Unusual electrical response of a poly(aniline) composite film on exposure to a basic atmosphere and its application to sensors
Xingfa Ma*ac, Guang Li*b, Mang Wanga, Ru Baia, Feng Yanga and Hongzheng Chena
aDepartment of Polymer Science and Engineering, State Key Lab of Silicon Materials, Zhejiang University, Hangzhou, 310027, P. R. China. E-mail: xingfazju@163.com; xingfamazju@yahoo.com.cn; Fax: +86-571-87951635; Tel: +86-571-87952557
bDepartment of Biomedical Engineering, State Key Lab of Biosensors, Key Lab of Biomedical Engineering of Ministry of Education of China, Zhejiang University, Hangzhou, 310027, P. R. China. E-mail: guangli@cbeis.zju.edu.cn; Fax: +86-571-87951676; Tel: +86-13858068126
cShandong Research Institute of Non-metallic Materials, Jinan, 250031, P. R. China
First published on 24th November 2005
Abstract
An unusual electrical response of a poly(aniline) composite film on exposure to a basic atmosphere is reported in this paper. Based on this feature, it was possible to develop a novel kind of gas-sensor exhibiting high sensitivity, rapid response, good repeatability which can be easily recovered by purging with highly pure N2. In order to study the gas-response mechanism of this unusual electrical response, comparative tests were carried out on the gas-sensing properties of N-type and P-type silicon with porosity under similar conditions. The electric field-induced surface photovoltage spectrum (FISPS) on the poly(aniline) composite film before and after pretreatment was also examined. The experimental results indicated that the transition from P-type to N-type of a poly(aniline) composite film may exist as a result of a doping effect.
Introduction
Gas-sensors are an important part of electronic nose systems to simulate olfaction and can have potential applications in many fields, such as environmental monitoring, foodstuff industry, chemical industry, medical diagnostic, explosives and neurotoxin detection for counter-terrorism, detection for insecticides and drugs, and so on.1,2Currently, many researchers expect to obtain sensors with high sensitivity, rapid response, reversibility at room temperature and convenient operation. All these rely on new, highly sensitive materials and good fabrication technology. So far, the materials in these areas have focused on the metal oxides semiconductors,3–7 single-wall carbon tubes (SWNTs), mutil-wall carbon tubes (MWNTs),1,8–11conductive polymers,12–19 metal organic compounds,20–23 and so on. Among these materials, conductive polymers are one kind of sensitive material that can be operated at or near room temperature.
Because of the mechanical flexibility, environmental stability and controllable conductivity with an acid/base (doping/undoping), poly(aniline) has attracted considerable attention as one of the typical conductive polymers, and has a lot of potential applications in many fields, such as lightweight battery electrodes, electromagnetic shielding devices, anticorrosion coatings, and sensors.24–26
At present, along with the preparation of nano-structure materials and nano-electrical devices, studies on controlling the nano-structure and improving the properties of conductive polymers have become active. Template and non-template methods, nano-fiber seeding approaches, and so on, are applied for preparing conductive polymers with nano-structures.24–29 Some investigators pointed out that conductive polymers with nano-structures have potential applications for superb sensitive sensors, such as gas-sensors, due to the highly specific surface area and the excellent charge transmission. However, up to now, most of these researchers have focused on the preparation of materials and morphology characterization.27–29 Recently, Huang and co-workers investigated the sensing properties of sensors based on nanostructured poly(aniline) prepared using interface polymerization and interdigital gold electrodes to obtain good results.30–32 Therefore, these studies inspired us to develop some organic sensors with high sensitivity.
