Conversion of scallop viscera wastes to valuable compounds using sub-critical water
Omid
Tavakoli
and
Hiroyuki
Yoshida
*
Department of Chemical Engineering, College of Engineering, Osaka Prefecture University, 1-1 Gakuen-Cho, Sakai, Osaka, 599-8531, Japan. E-mail: yoshida @chemeng.osakafu-u.ac.jp; Fax: +81-72-254-9298; Tel: +81-72-254-9298
First published on 15th November 2005
Abstract
The large amount of seafood wastes discharged from related industries has become a serious problem. Since these wastes contain high concentrations of organic variables such as proteins and fat, a method for recovery of these useful materials would be a highly desirable process. In this work, sub- and supercritical water hydrolysis of scallop viscera wastes has been studied in a batch-type reactor. Experiments were performed at a temperature range of 443–673 K with the reaction time between 1–50 min. Through the hydrolysis reaction, this method produced valuable materials such as amino acids, organic acids, fat and oil phases, soluble proteins and peptides. At 513 K and 50 min, maximum amino acids (0.15 kg per kg dry scallop waste) were obtained, while maximum organic acids were found at 553 K and 40 min (0.08 kg per kg dry scallop waste). The optimum temperature for amino and organic acids was close to the temperature at which the ion product of water is maximum. Among amino acids, glycine was the most abundant while in organic acids pyroglutamic acid was most plentiful. Experimental results demonstrated that this technique has great potential for practical application because it was not only energy saving, environmentally friendly and cost effective but also produced many useful materials with zero emission.
Introduction
As a new green conversion method, reactions in sub-critical water have been gaining more attention of late. Sub-critical water can provide a new reaction medium and/or act as a catalyst in many chemical reactions. Changes in physical and chemical properties of water under sub-critical conditions, especially in hydrogen bond, ion product and dielectric constant, could facilitate reactions with a wide range of organic compounds resulting in many useful materials.1–4One of the great challenges in the treatment of organic wastes is related to seafood wastes from seafood and fishery industries world wide. Prevention of marine pollution from dumping of organic wastes provided by the London treaty5,6 of 1996 and other new environmental legislation restricted the disposal of these wastes. Immediately after 1996, incineration became an alternative procedure to process seafood wastes. In addition to its very expensive cost, incineration showed two major disadvantages. First, because seafood wastes contain a high level of protein, incineration destroyed these potentially useful materials. Second and also more importantly, some seafood wastes such as those of scallop and squid contain high concentrations of toxic metals and incineration could cause additional environmental problems. Another alternative methods to deal with such wastes is the supercritical water oxidation which decomposes almost all organic compounds mainly to carbon dioxide, water and nitrogen. Furthermore it suffers some major disadvantages including corrosion problems, expensive materials and lack of recycling and resource recovery.7–9 Therefore, a better, cheaper and cleaner process for the conversion of seafood wastes to useful materials would be highly beneficial.
Yoshida et al. have first showed that hydrolysis in sub-critical water could be an ideal new chemical process to treat seafood wastes.10–13 Using sub-critical water hydrolysis, some useful materials such as amino acids, organic acids, fatty acids, oil phase, and other materials could be produced from fish and squid wastes.10–16
Other research has demonstrated the possibility of using sub-critical water technology to convert organic wastes to useful substances and resources. Some recent studies have been reported on the treatment of organic wastes,17–19 sewage sludge,20–21 decomposition of glucose, fructose and cellobiose,22 hydrolysis of cellulose,23–24 amino acid decomposition,25 and hydrolysis of methyl t-butyl ether.26
In Japan alone, the production of scallops in the year 1998 reached 550![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tonnes. With a conversion factor of waste as 40% of meat and internal organs, this large scale production resulted in large amounts of waste containing proteins on an annual basis. Therefore, a green and/or clean conversion method of those wastes to useful materials is extremely urgent for this country.
000 tonnes. With a conversion factor of waste as 40% of meat and internal organs, this large scale production resulted in large amounts of waste containing proteins on an annual basis. Therefore, a green and/or clean conversion method of those wastes to useful materials is extremely urgent for this country.
