Novel quaternary ammonium ionic liquids and their use as dual solvent-catalysts in the hydrolytic reaction
Jianyang
Weng
,
Congmin
Wang
,
Haoran
Li
* and
Yong
Wang
Department of Chemistry, Zhejiang University, Hangzhou, 310027, P. R. China. E-mail: lihr@zju.edu.cn; Fax: +86-571-8795-1895; Tel: +86-571-8795-2424;
First published on 14th November 2005
Abstract
Ionic liquids (ILs) are no longer just a class of esoteric compounds, but are proving to be valuable and useful in a multitude of different applications. Herein, novel quaternary ammonium ionic liquids have been synthesized and characterised. These ionic liquids are Brønsted acidic, available from cheap raw materials and easy to prepare. They have been used both as a catalyst and environmentally benign solvent for the hydrolytic reaction of 1,1,1,3-tetrachloro-3-phenylpropane, eliminating the need for a volatile organic solvent and additional catalyst. The results clearly demonstrate that these ILs can be easily separated and reused without losing their activity and quality. Also, the yields obtained with this methodology are significantly increased in comparison with those reported in organic solvents to date.
Introduction
Ionic liquids (ILs) are very attractive and environmentally acceptable solvents because they have very low vapor pressure and are stable in a wide temperature range.1–3 Therefore, they can be used as environmentally benign solvents for a number of chemical processes, such as separations,4 reactions,5 homogeneous two-phase catalysis6 and extractions.7 The current emphasis on novel reaction media is motivated by the need for efficient methods for replacing of toxic or hazardous solvents and catalysts. The use of ionic liquids as novel reaction media may offer a convenient solution to both the solvent emission and the catalyst recycling problem.8Seddon et al.9 cooperated with many chemistry companies, such as BP, and manufactured the linear alkyl benzene based on ionic liquids as reaction media. Furthermore, after the announcement of the first industrial process involving ionic liquids by BASF (BASIL10 process) in 2003 the potential of ionic liquids for new chemical processes and technologies is beginning to be recognized. However, currently the industry application of ionic liquids is limited because of the high cost of ionic liquids, the difficulty in separations or recycling, and so on. In this paper, novel quaternary ammonium ionic liquids, which are Brønsted acidic and easy to prepare from cheap amines, have been synthesized and used both as catalysts and environmentally benign solvents for the hydrolytic reaction of 1,1,1,3-tetrachloro-3-phenylpropane to prepare cinnamic acid, Fig. 1.
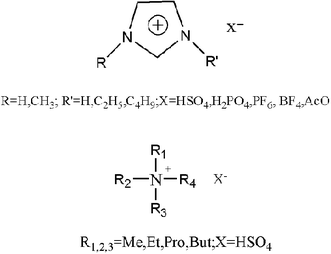 | ||
| Fig. 1 Structures of two sorts of ionic liquids. | ||
Cinnamic acid is a highly valuable class of fine chemicals with applications in polymer formulations, medication, pesticide, sensitive resin and plastics as well as general organic synthesis. It is a key intermediate in the synthesis of Segontin, Cinnarizine, chlorobenzene ammonic butyric acid and so on. Several methods are reported to prepare cinnamic acid: the Perkin reaction,11–15 halo-benzene with acrylic acid,16,17 benzoaldehyde with acetone,18 benzoaldehyde with ketene or acetic acid,19 the preparation from cinnamic aldehyde by air-oxidation,20 using microwave irradiation for the Knoevenagel–Doebner synthesis of cinnamic acid,21 styrene with carbon monoxide or carbon dioxide,22 the hydrolyzation of 1,1,1,3-tetrachloro-3-phenylpropane from styrene and CCl4,23 and a new direct synthesis from aromatic aldehydes and aliphatic caboxylic acid in the presence of sodium borohydride.24 Today, the hydrolyzation of 1,1,1,3-tetrachloro-3-phenylpropane is the one of the main routes for industrial production of cinnamic acid because the yield is high and CCl4 can be used up. CCl4 can not be used as solvent any longer and another product must be used because CCl4 may destroy the ozonosphere, and it is harmful to the human body. In the hydrolyzation of 1,1,1,3-tetrachloro-3-phenylpropane, solvents such as acetic acid and a strong acid catalyst such as sulfuric acid are needed. The yield of the hydrolytic reaction is low due to the use of the strong acid. Despite numerous attempts to overcome these drawbacks, no benign methods have been used for the synthesis of cinnamic acid.
