Assessing the factors responsible for ionic liquid toxicity to aquatic organisms via quantitative structure–property relationship modeling
David J. Coulinga, Randall J. Bernotb, Kathryn M. Dochertyb, JaNeille K. Dixona and Edward J. Maginn*a
aDepartment of Chemical and Biomolecular Engineering, University of Notre Dame, Notre Dame, IN, USA. E-mail: ed@nd.edu; Fax: +1 574 631 5687; Tel: +1 574 631 5687
bDepartment of Biological Sciences, University of Notre Dame, Notre Dame, IN, USA. Tel: +1 574 631 6522
First published on 30th November 2005
Abstract
Using previously published toxicity data as well as a small set of heretofore-unpublished results, quantitative structure–property relationship models are developed to assess the factors that govern the toxicity of a range of different ionic liquids to two aquatic organisms (Vibrio fischeri and Daphnia magna). With at most four molecular descriptors, log10 EC50 and log10 LC50 data are reproduced with an R2 of 0.78–0.88. Besides the well-established link between toxicity and alkyl chain length on imidazolium, pyridinium and quaternary ammonium-based ionic liquids, the models predict that toxicity increases slightly with the number of nitrogen atoms in an aromatic cation ring. All other things being equal, toxicity is expected to show the trend with cation type of ammonium < pyridinium < imidazolium < triazolium < tetrazolium. In addition, toxicity is expected to decrease with ring methylation as well as with an increase in the number of negatively charged atoms on the cation. The anion plays a secondary role in toxicity for the compounds studied here, although the presence of positively charged atoms on the anion are predicted to slightly increase toxicity.
Introduction
Ionic liquids (ILs), defined as pure ionic compounds with melting points below 100 °C, have drawn a great deal of interest due to their unique properties. Such properties include excellent solvation ability for a wide range of compounds, high thermal stability and immeasurably low vapour pressure. This last property has sparked interest in the potential environmental benefits of these compounds over conventional volatile organic solvents.1 While ILs cannot volatilise and lead to air pollution, they have at least some miscibility with water.2,3 Many ILs are minimally retained by geologic adsorption in non-interlayer clay systems,4 suggesting that their release into the environment could lead to their unimpeded transport through subsurface groundwater. Thus, ILs may pose environmental risks to aquatic ecosystems, but the potential impact of ILs on aquatic organisms is largely unknown. Knowledge of IL toxicity to aquatic organisms is necessary before an accurate assessment of their true environmental influence can be made. For this reason, ILs based on the pyridinium, imidazolium and pyrrolidinium cation have been nominated to the United States National Toxicology Program (NTP) for toxicological testing, based upon their widespread interest as possible alternatives to organic solvents.5Several recent toxicity studies have documented IL effects on organisms. Li et al.6 showed that polymeric materials with pyridinium functionality can exhibit significant toxicity to bacteria. Pernak et al. observed that antimicrobial activity increased as the alkyl chain length increased on pyridinium, imidazolium, and quaternary ammonium salts.7–11 This trend was also observed with the marine bacterium Vibrio fischeri for alkylimidazolium cations paired with BF4−, PF6− and Cl− anions12 as well as for various alkylimidazolium and alkylpyridinium cations.13 A similar trend of increasing toxicity with increasing alkyl chain length was observed for mammalian cell cultures12 and higher organisms, including the soil nematode Caenorhabditis elegans14 and the freshwater snail Physa acuta15 and was consistent among different cation and anion combinations.16 This result is perhaps not surprising, given that cetylpyridinium chloride is a well-known anti-microbial agent.17 Other studies include the effects of ionic liquids on enzyme activity,18 survivorship and life history of the freshwater crustacean Daphnia magna,19 and behavior of Physa acuta.15
Varying the anion has a minimal effect on the toxicity of pyridinium and imidazolium salts, which suggests that toxicity is largely driven by the cation,7,12–14 although recent studies indicate that the anion can play a role in toxicity.16
Experimental studies have been helpful in establishing general guidelines for the selection of ILs with low potential for toxicity. However, relatively little is still known about the toxicity of these materials as a class, especially when compared to conventional organic solvents. The general lack of knowledge and uncertainty surrounding the environmental impact of ILs is a major impediment to the adoption of these compounds by industry. In addition to generating data for existing ILs, there is a pressing need to be able to understand and predict the toxicity of compounds that could be made into ILs. Since IL research and development efforts are in their infancy, only a small fraction of the compounds that could be made into ILs have actually been synthesized. By being able to predict the factors that are responsible for increased or decreased toxicity, it should be possible to direct synthesis efforts along paths that will result in the development of IL classes that are safe both from an air and water pollution standpoint.
In this paper, we report experimental data on the aquatic toxicity of a range of ILs on two aquatic organisms. First, microbial toxicity was estimated using a standard Microtox Acute Toxicity test system to determine the effective concentration at 50% (or EC50) to the marine bacterium Vibrio fischeri. Second, toxicity to a freshwater crustacean (Daphina magna) was determined using 48 h acute toxicity bioassays. These data were then used to build a predictive toxicity model based upon quantitative structure–property relationship modeling (QSPR) methods. The model was used to elucidate the chemical and structural factors that govern the toxicity of these compounds to V. fischeri and D. magna. This information can be used as the basis for “design rules” in synthesizing ILs with minimal toxicity to aquatic organsims.