It is expected that sub-critical water as a powerful reactant and medium can be used for effective treatment of these scallop wastes. With regard to the proteins contained in scallop, some research is available in the literature, which deals with the major protein bands, protein–mineral interactions, and analysis of the proteins.27–29
The aim of this work is to investigate the feasibility of treatment of the valueless scallop viscera wastes in sub-critical water to produce valuable materials. From the results of our experiments, we propose an optimum procedure for hydrolysis conversion.
Materials and methods
Materials, reagents, and equipment
The waste of scallop internal organs (Patinopecten yessoensis) was supplied from Aomori prefecture (Japan). Before the experiments, the waste was homogenized with a Waring blender (Model 31 BL 92, Dynamic Corporation of America) for 10 min. The resulting homogenous wastes were stored in a freezer at 255 K. Water content of the sample was approximately 83.8%. All reagents were laboratory grade and water was Milli-Q which is deionized distilled double pure water (18.2 MΩ cm purity grade at 25 °C). A stainless tube (SUS 316, id 0.0075 m × 0.15 m) with Swagelok caps was used as a reactor (reactor volume 9.0 cm3).10–12Reaction and analytical methods
The reactions were conducted in the temperature range of 443–673 K and pressure range of 0.792–30 MPa. In each run, about 2.5 × 10−3 kg sample of scallop viscera wastes was used. The sample and Milli-Q water were put into the reactor and then the oxygen dissolved in the sample and the water was removed by purging argon gas to avoid the oxidation process. The reactor was then sealed and immersed in a preheated molten salt bath (Thomas Kagaku Co. Ltd., Tokyo, Japan) containing a mixture of potassium nitrate and sodium nitrate. Soaking the reactor into a water bath after the desired reaction time terminated the reaction.After the reaction, the aqueous phase was diluted to 50 cm3 with distilled pure water and was filtered through Millipore membranes (0.22 µm) to remove fat and oil droplets as well as water insoluble residues.
The amino acids content in the reaction products was measured using an amino acid HPLC (LC-10A, ISC-07/S 1504 column incorporated with a post-column labeling method, Shimadzu Corp.). The quantity of 17 amino acids was analyzed using a fluorescence detector (Shimadzu, RF-10A) at 350 nm of excitation wavelength and 450 nm of emission wavelength. Data acquisition and analysis software were provided by the instrument manufacturer. A mixture of sodium citrate, ethanol and perchloric acid (60%) at pH 3.31 was employed as the mobile phase. The HPLC was operated at an oven temperature of 333 K and a 0.6 ml min−1 flow rate of mobile phase with 45 min run times.
Organic acid HPLC (SCL-10A, Shimadzu Corp.) was used to determine the quantities of 7 organic acids with two serial ion-exclusion chromatography columns (Shim-pack SCR-102H, id 0.008 m × 0.3 m) equipped with a guard column (SCR-102H, id 0.006 m × 0.05 m) and a post-column pH-buffered electroconductivity detection (Shimadzu, CDD-6A). The mobile phase was 5 mM of p-toluenesulfonic acid solution at a flow rate of 0.8 ml min−1. A mixture of 5 mM p-toluenesulfonic acid, 20 mM of bis-tris and 100 mM of EDTA was used as post column reagent at a flow rate of 0.8 ml min−1. The column temperature was kept at 318 K.
To obtain total organic carbon (TOC) of the products, total carbon (TC), and inorganic carbon (IC) in aqueous samples were measured with a TOC analyzer (TOC-500, Shimadzu Corp.). Air was used as both a sparging and carrier gas at 150 ml min−1. The aqueous phase (0.01 cm3) was injected into the high temperature combustion tubes (953 K) prior to entering the infrared detector.
Carbon, hydrogen, nitrogen, and sulfur content in the raw scallop wastes and solid residual was measured with a CHNS corder (Perkin Elmer 2400). The samples were dried in an oven (348 K) for 2 days prior to the CHNS analysis.
