycloaddition to form the electron deficient Au-carbene species which subsequently underwent sequential fragmentation to give product 13 and to generate AuCl3 for the next cycle (Scheme 3). The fused polycyclic products obtained from this study were very stable during silica gel chromatography and prolonged storage at room temperature. We studied this reaction in 1,2-dichloroethane with different gold catalysts. AuCl3 with/without combining AgOTf catalyzed [3 + 2] cycloaddition,15 whereas a combination of AuCl3 with PPh3 did not catalyze any of these pathways (Table 1, entries 1–3). Triphenylphosphine might react with AuCl3 to destroy its catalytic activity or Lewis acidity. Au (+1) also catalyzed [3 + 2] cycloisomerization but albeit in low yield (Table 1, entry 4). Among the reaction conditions we have tried, gold (III) chloride with 3 mmol% in 1,2-dichloroethane solution at room temperature is the best suitable condition for the present study (Table 1, entry 1).
 | ||
| Scheme 3 | ||
|
|
|||
|---|---|---|---|
| Entry | Reaction conditions | T/°C, t/h | Yield (%) |
| 1 | AuCl3 | 25, 8 | 69 |
| 2 | AuCl3/AgOTf | 25, 8 | 43 |
| 3 | AuCl3/PPh3 | 80, 10 | Trace |
| 4 | AuCl | 25, 24 | 23 |
| 5 | AuBr3 | 25, 36 | 46 |
| 6 | AuCl3 nanoparticles | 25, 12 | 63 |
| 7. | AuCl3 nanoparticles | 25, 12 | 60 |
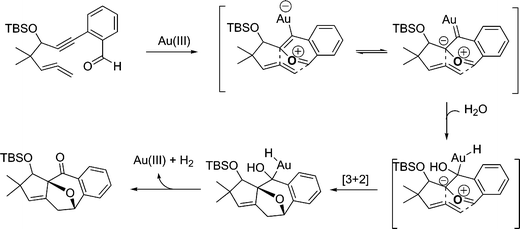
A plausible mechanism for the present metal catalyzed cycloaddition–cyclization reaction is AuCl3-complexation of o-1,6-allenylbenzaldehyde which is expected to form a zwitterion as proposed by Yamamoto et al.16 This intermediate on successive tandem [3 + 2] cycloaddition with tethered allene will lead to the observed oxabicyclic product and addition of water can occur at any stage, regenerating Au (III) and molecular hydrogen (Scheme 3).
The gold nanoparticles formed were adsorbed onto a glass substrate of plate dimension 1 × 1 cm2 and characterized by X-ray diffraction (XRD) (Siemens D-5005 diffractometer) using graphite-monochromatized Cu Kα radiation at 40 kV and 100 mA. Fig. 1a shows the X-ray diffraction pattern of gold nanoparticles recorded at room temperature. The location of planes corresponding to (111), (200), (220), (311) and (222) are in good agreement with the Joint Committee on Powder Diffraction Standards (JCPDS No. 040-784) reference diagrams for the corresponding bulk phases with lattice constant a = 4.087 Å. The satisfactory agreement among the ‘d’ (interplanar spacing) values confirms the presence of gold nanoparticles. In the XRD pattern the presence of the (111) reflection as a dominant peak along with the presence of reflections from planes of other types, indicates the formation of nanocrystallites with a moderate degree preferred orientation. Nothing other than a broad hump (15–40°), a characteristic peak due to the glass substrate, was detected in XRD spectrum. This observation reveals that the gold nanoparticles preferentially grew in 3D rather than 1D or 2D. An intense peak (111) was used to calculate the grain size using Scherrer's formula with a correction factor of 0.94 due to the specific geometry of grains and non-conducting nature of the substrate.17 The 12.4 nm grain size was calculated for as-formed gold nanoparticles.