Background
The Microtox Acute Toxicity test is an ASTM standard test method20 that provides a rapid means of determining the acute toxicity of aqueous compounds by measuring decreases in light output from the luminescent bacterium Vibrio fischeri NRRL B-11177. A decrease in luminescence is linked to a decrease in respiration, and serves as an indirect measure of the toxicity of the test compound. The Microtox Acute Toxicity test is used widely for determining the toxicity of single compounds, for monitoring industrial effluents in environmental water quality surveys, and in sediment contamination studies.21 An extensive database of individual chemicals tested using the Microtox method is readily available.21Daphnia magna are freshwater crustaceans often used as standard toxicity test organisms because they are easily cultured in the laboratory and are sensitive to a variety of pollutants.22Daphnia are used as model organisms in standard toxicity bioassays used by regulatory agencies (e.g., United States Environmental Protection Agency) to compare the relative toxicities of different compounds. In natural freshwater ecosystems, Daphnia are an important link between microbial and higher trophic levels,23 and have been the focus of hundreds of physiological, evolutionary, and ecological studies.24
Quantitative structure–property relationship (QSPR) modeling is based on the idea that chemical structure completely determines the physical properties of a given compound. Given a reasonable set of experimental data for the property of interest (a “training set”), QSPR relates these properties to the chemical structure of the compounds. QSPR modeling requires that the “structure” of the compounds be quantified through various molecular-based descriptors. Numerous descriptors have been proposed to capture the many different aspects of structure.25,26 In past studies, we have successfully applied the QSPR method to predict the melting point of IL compounds27 as well as the infinite dilution activity coefficients of various organic compounds in three different ILs.28 In this work, we show that the QSPR method can be used to effectively correlate and predict the toxicity of ionic liquids.
Experimental
Synthesis of ionic liquids
The materials used in this study, including source, grade, and purification method (if any), are as follows: sodium bromide (Aldrich ≥99.0%, used as received), 1-chlorobutane (Aldrich 99.5%, redistilled), 1-bromohexane (Aldrich ≥98.0%, redistilled), sodium dicyanoamide (Aldrich 96%, used as received), pyridine (Aldrich ≥99.0%, redistilled over KOH), 3,5-dimethylpyridine (Aldrich ≥99.0%, redistilled over KOH), 4-dimethylaminopyridine (Reilly 99%, used as received), 1-methylimidazole (Aldrich 99%, redistilled over KOH), and lithium bis(trifluoromethanesulfonyl)imide (3 M 97%, used as received).All the pyridinium and imidazolium ILs except 1-n-butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide ([bmim][Tf2N]) were synthesized in our laboratory using standard procedures.29–31 A typical synthesis involved mixing equal molar amounts of a nitrogen base and alkyl halide in a flask then stirring the mixture overnight. Acetonitrile was frequently used as a solvent for reactions, but some of the reactions were done neat. The solvent was removed from the IL under vacuum. If the IL was a solid at room temperature, it was recrystallised from acetonitrile/ethylacetate. ILs that are liquids at room temperature were dissolved in methylene chloride and stirred over activated charcoal to remove any coloured impurities. The solutions were then filtered and the solvent removed in vacuo. The dicyanoamide ILs were made by reacting freshly prepared silver dicyanoamide and the appropriate pyridinium or imidazolium halide. The silver halide was then filtered and the IL dried on a vacuum line. The identity of all ILs was confirmed by 1H and 13C NMR. Impurity levels of halide ions in the ILs synthesized in-house were measured using an Oakton Ion 510 meter with Cole-Parmer Ion Specific Probes. All values were less than 10 ppm for halogen. NMR results indicate that amine impurities were below the detection limit, but the NMR detection limit is roughly 5 wt%. Based on previous experience, the actual value is likely significantly less than this.
[bmim][Tf2N] was provided by Covalent Associates, with purity ≥99%, and was used as received. The quaternary ammonium ILs were purchased from Aldrich with the following purities: tetramethylammonium bromide (≥99%), tetraethylammonium bromide (≥99%), tetrabutylammonium bromide (≥99%), hexyltriethylammonium bromide (99%), and choline chloride (≥99%). Choline bis(trifluoromethanesulfonyl)imide was synthesized in-house by a metathesis reaction between choline chloride and lithium bis(trifluoromethanesulfonyl)imide. Purity was determined as described above. Trihexyl(tetradecyl)phosphonium bromide and tributylethylphosphonium diethyl phosphate were obtained from CYTEC and used as received.