The literature has indicated that most sensors made of poly(aniline) are based on the reversible reaction of an acid/base, for instance, the conductivity of poly(aniline) increases with acid and decreases with base. However, in our experiments, we found an interesting and unusual phenomena. Based on this feature, a novel gas-sensor, having high sensitivity, rapid response and good repeatability which can be easily recovered by purging with highly pure N2, in comparison with conventional poly(aniline) sensors, may be developed. Accordingly, this paper reports this abnormal behavior, discusses the sensitive mechanism, and also provides further evidence to support our previous reports.30–33
Experimental
Materials
Aniline (A.R.) was freshly distilled under vacuum prior to use, ammonium peroxydisulfate (A.R.), hydrochloride (A.R.), toluene (A.R.), n-hexane (A.R.), alcohol (A.R.), N-type and P-type silicon with porosity, and so on, are commercially available. Deionized filtered water was used in all studies.The interdigital electrodes are made of carbon paste, which are prepared with screen-printing technology, the gap and the length of electrode are 0.5 mm and 1 mm, respectively, the substrate of the electrode is a poly(propylene) plate with 0.5 mm thickness.
Preparation of sensing film
The coating mixture was prepared by adding equal mol aniline and ammonium peroxydisulfate in a hydrochloride solution. The gas-sensor was fabricated via the carbon electrode being immersed in the above mentioned mixture. The carbon electrode was taken out after 24 h of in-situ polymerization, and then was washed with deionized filtered water repeatedly, then dried at room temperature.Pre-treatment of poly(aniline) film
The device was put into an airproof test box (about 2.5 L), and desorbed with high-purity N2. A certain amount of trimethylamine or other base volatile solvent (about 0.2 mL) was injected into the test chamber with a syringe for deprotonation.Gas-response characterization of sensor
The gas-response characterization of the sensor to vapors has been reported in ref. 33 and 34.XRD characterization
X-ray diffraction patterns were recorded by a Rigaku D/max 2550 Pe diffractometer, equipped with a rotating anode X-ray generator, with Cu Kα monochromatic radiation at 40 kV and 300 mA. The samples were the films deposited on a glass substrate by in-situ polymerization.SPS and FISPS measurements
The SPS instrument was made by Jilin University (China). Monochromatic light was obtained by passing light from a 500 W xenon lamp (CHF XQ500W, Global xenon lamp power made in China) through a double-prism monochromator (Hilger and Watts, D 300, UK). The slit width of the entrance and exit is 1 mm. A lock-in amplifier (SR830-DSP, USA), synchronized with a light chopper (SR540, USA), was employed to amplify the photovoltage signal. The range of modulating frequency is from 20 to 70 Hz. The spectral resolution is 1 nm. The raw SPS data were normalized using the illuminometer (Zolix UOM-1S, China). The poly(aniline) casting film on the ITO glass was studied during the SPS and FISPS measurements, and the contact between samples and the indium tin oxide (ITO) electrode is not an ohmic contact since we carried out the measurement of the surface photovoltage. The construct of the photovoltaic cell is a sandwich structure (see Fig. 9 later, inset). It was ensured that the light penetrating depth was much less than the film layer thickness. The obvious absorption of the ITO electrode was observed at 400–950 nm. The potential of the irradiated electrode with respect to the back electrode denotes the signs of the applied electrical field.35,36Results and discussion
Electrical response behavior of protonation poly(aniline) film exposed to a basic atmosphere
The structure of the sensor in our experiments was reported in ref. 33.It was well known that the conductivity of poly(aniline) strongly depends on the doping and de-doping of an acid/base. The conductivity of poly(aniline) increases with the acid added and decreases with the base added. In our experiments, we found an unusual phenomena in conjuction to the above-mentioned rule. Exposed to a basic atmosphere, the current flowing through the protonated poly(aniline) film decreased suddenly at the beginning and then increased significantly. At this time, this film could be easily desorbed with high-purity N2 and be repeatedly utilized. This film was not only sensitive to an acid atmosphere (the current of film increasing), but also sensitive to a base (the current of film increasing as well). The essential difference was that the film exposed to an acid could not be desorbed with N2, and had to be recovered in a basic atmosphere. This gave us the idea that the conductivity of the de-protonated poly(aniline) film increased with the quantity of absorbed base. Based on this feature, it was possible to develop a novel gas-sensor having a high sensitivity, rapid response, good repeatability and being easy to recover by purging with highly pure N2. In this way, poly(aniline) could be used to develop two kinds of gas sensors for a basic atmosphere. One is based on the acid/base reversibility (the current of film increasing on exposure to an acid atmosphere and decreasing on exposure to a basic atmosphere). The other is based on the reversible process of polar vapor/N2 (the current of film increasing on exposure to a basic atmosphere and decreasing on exposure to a N2 atmosphere).33
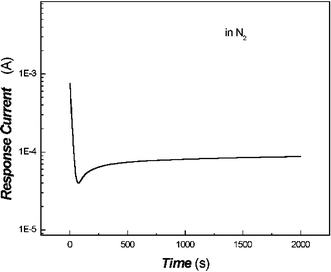
The conductivity dependence of the protonated poly(aniline) film on desorption with high-purity N2 is shown in Fig. 1.