We describe here the hydrolyzation of 1,1,1,3-tetrachloro-3- phenylpropane to synthesize cinnamic acid in different ionic liquids, Fig. 2. The process of hydrolyzation can be carried out without additional catalyst and solvent and the ionic liquids can be easily separated and reused. In particular, the effect of the ionic liquid, temperature, dosage and recycling on the yield is discussed.
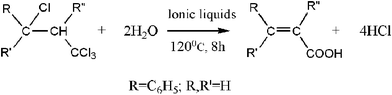 | ||
| Fig. 2 Hydrolytic reaction in ionic liquids. | ||
Experimental
Chemicals
Styrene, copper(I) chloride, carbon tetrachloride, acetic acid, sulfuric acid, acetone, n-butyl bromide, bromoethane, 1-chlorobulane, potassium hexafluorophosphate, sodium tetrafluoroborate, imidazole, 1-methylimimdazole, ethyl acetate, trimethylamine, diethylamine, triethylamine, n-tripropylamine, iso-propylamine, tri-n-butylamine, n-butylamine, and chloroform were all used as received unless otherwise stated.Instruments
NMR sectra were recorded on a 500 MHz Bruker spectrometer in DMSO, CDCl3 and calibrated with tetramethylsilane (TMS) as the internal reference. The purity was analyzed using a high pressure liquid chromatogram (HPLC-10 ATVP SHIMADZU). The column was Kromasil C18 (4.6 mm × 250 mm). An acetonitrile–water–acetic acid (100 ∶ 99 ∶ 1) mixture was used as solvent. IR measurements were performed on an FT-IR 470 Nicolet using KBr optics. The melting point was recorded on a digitial melting point apparatus (WRS-1A).Synthesis of ionic liquids
The ionic liquids of general type [amine][HSO4] were synthesized in the following way.The following four kinds of ionic liquids were synthesized as [Et2NH2][HSO4].
Other ionic liquids used in this paper were synthesized using standard literature methods.25
The hydrolyzation of 1,1,1,3-tetrachloro-3-phenylpropane in ionic liquids
The hydrolyzation of 1,1,1,3-tetrachloro-3-phenylpropane was investigated in several ionic liquids. The choice of ionic liquids were motivated by their being the most widely used, and therefore the most widely available (Fig. 2). These reactions were carried out in a 250 ml volumetric flask set in a recirculating heated bath and stirred with a magnetic stir bar. In a typical reaction 1,1,1,3-tetrachloro-3-phenylpropane (0.5 mol) was added to quaternary ammonium ionic liquids (trimethylamine sulfate [Me3NH][HSO4], 0.5 mol), and water (1.0 mol) was dropped into the flask, then the mixture was stirred at 120 °C for several hours. During the reaction, cinnamic acid and ionic liquid were mixed together, giving out hydrochloric acid from the liquid system, which was absorbed by water. At the end of the reaction, the mixture was treated with water. The products were separated by filtration as the ionic liquids could be dissolved in water while the cinnamic acid could not.The characterisation of cinnamic acid: 1H NMR (ppm, δH CDCl3): 6.46 (s, 1H), 7.40 (s, 1H), 7.41 (s, 1H), 7.55 (s, 1H), 7.80 (s, 1H), 11.19 (s, 1H); 13C NMR (ppm, δCCDCl3): 117.57; 128.91; 132.63; 147.37; 172.92. IR (cm−1): 3448.22, 3024.46, 2922.08, 2830.52, 1685.07, 1631.09, 1560.06, 1449.75, 1134.05, 762.08, 705.41. melting point: 132.5–134.5 °C.