Microtox experiments—methodology
Pure ILs were diluted to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 in sterile distilled water. Three replicated EC50 (effective IL concentration at which V. fischeri respiration was reduced by 50%) values at 15 min were determined for each IL. EC50 values were determined using the Microtox M500 Rapid Toxicity Testing System (Azur Environmental, Strategic Diagnostics, Newark, DE) and the ASTM standard toxicity protocol.20
000 mg L−1 in sterile distilled water. Three replicated EC50 (effective IL concentration at which V. fischeri respiration was reduced by 50%) values at 15 min were determined for each IL. EC50 values were determined using the Microtox M500 Rapid Toxicity Testing System (Azur Environmental, Strategic Diagnostics, Newark, DE) and the ASTM standard toxicity protocol.20Daphnia magna bioassays—methodology
Daphnia magna IL exposure bioassays were conducted as 48 h static acute tests according to standard procedures.32 Each IL exposure used D. magna neonates (age, <24 h) born from parthenogenic females grown in batch cultures. Eight neonates were placed in each of 30 glass beakers (250 mL), with five replicates for each of six treatment concentrations (control plus five IL concentrations). The number of living and dead neonates was noted at 24 and 48 h after the initiation of each trial. Neonates observed as motionless and without a discernable heartbeat were considered to be dead. Each trial was conducted at 20 ± 1 °C in the laboratory with a 16 ∶ 8 h light ∶ dark photoperiod. Ionic liquid test concentrations were based on preliminary range-finding tests of acute toxicity using concentrations from 0.001 to 100 mmol L−1. The median lethal concentration (LC50) and associated 95% confidence intervals were obtained by fitting dose–response curves to a normal model using the maximum likelihood method.33 The LC50 is the concentration of a chemical that causes death to 50% of the test organisms. Statistical analyses were performed using SAS Version 8.02 statistical software (SAS, Cary, NC, USA).Modeling details
The compounds used in the initial training set for Vibrio fischeri toxicity are listed in Table 1 with a superscript a, while those for Daphnia magna are listed with a superscript a in Table 2. The toxicity of compounds whose log EC50 values were greater than 2.0 could not be reliably determined; for the model, these compounds were assigned a log EC50 value between 2.16 and 2.19. A second QSPR equation was developed for V. fischeri using all the compounds in Table 1. Each compound was registered into the QSPR software (Accelrys, San Diego, CA, USA) by entering the cation and anion as separate entities. The geometries of both the cation and the anion were optimized separately and partial charges were calculated using MOPAC with the AM1 semi-empirical method.34–36| Compound | CAS # | log EC50 expt. | Ref. | log EC50 pred. |
|---|---|---|---|---|
| a Indicates members of initial training set; pw means present work. Predictions obtained with eqn (14). | ||||
| 1-n-Butylpyridinium chloridea | 1124-64-7 | 0.41 ± 0.08 | 13 | 0.13 |
| 1-n-Butylpyridinium dicyanoamidea | 827033-71-6 | 0.31 ± 0.10 | 13 | 0.13 |
| 1-n-Butyl-3-methylpyridinium dicyanoamidea | 712355-12-9 | −0.34 ± 0.05 | 13 | −0.43 |
| 1-n-Butyl-3,5-dimethylpyridinium dicyanoamidea | — | −0.62 ± 0.18 | 13 | −0.12 |
| 1-n-Butylpyridinium bromidea | 874-80-6 | 0.40 ± 0.01 | 13 | 0.13 |
| 1-n-Butyl-3-methylpyridinium bromidea | 26576-85-2 | −0.25 ± 0.13 | 13 | −0.43 |
| 1-n-Butyl-3,5-dimethylpyridinium bromidea | 26576-98-7 | −0.31 ± 0.09 | 13 | −0.12 |
| 1-n-Hexyl-3-methylpyridinium bromidea | 67021-56-1 | −0.94 ± 0.16 | 13 | −0.81 |
| 1-n-Octyl-3-methylpyridinium bromidea | — | −2.21 ± 0.05 | 13 | −2.56 |
| 1-n-Butyl-4-dimethylaminopyridinium bromide | 395677-61-9 | −0.68 ± 0.05 | pw | −0.89 |
| 1-n-Butyl-3-methylimidazolium dicyanoamidea | 44824-52-1 | 0.67 ± 0.10 | 13 | 0.47 |
| 1-n-Butyl-3-methylimidazolium chloridea | 79917-90-1 | 0.71 ± 0.14 | 13 | 0.47 |
| 1-n-Butyl-3-methylimidazolium bromidea | 85100-77-2 | 1.01 ± 0.05 | 13 | 0.47 |
| 1-n-Butyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imidea | 174899-83-3 | 0.39 ± 0.08 | pw | −0.25 |
| 1-n-Hexyl-3-methylimidazolium bromidea | 85100-78-3 | −1.58 ± 0.12 | 13 | −0.46 |
| 1-n-Octyl-3-methylimidazolium bromidea | 61545-99-1 | −2.37 ± 0.07 | 13 | −1.05 |
| Tetramethylammonium bromide | 64-20-0 | >2.0 | pw | 2.37 |
| Tetraethylammonium bromide | 71-91-0 | >2.0 | pw | 1.23 |
| Tetrabutylammonium bromidea | 1643-19-2 | 0.27 ± 0.07 | pw | −0.08 |
| Hexyltriethylammonium bromide | 13028-71-2 | −0.54 ± 0.16 | pw | −0.62 |
| Tetrabutylphosphonium bromide | 3115-68-2 | −0.29 ± 0.00 | pw | 0.76 |
| Tributylethylphosphonium diethylphosphate | 20445-94-7 | 0.07 ± 0.09 | pw | 0.21 |
| Trihexyl(tetradecyl)phosphonium bromide | 654057-97-3 | 0.41 ± 0.02 | pw | −0.21 |
| Choline chloride | 67-48-1 | >2.0 | pw | 2.29 |
| Choline bis(trifluoromethanesulfonyl)imide | 475998-66-4 | 1.15 ± 0.03 | pw | 1.57 |
| Compound | CAS # | log LC50 expt. | UCL | LCL | Ref. | log LC50 pred. |
|---|---|---|---|---|---|---|
| a Indicates members of training set; pw means present work. Predictions obtained with eqn (15). | ||||||
| 1-n-Octyl-3-methylpyridinium bromidea | — | −2.60 | 0.07 | 0.07 | pw | −3.22 |
| 1-n-Hexyl-3-methylpyridinium bromidea | 67021-56-1 | −2.41 | 0.59 | 0.43 | pw | −2.06 |
| 1-n-Butyl-3-methylpyridinium bromidea | 26576-85-2 | −1.24 | 0.11 | 0.08 | pw | −0.92 |
| 1-n-Octyl-3-methylimidazolium bromidea | 61545-99-1 | −4.33 | 0.33 | 0.25 | pw | −3.65 |