 | ||
| Fig. 1 Conductivity dependence of in-situ polymerized poly(aniline) film in an N2 flow. | ||
Fig. 1 shows that the conductivity of the protonated poly(aniline) film decreased slightly along with the desorption with high-purity N2, which was mainly due to the absorption of a certain amount of gas in air. It took a few seconds to reach a steady baseline. Afterwards it became very difficult to further desorb with N2 which results in the small decline.
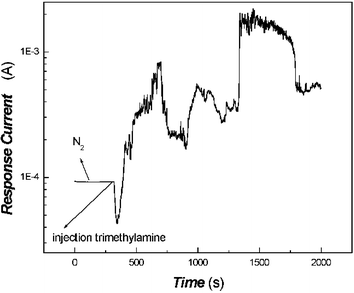
When the protonated poly(aniline) film had obtained a steady current baseline and was exposed to a trimethylamine atmosphere, the conductivity dependence of the poly(aniline) film on the exposure time gave an interesting phenonenon, as shown in Fig. 2.
 | ||
| Fig. 2 Conductivity dependence of in-situ polymerized poly(aniline) film on exposure to trimethylamine (1.54 × 10−6 mol mL−1). | ||
The current of the in-situ polymerized poly(aniline) composite film after desorption with N2 remained constant at the beginning, then the current decreased sharply when the film was exposed to a trimethylamine atmosphere. Afterwards, the current increased rapidly along with the adsorption of trimethylamine, achieving a level of protonation of poly(aniline) with no desorption via N2. This illustrates that the conductivity of poly(aniline) decreased along with an increasing extent of deprotonation. However, the increase of conductivity of poly(aniline) was very difficult to explain according to the usual reversible reaction of acids/bases and should be contributed to some other sensing mechanism.
The first exposure of poly(aniline) to a basic atmosphere acted as a pretreatment of the sensor in our research. In this way, the conductivity of poly(aniline) increases after the second, third, and fourth exposure to a basic atmosphere. The reversibility and repeatability were very good.
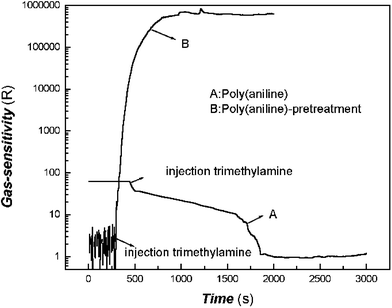
The comparative tests on gas-response between before and after pretreatment of poly(aniline) composite films to trimethylamine using the same sensor are shown in Fig. 3.
 | ||
| Fig. 3 Comparative tests on gas-response between before and after pretreatment of poly(aniline) composite films to trimethylamine (5.13 × 10−7 mol mL−1). | ||
Fig. 3 shows that the sensitivity and response speed after pretreatment were much higher than that before pretreatment. For the same sensor, before pretreatment, it took about 1000 s to reach 1 order of magnitude change of gas-sensitivity, about 1600 s to reach 2 orders, but after pretreatment, its response was very quick. It took about 120 s to reach 4 orders of magnitude, and about 260 s to reach 5 orders. These phenomena showed that the mechanism of the sensor based on the reversible reaction of the acid/base of poly(aniline) is a protonation and de-protonation process, but the unusual electrical conductivity response of poly(aniline) pretreated in our studies should be attributed to the van der Waals interactions. The latter is very similar to that of the polymer system filled with carbon black.37–40 According to previous literature, although the separation of amino acids had been carried out based on weak interactions of poly(aniline) with chromatographic technology,41 which acted as a stationary phase, some very similar amino acids can be identified. Therefore, it was meaningful to obtain a large measurable physical signal based on this weak interaction.