Results and discussion
Total yields of products as a function of reaction temperature and time
The temperature–time profiles of scallop waste inside the reactor demonstrated that after immersing the reactor into the salt bath, about 30 s is required to reach the expected reaction temperature. After the reaction, three phases of aqueous, oil, and fat were produced. Some parts of the wastes remained as a solid residual. The total amount of gases (such as CO2, H2O, N2 and O2) produced during sub-critical water reaction was very small and negligible. Fig. 1 shows photographs of the reaction products of scallop wastes at 553 K for different reaction times in the range of 1–40 min, respectively, compared to the one before the reaction. The changing of the color in the aqueous phase from dark to light brown with increasing reaction time means that the amount of soluble proteins and other organic compounds has gradually decreased in the aqueous phase. On the other hand, the amount of oil phase has increased with time. Fig. 2 illustrates the time course of the solid residual and the yields of fat and oil. The solid has decreased in volume due to the decomposition of the solid-proteins and the extraction of fat and oil. The yield of the fat phase has also decreased along with increasing of the oil phase. Fig. 3 demonstrates the effect of temperature on the solid residual and the yields of fat and oil. An increase in the temperature caused a dramatic decrease in the fat yield and the solid residual whereas the oil yield increased. As shown in these figures, the fat phase exhibited the largest yield among the three phases at 443 K, and through rising temperature this phase decomposed and some of its parts were converted to the oil-phase. Since oil and fat contain fatty acids, such as DHA and EPA, those phases could be considered as important sources to produce not only energy but also variable materials. The amount of solid residual, which were unreacted wastes, reached a plateau around 500 K. | ||
| Fig. 1 Photographs of the reaction products of the scallop wastes reacted at 553 K for different reaction times. | ||
 | ||
| Fig. 2 Time course of solid residual and yield of fat, and oil phases at 553 K. | ||
 | ||
| Fig. 3 Effect of reaction temperature on solid residual and yield of fat, and oil phases for a reaction time of 5 min. | ||
The contents of carbon, hydrogen, nitrogen, sulfur, oxygen, and ash in the dry raw scallop wastes were found to be 48.55%, 7.56%, 9.94%, 3.18%, 20.8%, and 10% respectively. These values provide information on scallop waste structure and describe the many organic molecules inside the waste, which contains carboxylic groups, amino groups, sulfur bonds and so on. Table 1 illustrates the effect of reaction temperature on the CHNS ratios (number of atoms to carbon) in the solid residual and the fat phase at 5 min as ratios of H/C, N/C and S/C. The values of H/C in both solid and fat phases are largest at 443 K. This value in the solid residual at 443 K and the one before reaction is close to 2. This means that they are almost single bond solid proteins. The values of H/C in the solid and the fat phase have gradually decreased with rising temperature. Decreasing this ratio could be helpful to find the structure of solid residual at different reaction points. This may suggest that carbon components of the solid during the sub-critical water hydrolysis reaction have gradually moved to aqueous, fat and oil phases in the form of organic substances. The nitrogen ratio also decreased and the ratio of sulfur is almost constant.
| Reaction temperature/K | Solid/kg per kg carbon | Fat phase/kg per kg carbon | ||||
|---|---|---|---|---|---|---|
| Ratio of no. of atoms to carbon | Ratio of no. of atoms to carbon | |||||
| H/C | N/C | S/C | H/C | N/C | S/C | |
| 443 | 1.94 | 0.23 | 0.029 | 1.76 | 0.17 | 0.022 |
| 473 | 1.69 | 0.23 | 0.026 | 1.73 | 0.17 | 0.022 |
| 493 | 1.64 | 0.21 | 0.025 | 1.68 | 0.16 | 0.023 |
| 513 | 1.71 | 0.19 | 0.024 | 1.68 | 0.17 | 0.025 |
| 553 | 1.57 | 0.12 | 0.025 | — | — | — |
| 573 | 1.52 | 0.11 | 0.024 | 1.69 | 0.11 | 0.024 |
| 593 | 1.55 | 0.1 | 0.022 | 1.73 | 0.064 | 0.025 |
| 613 | 1.61 | 0.11 | 0.023 | 1.65 | 0.059 | 0.02 |
| 653 | 1.35 | 0.093 | 0.022 | 1.55 | 0.06 | 0.022 |
| 673 | 1.22 | 0.09 | 0.021 | 1.41 | 0.054 | 0.025 |
Fig. 4 illustrates the temperature course of the yields of TOC, TC, and IC at a reaction time of 5 min in the aqueous phase. The course of TOC and TC are very similar to the course of ion product of water (Kw) versus temperature. The TC and TOC course showed the peaks of 0.73 and 0.68 kg C per kg C respectively around 513–533 K, where the ion product of water gives a maximum value. This means that the organic wastes were mainly decomposed by the hydrolysis reaction. In addition to the ion product of water which supplies the ability for water to act as an acid catalyst and enhancing the reaction rate, the dielectric constant of water provides a wide range of polarity to extract organic compounds. In other words, since in the supercritical area the above-mentioned properties became very small, the yield of TOC decreased to its lowest amount due to the thermal destruction of the organic compounds to some refractory acids and gasses. The increase in IC (such as carbonic acid) with rising temperature indicates that the effect of thermal decomposition increases with an increase in temperature.