| 1-n-Hexyl-3-methylimidazolium bromidea | 85100-78-3 | −2.22 | 0.48 | 0.43 | pw | −2.52 |
| 1-n-Butyl-3,5-dimethylpyridinium bromidea | 26576-98-7 | −1.01 | 0.05 | 0.06 | pw | −1.07 |
| 1-n-Hexyl-4-piperidinopyridinium bromide | — | −3.66 | 0.38 | 0.23 | pw | −5.59 |
| 1-n-Hexyl-4-dimethylaminopyridinium bromide | — | −3.28 | 0.40 | 0.23 | pw | −2.98 |
| 1-n-Hexyl-3-methyl-4-dimethylaminopyridinium bromide | — | −2.79 | 0.17 | 0.15 | pw | −2.65 |
| 1-n-Hexylpyridinium bromide | 74440-81-6 | −1.93 | 0.25 | 0.15 | pw | −2.13 |
| 1-n-Hexyl-2,3-dimethylimidazolium bromide | — | −2.19 | 0.48 | 0.27 | pw | −2.41 |
| 1-n-Butyl-3-methylimidazolium chloridea | 79917-90-1 | −1.07 | 0.07 | 0.06 | 19 | −1.34 |
| 1-n-Butyl-3-methylimidazolium bromidea | 85100-77-2 | −1.43 | 0.07 | 0.07 | 19 | −1.34 |
| 1-n-Butyl-3-methylimidazolium tetrafluoroboratea | 174501-65-6 | −1.32 | 0.16 | 0.08 | 19 | −1.34 |
| 1-n-Butyl-3-methylimidazolium hexafluorophosphatea | 174501-64-5 | −1.15 | 0.14 | 0.08 | 19 | −1.34 |
| Tetrabutylammonium bromidea | 1643-19-2 | −1.53 | 0.16 | 0.11 | pw | −1.33 |
| Tetrabutylphosphonium bromidea | 3115-68-2 | −2.05 | 0.37 | 0.36 | pw | −2.25 |
The descriptor pool included electronic, spatial, structural, thermodynamic, and topological descriptors. Descriptors were calculated for both the cation and anion separately. The same symbols were used for each descriptor, but those for the anion were designated with an asterisk. Values of the descriptors varied widely in magnitude. To enable the relative importance of a given descriptor to be easily determined from the magnitude of its coefficient, each descriptor set was scaled to the range [0,1] according to the following equation
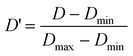 | (1) |
Correlations were generated using the scaled descriptors and a genetic function approximation (GFA) statistical method. Since the GFA generates many different correlations, correlations with higher R2 values were more favored. In addition, care was taken to ensure that the descriptors selected by the GFA were independent by using a correlation matrix and discarding descriptors that were highly correlated with other descriptors (i.e. had R2 values > 0.8). When such close correlations were observed, the descriptor deemed to have the most “physical” significance was retained.
The initial equation for the V. fischeri correlations was generated using sixteen training set compounds (Table 1). This equation was then applied to predict the toxicity of nine additional (“predicted”) compounds. A second equation was generated using all the V. fischeri data. A similar procedure was followed to generate the toxicity equation for D. magna. An initial equation was generated using twelve training set compounds (Table 2). This equation was used to predict the toxicity of five additional compounds. Since the initial equation accurately predicted the toxicity of these five additional compounds, only one equation was generated for D. magna.
Descriptor definitions
The following descriptor types and classes were used to generate toxicity correlations.E-state indices
The electrotopological state (E-state) accounts for both electrostatic and steric influences of individual atoms in a molecule. To determine an E-state index, an intrinsic value I is assigned based on the valences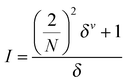 | (2) |
To this intrinsic value I a perturbation term ΔIij is added, in which
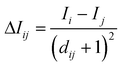 | (3) |
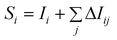 | (4) |
E-state sum descriptors were formed by summing E-state indices for the atoms of a given type. For example, [SaasN] is the sum of the E-state indices for all nitrogen atoms (N) with two aromatic (a) bonds and one single (s) bond.
Surface area descriptors
Solvent-accessible surface area (SASA) was calculated using a sphere of radius 0.15 nm to approximate the contact surface formed when a water molecule interacts with the IL.[PPSA2] is the total charge weighted positive surface area, and is computed by multiplying the partial positive solvent-accessible surface area by the total positive charge Q+:
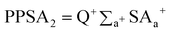 | (5) |
Likewise, [PNSA2] is the partial negative solvent-accessible surface area multiplied by the total negative charge Q−:
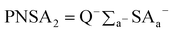 | (6) |
[DPSA2] is the total charge weighted partial positive solvent-accessible surface area minus the total charge weighted partial negative solvent-accessible surface area, given by
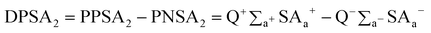 | (7) |
[PPSA3] is the atomic charge weighted positive surface area, given by
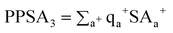 | (8) |
where qa+ is the positive partial charge on atom a. Likewise, [PNSA3] is the atomic charge weighted negative surface area, given by
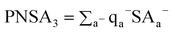 | (9) |
[DPSA3] is simply the difference between the atomic charge weighted positive and negative solvent-accessible surface areas:
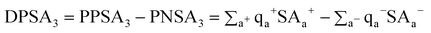 | (10) |
[RPCG] is the partial charge of the most positive atom divided by the total positive charge:
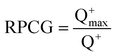 | (11) |
[RNCG] is the partial charge of the most negative atom divided by the total negative charge:
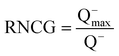 | (12) |
Shadow parameter
[Shadow-ν] is the ratio of the longest to the shortest side of the rectangle that envelops a molecular structure and at the same time maximizes the aspect ratio, assuming van der Waals radii for atoms and standard bond lengths.Results and discussion