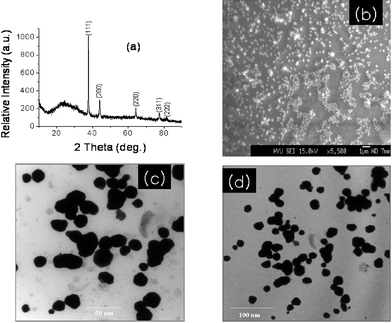 | ||
| Fig. 1 (a) XRD profile of as-deposited gold nanoparticles onto a 1 × 1 cm2 glass substrate, (b) SEM image, (c), (d), low and high magnified TEM images. | ||
In the SEM image18 (Fig. 1b), good film substrate coverage of gold nanoparticles in the form of monodispersed spheres with considerable void spaces are clearly observed. The resolution limit of the SEM made it difficult for us to calculate the exact nanoparticle size, where we preferred to use transmission electron microscopy (TEM).19 Representative TEM images of gold naoparticles for two different magnifications are shown in Fig. 1c and d, indicating a controlled growth of spherical gold nanoparticles. It can be seen in Fig. 1c that there exists a large number of gold nanoparticles with sphere-like surface morphology. Observation of the magnified image in Fig. 1d, shows that the gold nanoparticles are either isolated or in colony form. The average diameter of 12–14 nm was obtained which was found to be consistent with XRD data.
The recovered valuable gold catalyst, as 12–14 nm range spherical nanoparticles, was reused in the same reaction (Table 1, entries 6 and 7) as well as its applications in various fields.20
In summary, Au-catalyzed [3 + 2] intramolecular tandem cycloaddition–cyclization of 1,6-allenynebenzaldehydes with the formation of nanoparticles was shown. Thus we have demonstrated a very simple, eco-friendly, and more economical methodology for the synthesis of fused polycyclic ring systems from the corresponding o-1,6-allenynebenzaldehydes. We are currently pursuing the application of this methodology to the generation of a large library of polycyclic ring systems, which will be of interest to medicinal chemistry and in the construction of natural products.21
Acknowledgements
This work was supported by the Korean Science and Engineering Foundation (ABRL R14-2003-014-01001-0). AKG is thankful to the KOSEF for a Brain Pool fellowship.References
-
(a) A. S. K. Hashmi, L. Schwarz, J.-H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285–2287 CrossRef CAS
; (b) A. S. K. Hashmi, T. M. Frost and J. W. Bats, J. Am. Chem. Soc., 2000, 122, 11553–11554 CrossRef CAS
; (c) N. Asao, K. Takahashi, S. Lee, T. Kasahara and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 12650–12651 CrossRef CAS
.
-
(a) N. Asao, H. Aikawa and Y. Yamamoto, J. Am. Chem. Soc., 2004, 126, 7458–7459 CrossRef CAS
; (b) A. S. K. Hashmi, T. M. Frost and J. W. Bats, J. Am. Chem. Soc., 2000, 122, 11553–11554 CrossRef CAS
; (c) A. S. K. Hashmi, T. M. Frost and J. W. Bats, Catal. Today, 2002, 72, 19–27 CrossRef CAS
; (d) J. W. Dankwardt, Tetrahedron Lett., 2001, 42, 5809–5812 CrossRef CAS
.
- N. Asao, K. Sato, Menggenbateer and Y. Yamamoto, J. Org. Chem., 2005, 70, 3682–3685 CrossRef
.
- S. Shin, A. K. Gupta, C. Y. Rim and C. H. Oh, Chem. Commun., 2005, 4429–4431 RSC
.
-
(a) H. Urabe, T. Takeda, D. Hideura and F. Sato, J. Am. Chem. Soc., 1997, 119, 11295–112305 CrossRef CAS
; (b) C. Mukai, I. Nomura and S. Kitagaki, J. Org. Chem., 2003, 68, 1376–1385 CrossRef CAS
; (c) N. Cadran, K. Cariou, G. Hervé, C. Aubert, L. Fensterbank, M. Malacria and J. Marco-Contelles, J. Am. Chem. Soc., 2004, 126, 3408–3409 CrossRef CAS
; (d) A. Padwa, H. Lipka, S. H. Watterson and S. S. Murphree, J. Org. Chem., 2003, 68, 6238–6250 CrossRef CAS
; (e) K. Hiroi, T. Watanabe and A. Tsukui, Chem. Pharm. Bull., 2000, 48, 405–409 CAS
.