Results and discussion
Table 1 summaries the results of the hydrolytic reaction in the ionic liquids. It can be seen that the conversion and yield were better achieved in ionic liquids than in acetic acid as solvent. Not only the cation of ionic liquids but also the anion of ionic liquids affects the conversion and the yield of the hydrolytic reaction. In the case of sulfate acid ionic liquids only, the conversion and yield in the quaternary ammonium ionic liquids are better than those in the other ionic liquids from entries 1, 2, 3, 4, 5 and 6. The reaction appeared to be largely dependent on the anion of the ionic liquids. The yield and the conversion are better for [Bmim][HSO4] than for [Bmim][H2PO4] and [Bmim][PF6 ] from entries 3, 7 and 10. Similar results are obainted from entries 4 and 8, and entries 5 and 9. It was surprising that the hydrolyzation of 1,1,1,3-tetrachloro-3- phenylpropane could be carried out in neutral ionic liquids, which is different from the method according to the patent26 that it should be carried out under acid conditions. Above all, the yield of cinnamic acid was greater in sulfate acid ionic liquids.| Entry | Solvent | T/°C | Conversionb (%) | Yield (%) |
|---|---|---|---|---|
| a Reactions were performed at 120 °C. b Conversions after 8 h. c The reaction was in acetic acid as solvent and sulfuric acid as catalyst; conversion after 15 h. | ||||
| 1 | [Me3NH][HSO4] | 120 | 100 | 87 |
| 2 | [Et2NH2][HSO4] | 120 | 100 | 88 |
| 3 | [Bmim][HSO4] | 120 | 100 | 89 |
| 4 | [Emim][HSO4] | 120 | 100 | 88 |
| 5 | [Hmim][HSO4] | 120 | 100 | 89 |
| 6 | [ImI][HSO4] | 120 | 100 | 88 |
| 7 | [Bmim][PF6] | 120 | 87 | 84 |
| 8 | [Emim][BF4] | 120 | 89 | 83 |
| 9 | [Hmim][OAc] | 120 | 90 | 82 |
| 10 | [Bmim][H2PO4] | 120 | 90 | 85 |
| 11c | Acetic acid | 120 | 98 | 85 |
An important feature of these reactions in ionic liquids is that there is no evidence for significant formation of a side reaction. There was good conversion in the sulfate acid ionic liquids.
In order to obtain a higher yield, different quaternary ammonium ionic liquids were used in the hydrolytic reaction. Table 2 shows the effect of the ionic liquid on the conversion and the yield. We found that [Et3NH][HSO4] might be best for the hydrolyzation of 1,1,1,3-tetrachloro-3-phenylpropane in all the quaternary ammonium ionic liquids.
The effort required to find the influence of dosage and temperature for [Et3NH][HSO4] was great. Firstly, we investigated the influence of dosage of ionic liquids, which was recorded from 0.25 mol to 2.00 mol; see Table 3 entries 1 to 6. The higher dosage of [Et3NH][HSO4], the better conversion and yield of 1,1,1,3-tetrachloro-3-phenylpropane and the higher yield of cinnamic acid obtained. However, the result did not continue improving when the dosage reached 1.00 mol or greater. Entries 3, 7, 8, 9 and 10 in Table 3 show that the temperature appears to affect the reaction. Low temperature (<100 °C) led to poor conversion levels. When the temperature was so high that 1,1,1,3-tetrachloro-3-phenylpropane and cinnamic acid decomposed by themselves, the yield decreased significantly. In contrast, the desired cinnamic acid was obtained with good results at 120 °C.
| Entry | Dosage/mol | T/°C | conversionb (%) | Yield (%) |
|---|---|---|---|---|
| a Reactions were performed at 120 °C, the dosage of 1,1,1,3-tetrachloro-3-phenylpropane was 1.00 mol. b Conversions after 8 h. | ||||
| 1 | 0.25 | 120 | 85 | 54 |
| 2 | 0.50 | 120 | 95 | 82 |
| 3 | 1.00 | 120 | 100 | 90 |
| 4 | 1.25 | 120 | 100 | 90 |
| 5 | 1.50 | 120 | 100 | 90 |
| 6 | 2.00 | 120 | 100 | 90 |
| 7 | 1.00 | 140 | 100 | 86 |
| 8 | 1.00 | 160 | 100 | 60 |
| 9 | 1.00 | 100 | 60 | 37 |
| 10 | 1.00 | 80 | 36 | 12 |
The product of the reaction resulted in a one phase system after reaction. Water was added into this system once the reaction was completed. Filtration was sufficient to remove products from the solution because the ionic liquids could dissolve in water while the cinnamic acid could not. The ionic liquids could be recycled after separating away most of the water under vacuum which was left in the liquid. Also, it did not affect the recycling of the ionic liquid with a small amount of water in the ionic liquid. The hydrolyzation of 1,1,1,3-tetrachloro-3-phenylpropane to produce cinnamic acid was repeated ten times. It can be seen (Table 4) that the conversions and the yield were not reduced after being reused ten times and [Et3NH][HSO4] could have the potential to be used more than ten times.
