Highly efficient and clean synthesis of 3,4-epoxytetrahydrofuran over a novel titanosilicate catalyst, Ti-MWW
Haihong
Wu
,
Lingling
Wang
,
Haijiao
Zhang
,
Yueming
Liu
,
Peng
Wu
* and
Mingyuan
He
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, Department of Chemistry, East China Normal University, North Zhongshan Rd. 3663, Shanghai, 200062, P. R. China. E-mail: pwu@chem.ecnu.edu.cn
First published on 1st November 2005
Abstract
A much greener synthesis method is discovered for the synthesis of 3,4-epoxytetrahydrofuran (ETHF), an important synthetic intermediate for stereo-controllable synthesis of complex organic compounds. 3,4-ETHF is synthesized with a conversion >95% and a selectivity >99% through a heterogeneous epoxidation of 2,5-dihydrofuran (DHF) with hydrogen peroxide in the liquid phase over a novel titanosilicate catalyst, Ti-MWW.
Introduction
3,4-Epoxytetrahydrofuran (ETHF) is becoming an increasingly important intermediate in chemical syntheses. The polymerization of 3,4-ETHF gives linear polymers having intrinsic viscosity and good hydrophilicity.1 It also serves as an important class of substrate for new desymmetrisation methodologies,2,3 and also provides a useful building block of the tetrahydrofuran ring in combinatorial chemistry for pharmaceutical applications.4,5 For example, it is a key synthesis component for the HIV-protease inhibitor nelfinavir6 and the cysteine protease cathepsin K which has been postulated as a target for the treatment of osteoporosis.7 Thus, 3,4-ETHF tends to be versatile in the stereo-controllable synthesis of complex organic compounds and is receiving considerable research interest because of the promising applications.3,4-ETHF is usually prepared by oxidizing 2,5-dihydrofuran (DHF) with peracids such as m-chloroperbenzoic acid (CPBA)8 and trifluoroperacetic acid,9 and also using urea hydrogen peroxide together with phthalic anhydride.4 These stoichiometric oxidations suffer major disadvantages such as production of a large quantity of toxic by-products, slow reaction rates, low product selectivity, and difficulty in product separation. The chlorohydrin method, widely used for various epoxides, has also been applied to the synthesis of 3,4-ETHF via ring-closing of trans-4-chloro-tetrahydrofuran-3-ol.9,10 On the other hand, an improved method has been proposed to obtain the corresponding halohydrin first through the reaction of 2,5-DHF with 1,3-dibromo-5,5-dimethylhydantoin, and then 3,4-ETHF via dehalogenation.11 However, the product yield is still not as high as expected, and more importantly, the procedure is not environmentally benign because poisonous and corrosive halogens are involved in both reactants and by-products.
The discovery of titanosilicate (with TS-1 as a representative), which is capable of catalyzing a variety of reactions under mild conditions using aqueous H2O2 as an oxidant and co-producing essentially water as the only by-product, opens up the possibility of developing environmentally friendly chemical processes.12 Concerning the epoxidation of alkenes, the medium pore system (10-membered ring, MR) of TS-1 produces steric hindrance particularly for cyclic substrates with relatively bulky molecular dimensions. Furthermore, the fact that TS-1 favors the protic solvent methanol makes the solvolysis of epoxide unavoidable, which results in useless by-products and then lowers the epoxide selectivity significantly.13 In fact, catalyst of TS-1 monoliths have been used in the attempted epoxidation of 2,5-DHF in methanol.14 By prolonging the reaction time and using an excess of H2O2, 2,5-DHF is converted almost completely in methanol solvent but the selectivity for 3,4-ETHF, initially not high enough (<80%), drops continuously to ca. 70%. Therefore, to synthesize 3,4-ETHF more efficiently and cleanly using an aqueous H2O2-involving system, it is crucial to design and prepare a new titanosilicate catalyst superior to TS-1 in both the pore system and the nature of the crystalline framework.
Recently, we developed a novel titanosilicate with the MWW structure, Ti-MWW.15 Since it contains large pores of 12 MR side pockets and supercages both of which serve as open reaction spaces for cyclic molecules, and its unique hydrophilic/hydrophobic nature preserves its high catalytic performance in aprotic solvents or even water,16 Ti-MWW is thus considered to be a suitable candidate for the epoxidation of cyclic alkenes of 2,5-DHF. We communicate in this paper an efficient and environmentally friendly way for the selective synthesis of 3,4-ETHF by using the Ti-MWW–H2O2 system.