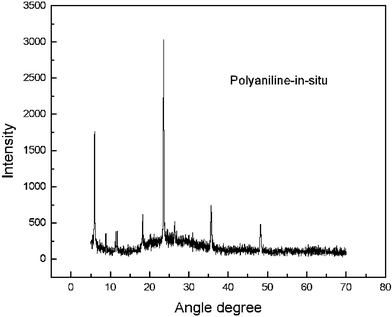
The sensing mechanisms mentioned above might be the result of grain boundary effects. It is well known that the poly(aniline) composite after deprotonation has a high resistance. When it absorbs chemical vapors, the potential barrier between intercrystallite boundaries is overcome. This leads to an increase of the film’s conductivity. This mechanism can also be explained by the XRD results as shown in Fig. 4.
 | ||
| Fig. 4 XRD results of in-situ polymerized poly(aniline). | ||
As shown in Fig. 4, the in-situ polymered poly(aniline) film has a multi-crystal structure. This supports the sensing mechanism mentioned above.
On the other hand, poly(aniline) is an important electroactive material. When a voltage is applied, greater delocalization and polarization of the poly(aniline) will be induced, leading to the absorption ability of the poly(aniline) film to chemical vapors naturally increasing. Consequently, the response current of the poly(aniline) film increases. Of course, the real sensing mechanism still needs further investigation.
Effects of trimethylamine concentration on the gas-response of the sensor
The effects of trimethylamine concentration on the gas-response of the sensor to trimethylamine are shown in Fig. 5.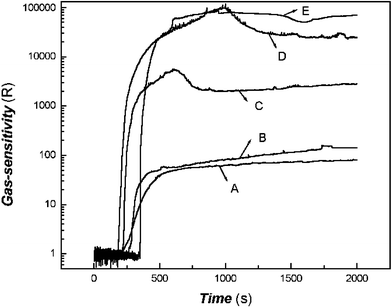 | ||
| Fig. 5 Effects of trimethylamine concentration on the gas-response of the sensor to trimethylamine (A: 3.21 × 10−8 mol mL−1; B: 6.41 × 10−8 mol mL−1; C: 2.56 × 10−7 mol mL−1; D: 5.13 × 10−7 mol mL−1; E: 1.54 × 10−6 mol mL−1). | ||
As shown in Fig. 5, it is obvious that the response magnitude as well as the response rate decrease with decreasing concentration. These results might offer up some valuable information about quantitative analysis.
Selectivity of the sensor
The gas-sensitivity of the poly(aniline) composite film to a series of vapors (trimethylamine, triethylamine, ammonia, formaldehyde, alcohol, toluene, and n-hexane) was examined, and the blank comparison test was also carried out considering the effect of moisture on the sensitivity by H2O injection instead of trimethylamine. The results are shown in Fig. 6.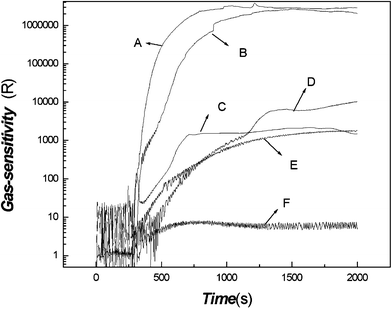 | ||
| Fig. 6 The gas-response of the poly(aniline) composite film to a series of vapors (A: trimethylamine; B: triethylamine; C: ammonia; D: formaldehyde; E: alcohol; F: H2O). | ||
Fig. 6 shows that distinct differences of the gas-sensitivity and response speed to similar vapors can be observed. The value of the gas-sensitivity and the response speed to trimethylamine were much higher than those of other similar vapors so that the composite film might be used to distinguish trimethylamine and some similar vapors with the aid of a sensor array and an artificial neural network system.