Toxicity for Vibrio fischeri—experimental results
The trend of increasing toxicity with increasing alkyl chain length of a substituted alkyl chain was observed in both pyridinium- and imidazolium-based ILs.13 Ionic liquid toxicity to V. fischeri has been linked with the cation structure and branching,8,12,13 and was reflected in the EC50 values of the starting compounds for IL synthesis (Table 3). Salts used for anion substitution, such as sodium bromide and sodium dicyanoamide, were less toxic to V. fischeri than the compounds used for synthesis of the cation. The addition of a butyl chain to the C-1 carbon of the pyridinium or imidazolium cation slightly increased IL toxicity to V. fischeri relative to starting compounds such as 3-methyl pyridine and 1-methylimidazole. The addition of a hexyl or octyl chain further increased the toxicity of both pyridinium and imidazolium cation compounds from their respective initial starting compounds.| Compound | CAS # | log10 EC50 | Ref. |
|---|---|---|---|
| Methanol | 67-56-1 | 3.50 | 21 |
| Acetonitrile | 75-05-8 | 2.77 | 21 |
| Acetone | 67-64-1 | 2.52 | 21 |
| Benzene | 71-43-2 | 0.14 | 21 |
| Phenol | 108-95-2 | −0.49 | 21 |
| Methyl tertiary butyl ether | 1634-04-4 | −0.89 | 21 |
| Sodium bromide | 7647-15-6 | 2.29 | pw |
| 1-Chlorobutane | 109-69-3 | 1.92 | pw |
| 1-Bromobutane | 111-25-1 | 0.95 | pw |
| Sodium dicyanoamide | 1934-75-4 | 1.72 | pw |
| Pyridine | 110-86-1 | 0.87 | pw |
| 3-Methylpyridine | 108-99-6 | 0.07 | 13 |
| 3,5-Dimethylpyridine | 591-22-0 | −0.36 | pw |
| 4-Dimethylaminopyridine | 1122-58-3 | −0.41 | pw |
| 1-Methylimidazole | 616-47-7 | 1.17 | 13 |
A similar trend of increased toxicity with increasing alkyl chain length was observed for a series of quaternary ammonium ILs (Table 1). These results reflect previous experimental and theoretical findings that quaternary ammonium chloride antimicrobial effects are related to the lipophilicity of the cation.9 The quaternary ammonium compounds examined in this study were also less toxic to V. fischeri than the pyridinium and imidazolium compounds tested. Choline chloride and choline dicyanoamide were significantly less toxic than pyridinium and imidazolium ILs with the same anions.
Many of the ILs tested, excluding those substituted with long alkyl chains, were less toxic to V. fischeri than some traditional industrial solvents such as methyl tertiary butyl ether, phenol and benzene (Table 3). However, our results also indicate that many of the imidazolium and pyridinium compounds were more toxic to V. fischeri than common high-volume solvents such as acetonitrile, acetone and methanol. Some exceptions include low alkyl chain length quaternary ammonium ILs and those ILs containing choline as the cation, which were relatively nontoxic to V. fischeri. The data suggest that choline or quaternary ammonium solvents may be more environmentally friendly alternatives than both aromatic ILs and some traditional industrial solvents. We note, however, that single species acute toxicity is not the only measure of the environmental impact of a chemical. Other factors not investigated here, including biodegradation and bioaccumulation, are also important and need to be studied before firm conclusions can be drawn.
Toxicity for Vibrio fischeri—QSPR results
The following 4-parameter equation was obtained for EC50 values of V. fischeri using the training set data| log10 EC50/mmol L−1 = 6.82837 − 4.80633[SaaCH] − 3.2043[SaasN] − 6.55084[DPSA2] − 1.38266[SaasC] | (13) |
There are four descriptors in the correlation: [DPSA2], [SaasN], [SaaCH], and [SaasC]. [SaasN], [SaaCH], and [SaasC] are all sums of E-state indices for atoms of a given type. [SaasN] is the sum of the E-state indices for all nitrogen atoms (N) with two aromatic (a) bonds and one single (s) bond. Similarly, [SaaCH] is the sum of the E-state indices for all carbon atoms with two aromatic bonds and one hydrogen (CH) bond, and [SaasC] is the E-state sum for all carbon atoms with two aromatic bonds and one single bond.
Each descriptor is preceded by a negative coefficient, indicating that each structural aspect encoded in the descriptors leads to higher toxicity at higher incidence. [DPSA2] is highly correlated with the length of the alkyl chain on the aromatic cations, since the partial positive surface area increases as the length of the alkyl chain increases, while the partial negative surface area remains essentially constant with alkyl chain length. Therefore, this descriptor is consistent with the experimental observation that aromatic cations with longer alkyl chains were more toxic (i.e. 1-n-hexyl-3-methylimidazolium bromide was more toxic than 1-n-butyl-3-methylimidazolium bromide). Since [SaasN] describes compounds with nitrogen atoms having two aromatic (a) bonds and one single (s) bond, it indicates that toxicity increased as the number of nitrogen atoms of this form increased. Imidazolium cations, with two nitrogen atoms of this form, are predicted by this equation to be more toxic than pyridinium cations, which only have one nitrogen atom of this form. For example, 1-n-hexyl-3-methylpyridinium bromide was less toxic than 1-n-hexyl-3-methylimidazolium bromide. Following this reasoning, triazolium-based ionic liquids should be more toxic than those with imidazolium cations, although there are no experimental toxicity data that we know of for triazolium-based ionic liquids. In addition, the correlation predicts that quaternary ammonium cations are less toxic than those with cations consisting of nitrogen-bearing rings.