Experimental
Ti-MWW (Si/Ti = 55) was prepared following a post-synthesis method reported previously.17 For control experiments, TS-1 (Si/Ti = 51) was synthesized by the method widely adopted.18The structures of the titanosilicates were checked with X-ray diffraction patterns recorded on a Bruker D8 AVANCE diffractometer (Cu-Kα). The chemical analyses were carried out by inductively coupled plasma (ICP) on a Thermo IRIS Intrepid II XSP atomic emission spectrometer. UV-visible spectra were collected on a Shimadzu UV-2550 spectrophotometer using Spectralon® as a reference to characterize the coordination states of Ti species.
The oxidation of 2,5-DHF (>98%, TCI, Japan) with H2O2 was carried out batchwise in a 25 mL flask connected to a cooling condenser. In a typical run, 5 mmol of 2,5-DHF, 5 mL of solvent and 0.07 g catalyst were charged into the flask. H2O2 (30 wt%, 5 mmol) was then added to the mixture to begin the reaction at 333 K under vigorous agitation. After the catalyst solid was removed, the liquid phase was analyzed on a gas chromatograph (Shimadzu GC-14B) equipped with a DB-1 capillary column. The absolute amount was quantified using cyclohexanone as an internal standard. The amount of unconverted H2O2 was determined by the standard titration method using a 0.1 M Ce(SO4)2 solution. The products were verified using authentic chemicals commercially available or determined on a gas chromatograph-mass spectrometer (Agilent HP6890/5973N).
Results and discussion
Fig. 1 shows the XRD patterns and UV-visible spectra of Ti-MWW and TS-1. Both samples had high crystallinity and contained essentially isolated tetrahedral Ti species in the framework as indicated by the single UV-visible band at 220 nm. Thus, the two samples were qualified as liquid-phase oxidation catalysts with H2O2.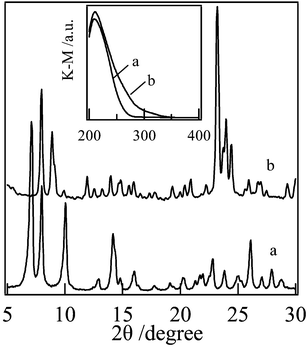 | ||
| Fig. 1 Representative XRD patterns and UV-visible spectra (inset) of Ti-MWW (a) and TS-1 (b). | ||
The products from the epoxidation of 2,5-DHF greatly depend on the reaction conditions such as the kind of catalyst and solvent employed. After analyzing the products with GC-MS carefully together with using authentic samples, a clear diagram of product distribution was obtained (Fig. 2). The two solvolysis products were determined by synthesizing the corresponding chemicals from 3,4-ETHF in water or MeOH using H-ZSM-5 zeolite as a solid catalyst (333 K, 5 h). The other products particularly those produced from the allylic reactions were deduced based on the fact that the final products of allylic reactions, 5H-furan-2-one and maleic anhydride, were actually obtained as confirmed by authentic samples, and that chemicals with the same mass numbers were detected on GC-MS. The main product was 3,4-ETHF as a result of the oxidation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond. The acid sites contained within the titanosilicate catalysed the reaction of 3,4-ETHF with water or alcohols to give solvolysis products. The oxidation occurred also on allylic positions to give the corresponding products which underwent successive oxidation by H2O2 and/or solvolysis by protic solvents. A product resulting from the direct addition reaction of alcohol on the allylic positions of 2,5-DHF was also observed. To evaluate the catalytic results simply among the catalysts and solvents, the products have been sorted into three groups, i.e. 3,4-ETHF, allylic reaction products and solvolysis products of 3,4-ETHF.
C bond. The acid sites contained within the titanosilicate catalysed the reaction of 3,4-ETHF with water or alcohols to give solvolysis products. The oxidation occurred also on allylic positions to give the corresponding products which underwent successive oxidation by H2O2 and/or solvolysis by protic solvents. A product resulting from the direct addition reaction of alcohol on the allylic positions of 2,5-DHF was also observed. To evaluate the catalytic results simply among the catalysts and solvents, the products have been sorted into three groups, i.e. 3,4-ETHF, allylic reaction products and solvolysis products of 3,4-ETHF.