-
(a) K. M. Brummond and J. Lu, J. Am. Chem. Soc., 1999, 121, 5087–5088 CrossRef CAS
; (b) K. M. Brummond, J. Lu and J. Petersen, J. Am. Chem. Soc., 2000, 122, 4915–4920 CrossRef CAS
; (c) K. M. Brummond, D. P. Curan, B. Mitasev and S. Fischer, J. Org. Chem., 2005, 70, 1745–1753 CrossRef CAS
.
- S. Qilong and G. B. Hammond, J. Am. Chem. Soc., 2002, 124, 6534–6535 CrossRef CAS
.
- K. M. Brummond and D. Chen, Org. Lett., 2005, 16, 3473–3475 CrossRef
.
-
(a) K. M. Brummond, H. Chen and J. L. Kent, J. Org. Chem., 1998, 63, 6535–6526 CrossRef CAS
; (b) K. M. Brummond, A. D. Kerekes and H. Wan, J. Org. Chem., 2002, 67, 5156–5163 CrossRef CAS
.
-
(a) K. M. Brummond, H. Chen, P. Sill and L. You, J. Am. Chem. Soc., 2002, 124, 15186–15187 CrossRef CAS
; (b) S. Shin and T. V. RajanBabu, J. Am. Chem. Soc., 2001, 123, 8416–8417 CrossRef CAS
; (c) T. Shibata, Y. Takesue, S. Kadowaki and K. Takagi, Synlett, 2003, 268–270 CrossRef CAS
; (d) C. H. Oh, S. H. Jung and C. Y. Rhim, Tetrahedron Lett., 2001, 42, 8669–8671 CrossRef CAS
; (e) C. H. Oh, S. H. Jung, D. I. Park and J. H. Choi, Tetrahedron Lett., 2004, 45, 2499–2501 CrossRef CAS
; (f) A. K. Gupta, C. Y. Rhim and C. H. Oh, Tetrahedron Lett., 2005, 46, 2247–2250 CrossRef CAS
.
- C. H. Oh, D. I. Park, S. H. Jung, V. R. Reddy, A. K. Gupta and Y. M. Kim, Synlett, 2005, 13, 2092–2094 CrossRef
.
- In fact, the Huisgen-type [3 + 2] cycloaddition transition states of carbonyl ylides and alkynes have been known for more than 30 years.
(a) R. Huisgen, Angew. Chem., Int. Ed. Engl., 1968, 7, 321–323 CrossRef CAS
; (b) H. Hamberger and R. Huisgen, J. Chem. Soc. D, 1971, 19, 1190 RSC
.
- Typical experimental procedure: Into a 10 mL round bottomed flask was placed 1 mmol of 1,6-allenynebenzaldehyde and 3 mol% AuCl3. To this, 1,2-dichloroethan (2 mL) was added through the glass syringe. The reaction mixture was first stirred for 10 min at 0 °C and then allowed to warm to room temperature. After completion of the reaction, the reaction mixture was decanted and the nanoparticles were thoroughly washed with 1–2 mL of 1,2-dichloroethane to be reused for the next time. The solvent thus decanted was evaporated and subjected to column chromatography to obtain pure compound 13
.