[SaaCH] and [SaasC] both describe carbon atoms with two aromatic bonds. Because the coefficient in front of [SaaCH] is larger in magnitude than that of [SaasC], carbon atoms with two aromatic bonds and one bonded hydrogen atom are predicted to be more toxic than carbon atoms with two aromatic bonds and a single bond to an alkyl group. This suggests that adding methyl groups to the aromatic rings may help decrease the toxicity. However, one must be careful drawing too many qualitative conclusions with such correlations, since the partial positive surface area would also tend to increase with an additional methyl group. Notably, all descriptors in Equation 25 are cation descriptors. This is in accord with the experimental finding that IL toxicity to V. fischeri is mainly cation-dependent.
Eqn (13) fits the training set data very well (R2 = 0.887) but the toxicity of the additional (“predicted”) compounds not used in the correlation is not modeled with similar high accuracy (Fig. 1). These “predicted” compounds were not used in the training set because their experimental values were not known at the start of the modeling work. The inadequacy of eqn (13) for these compounds is not surprising, since the majority of the training set ILs have aromatic cations (imidazolium or pyridinium) while the majority of the new “predicted” compounds have ammonium or phosphonium cations. The correlation only yields accurate toxicity predictions for compounds of the same class as the training set, as indicated by the fact that the “predicted” compound most accurately modeled with eqn (13) was 1-n-butyl-4-dimethylaminopyridinium bromide, which contains an aromatic pyridinium cation. Interestingly, however, this particular predicted compound also contains an amino group, unlike any of the training set compounds.
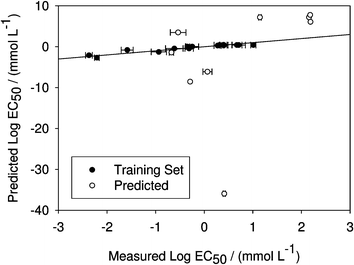 | ||
| Fig. 1 Predicted versus measured log10 EC50 values of different ionic liquids for Vibrio fischeri using eqn (13). While the training set data is reproduced well (R2 = 0.887), the model fails to capture the toxicity of predicted compounds. Note the scale for predicted EC50. | ||
Given the diversity of the compounds available from the experimental data, a new correlation was developed using all of the data
| log10 EC50/mmol L−1 = 0.885055 + 1.90609[RNCG] − 3.81771[Shadow-ν] − 1.13277[RPCG*] | (14) |
Eqn (14) fits the training set data very well (R2 = 0.782; Fig. 2) and contains additional information which can help shed light on important features responsible for toxicity. The three new descriptors are [RNCG], [Shadow-ν], and [RPCG*]. Although [Shadow-ν] is related to the size of the cation, it is more an indicator of whether the molecule is spherical or cylindrical in shape. The negative [Shadow-ν] coefficient indicates that IL toxicity to V. fischeri is more likely to be higher in long, asymmetric cations.
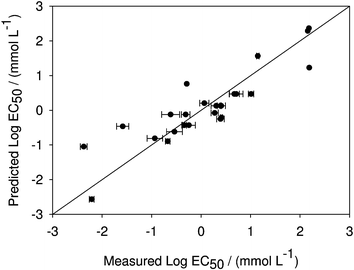 | ||
| Fig. 2 Predicted versus measured log10 EC50 of different ionic liquids for Vibrio fischeri using eqn (14). All the experimental data were used to generate this correlation, and an acceptable result is achieved for a diverse set of compounds (R2 = 0.782). | ||
[RNCG] is a charged partial surface area descriptor that increases with highly concentrated or localized negative charge. Because it is a cation descriptor, and because of its positive coefficient, eqn (14) indicates that IL toxicity should decrease with an increase in the localization of negatively charged atoms in the cation (such as the oxygen atom in [choline]). [RPCG*], while similar to [RNCG], is different in two key ways. First, it increases with highly localized positive charge, not negative charge. Second and more importantly, it is an anion descriptor. Unlike the [RNCG] descriptor, which indicated that a localization of negative charge in the cations tends to decrease the toxicity of the compound, the negative coefficient for [RPCG*] indicates that localiazation of positive charge on anions tends to increase the toxicity of the compound. Therefore, monatomic anions such as bromide and chloride are predicted to be less toxic than very large anions containing regions of positive charge, such as bis(trifluoromethanesulfonyl)imide.
Toxicity for Daphnia magna—experimental results
Ionic liquid toxicity to D. magna ranged from the highly toxic 1-n-octyl-3-methyl imidazolium bromide (LC50 = 0.00004 mmol L−1) to the less toxic 1-n-butyl-3,5-dimethyl pyridinium bromide (LC50 = 0.097 mmol L−1; Table 2). In general, ionic liquids with longer alkyl chain substituents had toxicities comparable to phenol, while those with shorter substituents (e.g., 1-n-butyl-3-methylimidazolium bromide) were more toxic to D. magna than benzene and methanol.19Toxicity for Daphnia magna—QSPR results
The following equation was developed using training set compounds to model ionic liquid toxicity to Daphnia magna| log10 LC50/mmol L−1 = 1.37806 − 3.62486[DPSA3] − 1.50205[SaasN] − 1.54858[SaaCH] | (15) |
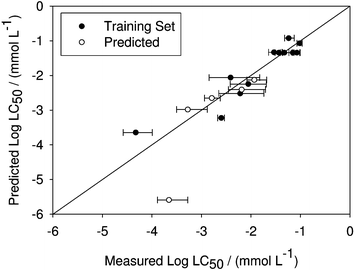 | ||
| Fig. 3 Predicted versus measured values of log10 LC50 of different ionic liquids for Daphnia magna. The model is based on eqn (15) using only the training set compounds (R2 = 0.862). | ||
Both E-state sums [SaaCH] and [SaasN] are found in the D. magna correlation as well. Because of their negative coefficients, the E-state sums indicate the same behaviors as the V. fischeri data. The negative [SaasN] coefficient indicates that imidazolium cations tend to be more toxic than pyridinium cations, which are more toxic than quaternary ammonium cations. The negative [SaaCH] coefficient indicates that methylating the aromatic carbon atoms may reduce the toxicity of the compounds. Noticeably absent from this correlation is [SaasC], suggesting that methylating the aromatic carbons may be more effective in reducing IL toxicity to D. magna than to V. fischeri.