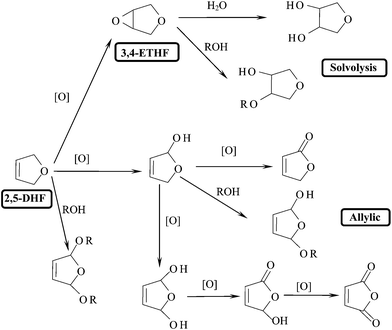 | ||
| Fig. 2 Reaction paths in the epoxidation of 2,5-DHF (R = Me, Et etc.). | ||
Table 1 compares the results from the catalytic epoxidation of 2,5-DHF in various solvents. The same reaction conditions other than the solvent were adopted for Ti-MWW and TS-1. The characters of the solvents had a great influence not only on the intrinsic activity of Ti species but also on the product distribution. Both Ti-MWW and TS-1 showed a clear but different solvent effect. The most favorable solvents for Ti-MWW were water and aprotic MeCN in which high conversion of 2,5-DHF (>95%), 3,4-ETHF selectivity (>99%) and efficiency for H2O2 utilization (>97%) were obtained (Table 1, no. 1 and 2). In protic solvents MeOH and EtOH, the catalytic activity of Ti-MWW retarded greatly (no. 3 and 4). Moreover, the selectivity to 3,4-ETHF was lowered greatly due to simultaneous allylic oxidation and solvolysis of 3,4-ETHF particularly in MeOH. The result that Ti-MWW favored MeCN with weak basicity in the present oxidation of 2,5-DHF is similar to the solvent effect previously observed on the epoxidation of simple alkenes, allyl alcohol and diallyl ether,15,16 which has been associated with the unique hydrophilic/hydrophobic nature of the Ti-MWW framework.
| Prod. sel. (mol%) | H2O2 (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | Cat. | Solvent | Conv. (mol%) | Oxide | Allylb | Othersc | Conv. | Eff.d |
| a Reaction conditions: cat., 0.07 g; 2,5-DHF, 5 mmol; H2O2 (30 wt%), 5 mmol; solvent, 5 mL; temp., 333 K; time, 2 h. b Allylic reaction products. c Solvolysis products of 3,4-ETHF. d H2O2 efficiency was calculated by relating all the oxidation products to the amount of H2O2 converted. The consumption of more H2O2 for the products formed involving second and third oxidations has been taken into account. e The molar ratio of 2,5-DHF to m-CPBA was 1 : 1.9, and the procedures followed the literature.8 | ||||||||
| 1 | Ti-MWW | MeCN | 97.2 | 99.8 | 0.2 | 0.0 | 99.8 | 98 |
| 2 | Ti-MWW | Water | 95.6 | 99.7 | 0.3 | 0.0 | 98.1 | 97 |
| 3 | Ti-MWW | MeOH | 60.1 | 62.3 | 18.3 | 19.4 | 69.4 | 80 |
| 4 | Ti-MWW | EtOH | 55.2 | 83.4 | 16.6 | 0.0 | 64.3 | 71 |
| 5 | TS-1 | MeCN | 62.7 | 80.8 | 19.2 | 0.0 | 94.0 | 60 |
| 6 | TS-1 | Water | 58.0 | 77.4 | 1.0 | 21.6 | 94.4 | 57 |
| 7 | TS-1 | MeOH | 71.3 | 74.0 | 14.8 | 11.2 | 99.3 | 72 |
| 8 | m-CPBAe | CH2Cl2 | 69.0 | 97.4 | 2.6 | 0.0 | ||
In comparison to Ti-MWW, TS-1 showed a lower catalytic activity, product selectivity and H2O2 efficiency whichever solvents were used. MeOH was obviously the most effective for TS-1 among the solvents examined from the viewpoint of 2,5-DHF conversion (no. 5–7). Nevertheless, MeOH promoted the solvolysis of 3,4-ETHF to a relatively high level. MeCN suppressed the solvolysis of 3,4-ETHF effectively but allowed the allylic reactions. On the other hand, the water solvent exhibited a reversal reaction phenomenon, making the selectivity to 3,4-ETHF also low. It should be noted that all these results obtained with TS-1 are very similar to those reported on a TS-1 monolith catalyst.14
The reason for the distinct solvent effects between Ti-MWW and TS-1 would lie in the different hydrophilic/hydrophobic nature of their frameworks. Ti-MWW prepared from a layered precursor contains many defect sites such as silanol groups as a result of incomplete dehydroxylation between the layers,15 while TS-1 with a highly crystalline structure has fewer silanol groups and is characteristic of higher hydrophobicity. Thus, TS-1 preferentially adsorbs MeOH and H2O2 molecules into hydrophobic channels where they interact with Ti active sites to form a five-membered ring Ti hydroperoxo species.12 These intermediate species then catalyze actively the oxidation of alkenes. In contrast, in the case of Ti-MWW, MeOH molecules and H2O2 presumably prefer to adsorb on its surface silanol groups rather than on the Ti sites, which leads to lower activity. Nevertheless, the aprotic solvent MeCN with weak basicity would neutralize partially the surface acidity. This then makes H2O2 and H2O avoid being adsorbed on the surface but diffuse easily into the channels to reach the Ti sites to accelerate the oxidation.