- Spectroscopic data of compound 13b: (Colorless syrupy liquid, Rf = 0.55, 5% EtOAc–hexane). IR (neat): 2957, 2928, 2857, 1704, 1603, 1463, 1211, 1174, 1145, 1023, 971cm−1; 1H NMR (CDCl3): ( 8.02 (d, J = 8.0 Hz, 1 H), 7.50 (t, J = 6.4 Hz ,1 H), 7.37 (d, J = 8.0 Hz, 1 H),7.19 (d, J = 8.0 Hz, 1H), 5.67 (s, 1 H), 5.50 (d, J = 6.8 Hz, 1 H), 4.03 (s, 1 H), 2.95 (dd, J = 9.2, 6.8 Hz, 1 H), 2.23 (d, J = 16.4 Hz, 1 H), 1.26 (s, 3 H), 1.17 (s, 3 H), 0.8 (s, 12 H), 0.05 (s, 3 H), −0.04 (s, 3 H); 13C NMR (CDCl3): (190.25, 147.13, 138.15, 137.15, 133.23, 130.99, 127.87, 127.25, 122.87, 98.91, 86.48, 77.32, 55.18, 32.633, 28.22, 22.65, 18.02, −4.71, −5.51; MS (m/z): 370, 355, 313, 295, 269, 221, 195, 155, 105, 75; HRMS: exact mass calculated for: C22H30O3Si (M+): 370.1964; found: 370.1960
.
-
(a) T. Yao, X. Zhang and R. C. Larock, J. Am. Chem. Soc., 2004, 126, 11164–11165 CrossRef CAS
; (b) R.-V. Nguyen, X.-Q. Yao, D. S. Bohle and C.-J. Li, Org. Lett., 2005, 7, 673–675 CrossRef CAS
; (c) X.-Q. Yao and C.-J. Li, J. Am. Chem. Soc., 2004, 126, 6884–6885 CrossRef CAS
.
-
(a) B. F. Straub, Chem. Commun., 2004, 1726–1728 RSC
; (b) N. Asao, K. Takahashi, S. Lee, T. Kasahara and Y. Yamamoto, J. Am. Chem. Soc., 2002, 124, 12650–12651 CrossRef CAS
.
- R. S. Mane and Sung-Hwan Han, Electrochem. Commun., 2005, 7, 205–207 CrossRef CAS
.
- For the SEM analysis, the gold nanoparticle film was coated with a 10 nm platinum layer using a Polaron scanning electron microscopy (SEM) sputter coating unit E-2500, before taking the image
.
- Samples for TEM investigations were prepared by putting an aliquot of dichloromethane solution of gold nanoparticles onto an amorphous carbon substrate supported on a copper grid. The excess liquid was then wicked away with tissue, and the grid was allowed to dry at room temperature
.
- M. Chrisitne and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS
.
- Natural products:
(a) M. George, H. Rodgers, Z. Yong, T. Xinrong, F. T. Lee, F. Jing and D. Sam, J. Org. Chem., 1996, 61, 8169–8185 CrossRef CAS
; (b) K. A. Ghosh, C. Mukhopadhyay and U. R. Ghatak, J. Chem. Soc., Perkin Trans. 1, 1994, 3, 327–332 RSC
; (c) T. Ohta, E. Yuichi, K. Rikako and N. Shigeo, Heterocycles, 1994, 38, 55 CrossRef CAS
; (d) M. F. MacKay, B. G. I Martin and P. R. Phyland, Acta Crystallogr., Sect. C, 1997, C53, 1497 CrossRef CAS
; (e) T. L. Matthew, B. G. John, B. S. Jeffrey, D. K. Gary, L. Robert, L. O. Daniel, L. J. Deann and H. K. Terrence, US Pat. Search PubMed
, 151792, 2004; (f) M. Hideaki and N. Mitsutaka, Tetrahedron Lett., 2002, 43, 2913–2917 CrossRef
; (g) G. Saha, S. R. Supti and S. Ghosh, Tetrahedron, 1990, 46, 8229–8236 CrossRef CAS
; (h) D. Soumitra, B. Gopa and U. R. Ghatak, J. Chem. Soc., Perkin Trans. 1, 1990, 5, 1453–1458 RSC
; (i) H. Shinichi, K. Toshimi and H. Yoshiyuki, Phytochemistry, 1985, 24, 1545–1551 CrossRef CAS
; (j) H. Hong, N. Neamati, H. E. Winslow, J. L. Christensen, A. Orr, Y. Pommier and G. W. Milne, Antiviral Chem. Chemother., 1998, 9, 461 Search PubMed
.
| This journal is © The Royal Society of Chemistry 2006 |