Eqn (15) not only models the training set data well, it also can be used to predict the toxicity of compounds left out of the training set (R2 = 0.775; Fig. 3). This is contrary to the case with Vibrio fischeri, where the toxicity predictions for left out data were poor. While this may be due to intrinsic differences between the two species, a more likely explanation is simply that the compounds left out of the Daphnia magna training set are more similar to the training set compounds than was the case with Vibrio fischeri.
The only compound not used in the training set whose toxicity was not predicted within error is 1-n-hexyl-4-piperidinopyridinium bromide ([hmppy][Br]). This is reasonable, since the additional ring on the cation of [hmppy][Br] make it the least similar compound with regard to the training set compounds. Nevertheless, the correlation does correctly place it as the most toxic of all the left out compounds. Moreover, if the [hmppy][Br] data point is ignored, the fit of the remaining data points improves slightly over that of just the training set data, with an R2 = 0.875.
It is also worthwhile to note that the inference that methylating the aromatic carbons could be effective in reducing toxicity to D. magna is consistent with the data. 1-n-butylpyridinium bromide is more toxic than 1-n-butyl-3-methylpyridinium bromide, which is more toxic than 1-n-butyl-3,5-dimethylpyridinium bromide. This applies to the left out compounds set as well—1-n-hexyl-4-dimethylaminopyridinium bromide is more toxic than 1-n-hexyl-3-methyl-4-dimethylaminopyridinium bromide. Given the accuracy with which eqn (15) represented all the data, a separate correlation using all the experimental data was not generated.
Conclusions
Toxicity data for a range of different ILs on V. fischeri and D. magna have been presented. Using these data, correlative and predictive equations were generated using quantitative structure-property relationship modeling. The models performed well, with R2 values ranging from 0.78 to 0.88, using at most four molecular descriptors. In accordance with experimental trends, the models predict that increasing alkyl chain length increases the IL toxicity for both V. fischeri and D. magna, and that cation properties have a larger effect on toxicity than anion properties. Many ILs are similar to cationic surfactants,37 which are known to induce polar narcosis38 due to their ability to be incorporated into biological membranes.39 Therefore, longer alkyl chains may be incorporated into the polar headgroups of the phospholipid bilayer, which are the major structural components of membranes.40 Narcosis then results because membrane-bound proteins are disrupted by the toxicant. Many of the imidazolium and pyridinium compounds were more toxic to V. fischeri than common high-volume solvents such as acetonitrile, acetone and methanol. Some exceptions include low alkyl chain length quaternary ammonium ILs and those ILs containing choline as the cation, which were relatively nontoxic to V. fischeri. The data suggest that choline or quaternary ammonium solvents may be more environmentally friendly alternatives than both aromatic ILs and some traditional industrial solvents.Additional insight into other less obvious factors influencing toxicity was also obtained. For both organisms, toxicity was predicted to increase slightly with the number of nitrogen atoms having two aromatic bonds and one single bond. Thus, the model correctly indicates that ammonium cations are less toxic than pyridinium cations, which are slightly less toxic than imidazolium cations. If true, this would mean that triazolium-based ILs would be even more toxic than imidazolium-based compounds, although we are unaware of any data to support this. The model predicts that IL toxicity to V. fischeri should decrease as the number of negatively charges atoms on the cation increases. This result is consistent with the experimental finding that the choline cation (with a negatively charged oxygen atom) is relatively non-toxic. Although anions play a secondary role in determining toxicity, the models indicate that the presence of positively charged atoms in the anion leads to higher toxicity than those systems with a single negative anion atom. The correlations also indicate that methylating the aromatic ring of the cation should reduce toxicity, and that this effect will be larger for D. magna than V. fischeri. The predictions from the present study suggest several additional ionic liquid classes that should be evaluated for aquatic toxicity.
Acknowledgements
Funding for this work was provided by the Indiana 21st Century Research and Technology Fund and the U. S. National Oceanic and Atmospheric Administration. Dr Mark Muldoon is acknowleged for having synthesized many of the compounds examined in this study. David Eike and Profs. Joan Brennecke, Charles Kulpa Jr. and Gary Lamberti are acknowleged for their many helpful discussions and comments. Mike Brueseke assisted with Daphnia bioassays.References
- Ionic Liquids: Industrial Applications to Green Chemistry, ed. R. D. Rogers and K. R. Seddon, American Chemical Society Symposium Series 818, 2002 Search PubMed.
- J. L. Anthony, E. J. Maginn and J. F. Brennecke, J. Phys. Chem. B, 2001, 105, 10942 CrossRef CAS.
- J. McFarlane, W. B. Ridenour, H. Luo, R. D. Hunt, D. W. DePaoli and R. X. Ren, Sep. Sci. Technol., 2005, 40, 1245 CrossRef CAS.
- D. J. Gorman-Lewis and J. B. Fein, Environ. Sci. Technol., 2004, 38, 2491 CrossRef CAS.
- National Toxicology Program (NTP) and National Institute of Environmental Health Sciences (NIEHS), Review of Toxicological Literature for Ionic Liquids, prepared by Integrated Laboratory Systems Inc., Research Triangle Park, NC, May 2004.