The above results prove that Ti-MWW is obviously superior to TS-1 in catalytic activity, epoxide selectivity, and H2O2 efficiency when choosing MeCN or water as a solvent. In the case of TS-1, the medium pores of 10-MR are assumed to produce more steric hindrance to 2,5-DHF with a relative bulky molecular size, which then lowers the catalytic activity. The higher intrinsic activity of Ti active species of Ti-MWW should be due to its more open pore system such as 12-MR side cups and supercages which serve as open reaction spaces and are favorable for inside channel diffusion and access to Ti active sites of bulky molecules of cyclic alkenes.
For control experiment, the homogeneous oxidation of 2,5-DHF with m-CPBA has been carried out following the same procedures reported in the literature.8 The stoichiometric reaction gave a conversion for 2,5-DHF and a selectivity to 3,4-ETHF of 69.0 and 97.4%, respectively (Table 1, no. 8), which obviously is much lower than those obtained in the Ti-MWW–H2O2 system.
Our research interest was attracted by the fact that the water solvent shows comparably high efficiency to MeCN in the epoxidation of 2,5-DHF over Ti-MWW catalyst since this would lead to a more environmentally benign process for the synthesis of 3,4-ETHF. Fig. 3 shows the time course for the 2,5-DHF epoxidation in the presence of water. The 2,5-DHF conversion increased rapidly with the reaction time, reached 85% at 15 min and finally 99% at 180 min. The selectivity for 3,4-ETHF and the H2O2 efficiency were always over 99% and 95%, respectively.
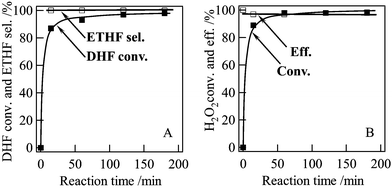 | ||
| Fig. 3 Changes of 2,5-DHF conversion and 3,4-ETHF selectivity (A), and H2O2 conversion and efficiency (B) with the reaction time. Reaction conditions: Ti-MWW, 0.07 g; 2,5-DHF, 5 mmol; H2O2 (30 wt%), 5 mmol; water, 5 mL; temp., 333 K. | ||
The recycling of the Ti-MWW catalyst in the epoxidation of 2,5-DHF has been investigated (Fig. 4). After the used catalyst was gathered and regenerated by washing with acetone and subsequent drying at 393 K, it was reused in the reaction under the same conditions. After being reused three times, Ti-MWW showed a conversion of 2,5-DHF of 87% which was slightly lower than that of the fresh catalyst (97%). However, the conversion was totally restored after burning off the organic species of high boiling points deposited inside the zeolite pores by a further calcination at 773 K in air. During the examination of the catalyst recycling, a high selectivity for 3,4-ETHF was always maintained. The used Ti-MWW showed nearly the same XRD and UV-visible spectra as those of the fresh one, indicating an unchanged nature of Ti active species and crystalline structure. These results prove the stability and reusability of Ti-MWW for the present reaction.