It is well known that trimethylamine, triethylamine and ammonia are materials with electron donors, and the ability of the electron donor of methyl and ethyl groups is different to lead to that of the interaction between film and adsorbed molecular gas, resulting in the variation of response current.
Regarding the effect of moisture on the gas-response, this influence was very small although the baseline shifts a little. For other vapors, such as toluene and n-hexane, there were no responses at all.
Repeatability of the sensor
The repeated measurement curve of the sensor is depicted in Fig. 7, which shows that the composite film has good repeatability.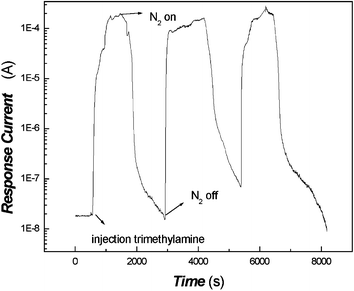 | ||
| Fig. 7 The cycle test examination of the poly(aniline) composite film to trimethylamine (5.13 × 10−7 mol mL−1). | ||
Fig. 7 also suggests that the present gas-sensing mechanism is possibly based on the physical adsorption/desorption process which induces the resistance change of the film.
Mechanism discussion
The preparation of poly(aniline) was generally carried out in acid media, so the product obtained was usually emeraldine salt, which had good conductivity. During the process of deprotonation, there will be several redox forms of poly(aniline), such as leucoemeraldine base (fully reduced form), emeraldine base (half-oxidized form), conducting emeraldine salt (half-oxidized and protonated form), and pernigraniline base (fully oxidized form).42 Therefore, its mechanism of gas-sensitivity to vapors will be complex.31The main reasons for gas-sensitivity should be attributed to the interaction between the sensing film and adsorbed molecular gas, including strong interactions (chemical bond) and weak interactions (such as hydrogen bonding, van der Waals force, and so on). For a strong interaction system, the recovery was generally very difficult, and for a weak interaction system, the recovery was easy at room temperature with highly pure N2. For the sensor based on the reversible reaction of the acid/base of poly(aniline), the mechanism was a protonation and deprotonation process. The unusual electrical response of poly(aniline) pretreated in our studies should be attributed to the van der Waals interactions.
When the poly(aniline) film was exposed to basic atmosphere again (second, third, fourth time), because the surface of the poly(aniline) base still adsorbed the vapors and the resistance of the poly(aniline) base was very large, the resistance of the adsorbed gas film varies so that the response current increases. In addition, since the electronegativity of the nitrogen of the poly(aniline) base was very large, the radius of the nitrogen atom was small, and nitrogen atom had a lone electron, the interaction between the surface of the poly(aniline) base and polar vapors would contain hydrogen bonding and a strong van der Waals force. Moreover, poly(aniline) was a typical electroactive polymer. When a voltage was applied, the poly(aniline) film adsorbed many more polar vapors by the inducement effects of the electric field. All these were possible reasons resulting in the electrical response of the film.
In order to illustrate this problem, we carried out comparative tests under similar conditions through a series of polar vapors (such as alcohol, toluene, n-hexane, and so on). The results indicated that the increase of the poly(aniline) conductivity after pretreatment strongly depended on the polarity of adsorbed vapors. The bigger the polarity of vapor, the larger the conductivity increased. For the non-polar or weak polar vapors (such as n-hexane, toluene), there was a small response and for alcohol, it still had a good response although the sensitivity was lower than that of trimethylamine. These results agree well with the above discussions.