Conclusion
For the application in industry, many ionic liquids are expensive. In this paper, some quaternary ammonium ionic liquids were synthesized, which were relatively cheap and easy to prepare. These ionic liquids were used in the hydrolytic reaction of 1,1,1,3-tetrachloro-3-phenylpropane to cinnamic acid without the use of tradition volatile organic compounds (VOCs) such as acetic acid and additional catalyst. The reactions proceeded well in sulfate ionic liquids and cinnamic acid is obtained with high conversion and yield, especially in the quaternary ammonium ionic liquids. The effect of the ionic liquids, temperature, dosage and recycling on the yield was discussed. Good conversion and high yield of cinnamic acid could be obtained with a 1 ∶ 1 mole ratio under 120 °C for 8 h with [Et3NH][HSO4]. These ionic liquids provide a good alternative for the industrial synthesis of cinnamic acid and this method has been applied by Juhua Group Corporation Lanxi Agricultural Chemistry Factory.Acknowledgements
We gratefully acknowledge financial support from the Natural Science Foundation of China (NO.20434020).References
- P. Wasserscheid and W. Keim, Angew. Chem., 2000, 112, 3926 CrossRef; P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef; P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3773 CrossRef; P. Wasserscheid and T. Welton, Ionic liquids in synthesis, Wiley-VCH, Weinheim, 2003, p. 364 Search PubMed.
- J. S. Wilkes, J. A. Levisky, R. A. Wilson and C. L. Hussey, Inorg. Chem., 1982, 21, 1263 CrossRef CAS; J. S. Wikes and M. J. Zaworotko, Chem. Commun., 1992, 965 RSC.
- Ionic liquids as green solvent: progress and prospects, ed. R. D. Rogers and K. R. Seddon, ACS Symposium Series 856, American Chemical Society, Washington, DC, 2003, p. 599 Search PubMed.
- J. G. Huddleston, H. D. Williams, R. P. Swatloski, A. E. Visser and R. D. Rogers, Chem. Commun., 1998, 1765 RSC.
- R. Sheldon, Chem. Commun., 2001, 2399 RSC; C. J. Adams, M. J. Earle, G. Roberts and K. R. Seddon, Chem. Commun., 1998, 2097 RSC; C. M. Gordon and A. McCluskey, Chem. Commun., 1999, 1431 RSC.
- J. D. Holbrey and K. R. Seddon, Clean Prod. Process., 1999, 1, 223 CrossRef.
- S. Zhang, Q. Zhang and Z. C. Zhang, Ind. Eng. Chem. Res., 2004, 43, 614 CrossRef CAS; J. Esser, P. Wasserscheid and A. Jess, Green Chem., 2004, 6, 316 RSC.
- T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS; F. Liu, M. B. Abrams, R. T. Baker and W. Tumas, Chem. Commun., 2001, 5, 433 RSC; E. D. Bates, R. D. Mayton, I. Ntai and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 926 CrossRef CAS; S. K. Ritter, Chem. Eng. News, 2001, 79, 27; Y. Wang, H. R. Li, C. M. Wang and J. Hui, Chem. Commun., 2004, 1938 RSC; S. Gmouh, H. Yang and M. Vaultier, Org. Lett., 2003, 5, 3365 CrossRef CAS.
- M. J. Earle, P. B. McCormac and K. R. Seddon, Chem. Commun., 1998, 2245 RSC.