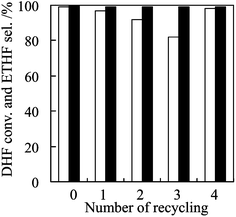 | ||
| Fig. 4 The 2,5-DHF conversion (blank) and 3,4-ETHF (black) when Ti-MWW was reused in the epoxidation of 2,5-DHF. Reaction conditions: cat., 0.07 g; 2,5-DHF, 5 mmol; H2O2 (30 wt%), 5 mmol; MeCN, 5 mL; temp., 333 K; time, 2 h. Regeneration: used catalyst was washed with acetone and dried at 393 K up to the third recycling, and was further calcined at 773 K in air for the fourth recycling. | ||
Conclusions
Ti-MWW with open reaction spaces and suitable hydrophilic/hydrophobic characters is a superior catalyst for the selective oxidation of 2,5-DHF with H2O2 to 3,4-ETHF. Ti-MWW shows extremely high efficiency and reusability in the presence of MeCN or water solvent. Especially, by using water as a solvent, the Ti-MWW–H2O2 catalytic system provides an effective, economic and environmentally friendly methodology for the synthesis of 3,4-ETHF. This would solve the problems of corrosion, toxicity and waste formation that the conventional methods are encountering.Acknowledgements
P.W. thanks the Program for New Century Excellent Talents in University (NCET). Financial support by the National Natural Science Foundation of China (Grants No. 20473027 and 20233030), 973 projects (2003CB615801) and Science and Technology Commission of Shanghai Municipality (05DJ1402, 05JC14069, 03DJ14005) is appreciated.References
- S. Imura and M. Ohtsuru, Jpn. Pat., 52 147 699, 1977 Search PubMed.
- D. M. Hodgson, M. A. H. Stent and F. X. Wilson, Org. Lett., 2001, 3, 3401 CrossRef CAS.
- D. M. Hodgson, M. A. H. Stent, B. Stefane and F. X. Wilson, Org. Biomol. Chem., 2003, 1, 1139 RSC.
- G. F. Lai, Synth. Commun., 2004, 34, 1981 CrossRef CAS.
- S. E. Schaus, J. F. Larrow and E. N. Jacobsen, J. Org. Chem., 1997, 62, 4197 CrossRef CAS; L. E. Martinez, J. L. Leighton, D. H. Carsten and E. N. Jacobsen, J. Am. Chem. Soc., 1995, 117, 5897 CrossRef CAS.
- S. E. Zook, J. K. Busse and B. C. Borer, Tetrahedron Lett., 2000, 41, 7017 CrossRef CAS.
- R. W. Marquis, Y. Ru, J. Zeng, R. E. L. Trout, S. M. LoCastro, A. D. Gribble, J. Witherington, A. E. Fenwick, B. Garnier, T. Tomaszek, D. Tew, M. E. Hemling, C. J. Quinn, W. W. Smith, B. G. Zhao, M. S. McQueney, C. A. Janson, K. D'Alessio and D. F. Veber, J. Med. Chem., 2001, 44, 725 CrossRef CAS.
- W. K. Anderson and R. H. Dewey, J. Med. Chem., 1977, 20, 306 CrossRef CAS; P. L. Barili, G. Berti and E. Mastrorilli, Tetrahedron, 1993, 49, 6263 CrossRef CAS; K. Ogasawara and O. Yamada, Jpn. Pat., 09 110 849, 1997 Search PubMed; B. Schmidt and H. Wildemann, Eur. J. Org. Chem., 2000, 18, 3145 Search PubMed.
- E. G. E. Hawkins, J. Chem. Soc., 1959, 248 RSC.
- W. Reppe, Justus Liebigs Ann. Chem., 1955, 596, 99.
- N. W. Boaz, US Pat., 6 162 924, 2000 Search PubMed.
- G. Bellussi and M. S. Rigguto, Stud. Surf. Sci. Catal., 2001, 137, 911 CAS.
- A. Corma, P. A. Esteve, A. Martinez and S. Valencia, J. Catal., 1995, 152, 18 CrossRef CAS.
- W. J. Kim, T. J. Kim, W. S. Ahn, Y. J. Lee and K. B. Yoon, Catal. Lett., 2003, 91, 123 CrossRef CAS.
- P. Wu, T. Tatsumi, T. Komatsu and T. Yashima, J. Phys. Chem. B, 2001, 105, 2897 CrossRef CAS; P. Wu, T. Tatsumi, T. Komatsu and T. Yashima, J. Catal., 2001, 202, 245 CrossRef CAS.
- P. Wu and T. Tatsumi, J. Catal., 2003, 214, 317 CrossRef CAS; P. Wu, Y. Liu, M. He and T. Tatsumi, J. Catal., 2004, 228, 183 CrossRef CAS.
- P. Wu and T. Tatsumi, Chem. Commun., 2001, 897 RSC.
- T. Taramasso, G. Perego and B. Notari, US Pat., 441 050, 1983 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