Trimethylamine was an electron donor and a typical biological base while the poly(aniline) salt usually belonged to a p-type semiconductor material. When the film of the poly(aniline) salt was first exposed to the atmosphere of trimethylamine vapor, the current of film decreased corresponding to the deprotonation process of poly(aniline). Afterwards, the proton was taken away by molecular trimethylamine along with desorption with highly pure N2. When the deprotonated poly(aniline) film was exposed to the trimethylamine vapor atmosphere once again, the film current increases due to a strong interaction of the van der Waals force. The question was: did this cause changes (transition from P-type to N-type because of a doping effect in the gas phase) in the conductive type of poly(aniline) during the two time exposure cycle to the trimethylamine vapor atmosphere? This problem still needed to be investigated further.
To examine this hypothesis, considering the N-type and P-type silicon to be one kind of mature semiconductor material, we carried out comparative tests under similar conditions via N-type and P-type silicon with porosity as sensitive materials. It was found that the current of the N-type silicon with porosity was increased when exposed to the trimethylamine vapor atmosphere, while the current of P-type silicon with porosity decreased under the same conditions. The results for comparison are shown in Fig. 8. This also indirectly supported our deduction.
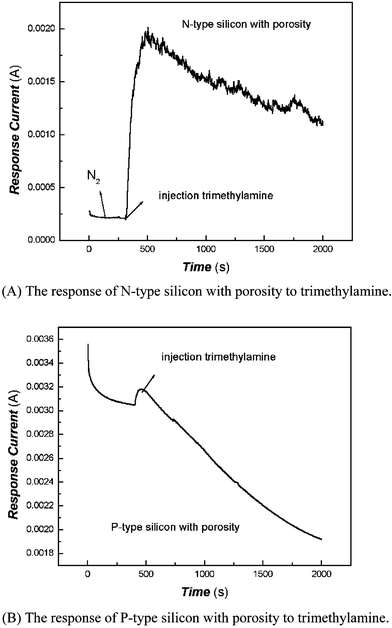 | ||
| Fig. 8 The response of N-type and P-type silicon with porosity to trimethylamine (5.13 × 10−7 mol mL−1). (A) The response of N-type silicon with porosity to trimethylamine. (B) The response of P-type silicon with porosity to trimethylamine. | ||
Further evidence of transition from P-type to N-type due to a doping effect in the gas phase was the result of the electric field-induced surface photovoltage spectrum (FISPS) on poly(aniline) composite films before and after pretreatment. It is well known that the FISPS is considered as one of the more common methods to examine different types of semiconductor. When the semiconductor films were applied with an electric field, their surface photovoltage signal varied. When it was applied with a reversed electric field, the surface photovoltage signal reversed. For P-type and N-type films, the surface photovoltage signal would be reversed. The scheme for the electric field-induced surface photovoltage spectrum (FISPS) experiment is shown in Fig. 9. The results are shown in Fig. 10.
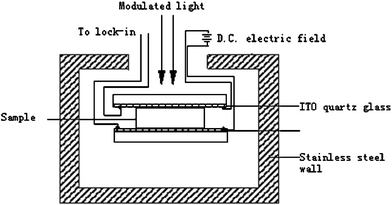 | ||
| Fig. 9 Scheme for the electric field-induced surface photovoltage spectrum (FISPS) experiment. | ||
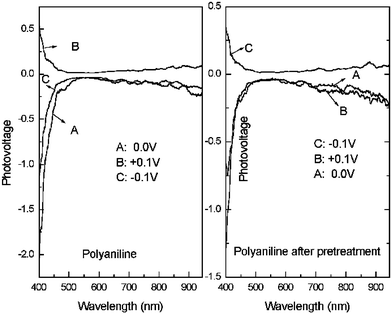 | ||
| Fig. 10 FISPS of poly(aniline) composite film before and after pretreatment. | ||
Fig. 10 shows that the results of FISPS on poly(aniline) before and after pretreatment agreed well with the hypothesis mentioned above. This also strongly supported the above discussion.
We had also carried out comparative experiments using nitrogen-based dopants such as ammonia and hydrazine for pre-treatment poly(aniline) film, the effects obtained were very similar. Of course, some details, such as the different oxidation states, need more discussions. Because the toxicity of hydrazine is very high, we usually did not use hydrazine as a pretreatment.