- M. Volland, V. Seitz, M. Maase, M. Flores, R. Papp, K. Massonne, V. Stegmann, K. Halaritter, R. Noe, M. Bartsch, W. Siegel, M. Becker and O. Huttenloch, Method for the separation of acids from chemical reaction mixtures by means of ionic liquids, PCT Int. Appl., WO 03/06225, 31. 7. 2003, BASF AG Search PubMed; M. Huttenloch, Chem. Eng. News, 2003, 81, 9.
- J. R. Johnson, Org. React., 1942, 1, 210; J. R. Jhonson, Org. Syn. Coll., 1955, 3, 426 Search PubMed; R. E. Ruckles, J. Chem. Educ., 1950, 27, 210 Search PubMed.
- E. Koepp and V. Fritz, Synthesis, 1987, 2, 177 CrossRef.
- R. Kern, DE Pat., DE 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 139
139![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 994, 1983.
994, 1983. - M. G.. Mihaiescu, N. Vasile and T. Sachelarie, RO Pat., RO 90
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 224, 1986.
224, 1986. - W. R. Peterson, US Pat., US 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 401
401![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 099, 1986.
099, 1986. - W. J. Houlihan and J. Nadelson, US Pat., US 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 870
870![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751, 1975; J. E. Telschow, US Pat., US 4
751, 1975; J. E. Telschow, US Pat., US 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571
571![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 432, 1986.
432, 1986. - N. A. Bumagin, N. P. Andryukhova and I. P. Beletskaga, Metalloorg. Khim., 1989, 2, 911 Search PubMed.
- J. H. Teles, N. Rieber, K. Breuer, D. Demuth, H. Hibst, H. Etzrodt and U. Rheude, US Pat., US 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 211
211![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 416, 1999.
416, 1999. - K. Karl, K. Thomas and S. Heinrich, DE Pat., DE 3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 437
437![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 624, 1986; G. Ihl, G. Roscher and N. Mayer, DE Pat., DE 3
624, 1986; G. Ihl, G. Roscher and N. Mayer, DE Pat., DE 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 743
743![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 616, 1988.
616, 1988. - B. Ramesh Babu and K.
K. Balasubramaniam, Org. Prep. Proced. Int., 1994, 26, 123 CrossRef CAS; E. Dalacanale, G. Bottaccio, S. Compolmi and F. Montamari, EP Pat., 0
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 146
146![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 373, 1985; H. Harada, EP Pat., EP 0
373, 1985; H. Harada, EP Pat., EP 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 170
170![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520, 1986.
520, 1986. - K. H. M. Sampath, R. B. V. Subba and R. P. Thirupathi, Org. Prep. Proced. Int., 2000, 32, 81 Search PubMed.
- H. Heinz, Y. Peres and A. Milchereit, J. Organomet. Chem., 1986, 307, c38 CrossRef CAS.
- I. Gerdlin, Pollena: Tluszcze, Srodki, Piorace Kosnet, 1987, 31, 13 Search PubMed; M. Hajek, J. Malek and P. Silhavy, CS Pat., 217
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 518, 1983; M. Hajek and P. Silhavy, DE Pat., DE 3
518, 1983; M. Hajek and P. Silhavy, DE Pat., DE 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524
524![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 475, 1986; M. Hajek, J. Malek and P. Silhavy, CS Pat., CS 209
475, 1986; M. Hajek, J. Malek and P. Silhavy, CS Pat., CS 209![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 347, 1981.
347, 1981. - I. C. Constantin, T. Fulga and O. Marioara, Tetrahedron Lett., 2003, 44, 3579 CrossRef.
- G. Y. Zhao, T. Jiang, H. X. Gao, B. X. Han, J. Huang and D. H. Sun, Green Chem., 2004, 6, 75 RSC; Lauric Ropel, S. B. Lionel, N. V. K. A. Sudhir, A. S. Mark and J. F. Brennecke, Green Chem., 2005, 7, 83 RSC; J. F. Dubreuil, K. Bourahla, M. Rahmouni, J. P. Bazureau and J. Hamelin, Catal. Commun., 2002, 3, 185 CrossRef CAS.
- M. Hàjek and P. Šilhav, US Pat., US 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806
806![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 681, 1998; CA, 104; 186139w.
681, 1998; CA, 104; 186139w.
| This journal is © The Royal Society of Chemistry 2006 |