- G. Li, J. Shen and Y. Zhu, J. Appl. Polym. Sci., 1998, 67, 1761 CrossRef CAS.
- J. Pernak, J. Kalewska, H. Ksycinska and J. Cybulski, Eur. J. Med. Chem., 2001, 36, 899 CrossRef CAS.
- J. Pernak, J. Rogoza and I. Mirska, Eur. J. Med. Chem., 2001, 36, 313 CrossRef CAS.
- J. Pernak and P. Chwala, Eur. J. Med. Chem., 2003, 38, 1035 CrossRef CAS.
- J. Pernak, I. Goc and I. Mirska, Green Chem., 2004, 6, 323 RSC.
- J. Pernak, K. Sobaszkiewicz and I. Mirska, Green Chem., 2003, 5, 52 RSC.
- J. Ranke, K. Molter, F. Stock, U. Bottin-Weber, J. Poczobutt, J. Hoffman, B. Ondruschka, J. Filser and B. Jastorff, Ecotoxicol. Environ. Saf., 2004, 58, 396 CrossRef CAS.
- K. M. Docherty and C. F. Kulpa, Jr., Green Chem., 2005, 7, 185 RSC.
- R. P. Swatloski, J. D. Holbrey, S. B. Memon, G. A. Caldwell, K. A. Caldwell and R. D. Rogers, Chem. Commun., 2004, 668 RSC.
- R. J. Bernot, E. E. Kennedy and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 1759 CrossRef CAS.
- B. Jastorff, K. Molter, P. Behrend, U. Bottin-Weber, J. Filser, A. Heimers, B. Ondruschka, J. Ranke, M. Schaefer, H. Schroder, A. Stark, P. Stepnowski, F. Stock, R. Stormann, S. Stolte, U. Welz-Biermann, S. Ziegert and J. Thoming, Green Chem., 2005, 7, 362 RSC.
- M. P. Edlind, W. L. Smith and T. D. Edlind, Antimicrob. Agents Chemother., 2005, 49, 843 CrossRef CAS.
- F. Stock, J. Hoffman, J. Ranke, R. Störmann, B. Ondruschka and B. Jastorff, Green Chem., 2004, 6, 286 RSC.
- R. J. Bernot, M. A. Brueseke, M. A. Evans-White and G. A. Lamberti, Environ. Toxicol. Chem., 2005, 24, 87 CrossRef.
- Standard Test Method for Assessing the Microbial Detoxification of Chemically Contaminated Water and Soil Using a Toxicity Test with a Luminescent Marine Bacterium, ASTM Designation: D 5660–96, 1996.
- K. L. E. Kaiser and V. S. Palabrica, Water Pollut. Res. J. Can., 1991, 26, 361 Search PubMed.
- D. M. M. Adema, Hydrobiologia, 1978, 69, 125 CrossRef.
- D. J. McQueen, J. R. Post and E. L. Mills, Can. J. Fish. Aquat. Sci., 1986, 43, 1571 CrossRef.
- D. G. Smith, Pennak's Freshwater Invertebrates of the United States: Porifera to Crustacea, Wiley, New York, 4th edn, 2001.
- Topological Indices and Related Descriptors in QSAR and QSPR, ed. J. Devillers and A. T. Balaban, Gordon and Breach Science Publishers, Amsterdam, 1999 Search PubMed.
- R. Todeschini and V. Consonni, Handbook of Molecular Descriptors, in Methods and Principles in Medicinal Chemistry, ed. R. Mannhold, H. Kubinyi and H. Timmerman, Wiley-VCH: Weinheim, 2000, vol. 11 Search PubMed.
- D. M. Eike, J. F. Brennecke and E. J. Maginn, Green Chem., 2003, 5, 323 RSC.
- D. M. Eike, J. F. Brennecke and E. J. Maginn, Ind. Eng. Chem. Res., 2004, 43, 1039 CrossRef CAS.
- P. Bonhote, A. P. Dias, N. Papageorgiou, K. Kalyanasundaram and M. Gratzel, Inorg. Chem., 1996, 35, 1168 CrossRef CAS.
- L. Cammarata, S. G. Kazarian, P. A. Salter and T. Welton, Phys. Chem. Chem. Phys., 2001, 3, 5192 RSC.
- C. P. Fredlake, J. M. Crosthwaite, D. G. Hert, S. N. V. K. Aki and J. F. Brennecke, J. Chem. Eng. Data, 2004, 49, 954 CrossRef CAS.
- United States Environmental Protection Agency, Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms, EPA 600/4-90/027F, 1993.
- M. C. Newman, Quantitative Methods in Aquatic Ecotoxicology, Lewis Publishers, Boca Raton, FL, 1995 Search PubMed.
- J. J. P. Stewart, MOPAC, version 6.0 for UNIX; Quantum Chemistry Exchange Program, Indiana University, Bloomington, IN, USA Search PubMed.
- J. J. P. Stewart, J. Comput. Chem., 1989, 10, 209 CrossRef CAS.
- J. J. P. Stewart, J. Comput. Chem., 1989, 10, 221 CrossRef CAS.
- J. Cross, Introduction to Cationic Surfactants, in Cationic Surfactants: Analytical and Biological Evaluation, ed. J. Cross and E. J. Singer, Marcel Dekker, New York, 1994, pp. 3–28 Search PubMed.
- D. W. Roberts and J. Costello, QSAR Comb. Sci., 2003, 22, 220 Search PubMed.
- M. J. Rosen, F. Li, S. W. Morrall and D. J. Versteeg, Environ. Sci. Technol., 2001, 35, 954 CrossRef CAS.
- D. W. Roberts and J. Costello, QSAR Comb. Sci., 2003, 22, 226 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