The results of FISPS on poly(aniline) before and after pretreatment with hydrazine are shown in Fig. 11.
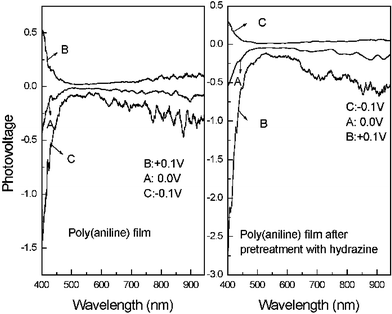 | ||
| Fig. 11 FISPS of poly(aniline) composite film before and after pretreatment with hydrazine. | ||
Fig. 11 also shows that the results of FISPS on poly(aniline) before and after pretreatment agreed well with the hypothesis mentioned above. Consequently, the sensing mechanism was mainly the result of grain boundary effects.
Conclusion
In summary, we examined an unusual electrical response of a poly(aniline) composite film exposed to a basic atmosphere. The results indicated that this kind of response strongly depended on the polarity of adsorbed vapors, and it was very easy to be recovered with N2, avoiding contaminating the surface of the sensing film via the reversible reaction of the acid/base in the gas phase. Consequently, it was possible to develop a new kind of sensor exhibiting high sensitivity and rapid response, which would have a good potential for application as an electronic nose or other sensor systems.Some evidence showed that the transition from P-type to N-type of a poly(aniline) composite film might exist because of doping effects in the gas phase.
Acknowledgements
The work was supported by the key project (No. 50433020) and the main international cooperation project from the NSFC.References
- S. Chopra and A. Pham, Appl. Phys. Lett., 2002, 80, 4632 CrossRef CAS.
- J. W. Gardrer, H. W. Shim and E. L. Hines, Sens. Actuators, B, 2000, 70, 19 CrossRef.
- M. A. Aronova, K. S. Chang and I. Takeuchi, Appl. Phys. Lett., 2003, 83, 1255 CrossRef CAS.
- E. Comini, G. Faglia and G. Sberveglieri, Appl. Phys. Lett., 2002, 81, 1869 CrossRef CAS.
- A. Khanna, R. Kumar and S. S. Bhatti, Appl. Phys. Lett., 2003, 82, 4388 CrossRef CAS.
- D. Lee, Y. T. Kim, J. Huh and D. Lee, Thin Solid Films, 2002, 416, 271 CrossRef CAS.
- V. Guidi, M. A. Butturi, M. C. Carotta, B. Cavicchi, M. Ferroni, C. Malagù, G. Martinelli, D. Vincenzi, M. Sacerdoti and M. Zen, Sens. Actuators, B, 2002, 84, 72 CrossRef.
- A. Modi, N. Koratkar, E. Lass, B. Wei and P. M. Ajayan, Nature, 2003, 424, 171 CrossRef CAS.
- J. Kong, N. R. Franklin, C. Zhou, M. G. Chapline, S. Peng, K. Cho and H. Dai, Science, 2000, 287, 622 CrossRef CAS.
- L. Valentini, I. Armentano and J. M. Kenny, Appl. Phys. Lett., 2003, 82, 961 CrossRef CAS.
- S. Chopra, K. McGuire, N. Gothard, A. M. Rao and A. Pham, Appl. Phys. Lett., 2003, 83, 2280 CrossRef CAS.
- M. M. Ayad, N. Salahuddin and M. A. Sheneshin, Synth. Met., 2003, 132, 185 CrossRef CAS.
- A. Göka, B. Sarý b and M. Talu, Synth. Met., 2004, 142, 41 CrossRef.
- O. A. Raitman, E. Katz, A. F. Bückmann and I. Willner, J. Am. Chem. Soc., 2002, 124, 6487–6496 CrossRef CAS.
- J. K. Avlyanov, J. Y. Josefowicz and A. G. MaceDiarmid, Synth. Met., 1995, 73, 205 CrossRef CAS.
- I. Sapurina, A. Riede and J. Stejskal, Synth. Met., 2001, 123, 503 CrossRef CAS.
- M. Matsuguchi, J. Io, G. Sugiyama and Y. Sakai, Synth. Met., 2002, 128, 15 CrossRef CAS.
- A. Riul Jr., A. M. G. Soto, S. V. Mello, S. Bone, D. M. Taylor and L. H. C. Mattoso, Synth. Met., 2003, 132, 109 CrossRef.
- V. V. Chabukswar, S. Pethkar and A. A. Athawale, Sens. Actuators, B, 2001, 77, 657 CrossRef.
- V. R. Albertini, A. Generosi, B. Paci, P. Perfetti, G. Rossi, A. Capobianchi, A. M. Paoletti and R. Caminiti, Appl. Phys. Lett., 2003, 82, 3868 CrossRef.
- T. Miyata, S. Kawaguchi, M. Ishii and T. Minami, Thin Solid Films, 2003, 425, 255 CrossRef CAS.
- E. van Faassen and H. Kerp, Sens. Actuators, B, 2003, 88, 329 CrossRef.
- N. A. Rakow and K. S. Suslick, Nature, 2000, 406, 710 CrossRef CAS.
- J. Huang and R. B. Kaner, J. Am. Chem. Soc., 2004, 126, 851 CrossRef CAS.
- X. Zhang, W. J. Goux and S. K. Manohar, J. Am. Chem. Soc., 2004, 126, 4502 CrossRef CAS.
- J. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817 CrossRef CAS.
- Y. Ma, J. Zhang, G. Zhang and H. He, J. Am. Chem. Soc., 2004, 126, 7097 CrossRef CAS.
- L. Zhang, Y. Long, Z. Chen and M. Wan, Adv. Funct. Mater., 2004, 14, 693 CrossRef CAS.
- A. D. W. Carswell, E. A. O'Rear and B. P. Grady, J. Am. Chem. Soc., 2003, 125, 14793 CrossRef CAS.
- J. Huang, S. Virji, B. H. Weiller and R. B. Kaner, J. Am. Chem. Soc., 2003, 125, 314 CrossRef CAS.
- S. Virji, J. Huang, R. B. Kaner and B. H. Weiller, Nano Lett., 2004, 4, 491 CrossRef CAS.
- J. Huang, S. Virji, B. H. Weiller and R. B. Kaner, Chem.–Eur. J., 2004, 10, 1314 CrossRef CAS.
- X. Ma, M. Wang, H. Chen, G. Li, J. Sun and R. Bai, Green Chem., 2005, 7, 507 RSC.
- S. Gupta and T. N. Misra, Sens. Actuators, B, 1997, 41, 199 CrossRef.
- J. Hong, J. Cao, J. Sun, H. Li, H. Chen and M. Wang, Chem. Phys. Lett., 2003, 380, 366 CrossRef CAS.
- Y. Lin, D. Wang, Q. Zhao, M. Yang and Q. Zhang, J. Phys. Chem. B, 2004, 108, 3202 CrossRef CAS.
- S. G. Chen, J. W. Hu, M. Q. Zhang, M. W. Li and M. Z. Rong, Carbon, 2004, 42, 645 CrossRef CAS.
- X. M. Dong, R. W. Fu, M. Q. Zhang, B. Zhang, J. R. Li and M. Z. Rong, Carbon, 2003, 41, 369 CrossRef.
- J. R. Li, J. R. Xu, M. Q. Zhang and M. Z. Rong, Carbon, 2003, 41, 2353 CrossRef CAS.
- X. M. Dong, R. W. Fu, M. Q. Zhang, B. Zhang and M. Z. Rong, Carbon, 2004, 42, 255.
- G. Gordon and A. Leon, Adv. Mater., 2002, 14, 953 CrossRef CAS.
- V. K. Milind, K. V. Annamraju, R. Marimuthu and S. Tanay, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 2043 CrossRef.
| This journal is © The Royal Society of Chemistry 2006 |