 | ||
| Fig. 4 Effect of temperature on total organic carbon (TOC), total carbon (TC) and inorganic carbon (IC) at a reaction time of 5 min in the aqueous phase. | ||
Fig. 5 shows the total yield of produced organic compounds, ammonia, and phosphoric acid in the aqueous phase against reaction temperature at 5 min. The production of organic compounds is evident from the total yield of amino acids and the total yield of organic acids, which as illustrated peaks at around 513 K (0.093 kg per kg dry wastes) and 573 K (0.076 kg per kg dry wastes) respectively. The total yield of amino, organic acids and ammonia in the aqueous phase showed a peak at around 513 K. Since the ion product of water shows a similar peak at around 513–533 K under saturated vapor pressure, these results suggest that 513 K is the most favorable temperature for hydrolysis of proteins leading to the production of amino acids. In temperatures above 513 K, the yields of amino acids decreased due to the decrease of the ion product of water. Some of them may decompose to organic acids. Because of these reactions, the total yield of organic acids showed a peak at around 573 K, which was higher than the peak of the total yield of amino acids. Phosphoric acid showed a peak at about 493 K. The yield of ammonia in the aqueous phase gradually increased to 653 K (0.0115 kg per kg dry wastes) as a result of amino acid decomposition.
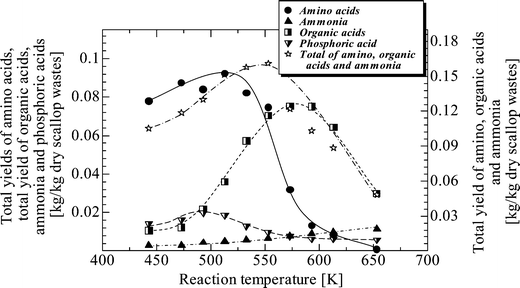 | ||
| Fig. 5 Effect of temperature on total yield of amino acids, total yield of organic acids, yield of ammonia, yield of phosphoric acid and total yield of amino, organic acids and ammonia at a reaction time of 5 min in the aqueous phase. | ||
The total yield of major compounds in the aqueous phase as a function of reaction time for the two reaction temperatures of 513 and 553 K are summarized in Table 2. At 513 K with an increase in time the total yields of amino and organic acids continually increased up to 50 min. That the yield of TOC reached a plateau after 3 min may be due to the equal appearance of compounds containing carbon in the aqueous phase, which were produced by the decomposition of solid proteins. At 553 K, the total yield of amino acids and TOC decreased after peaks at 5 min (0.085 kg per kg dry wastes and 0.69 kg C per kg C, respectively). The total yield of organic acids increased and reached a plateau above 10 min (0.077 kg per kg dry wastes). The total yield of amino, organic and phosphoric acids showed a similar curve to TOC. This means that at high reaction temperatures, the decomposition rate of soluble proteins to amino acids in the aqueous phase, which were produced by the decomposition of solid protein, was very fast. There may be little soluble proteins in the aqueous phase at high temperatures such as 553 K. The yield of phosphoric acid for both temperatures gradually decreased with time.
| Reaction temperature/K | Time/min | Total yield/kg per kg dry scallop wastes | ||||
|---|---|---|---|---|---|---|
| Amino acids | Organic acids | Phosphoric acid | Amino, organic, phosphoric acids | TOC | ||
| 513 (3.35 MPa) | 1 | 0.108 | 0.018 | 0.017 | 0.143 | 0.545 |
| 3 | 0.101 | 0.029 | 0.020 | 0.151 | 0.693 | |
| 5 | 0.093 | 0.037 | 0.019 | 0.149 | 0.686 | |
| 7 | 0.094 | 0.041 | 0.018 | 0.153 | 0.693 | |
| 10 | 0.097 | 0.046 | 0.017 | 0.161 | 0.684 | |
| 15 | 0.110 | 0.052 | 0.016 | 0.179 | 0.710 | |
| 20 | 0.115 | 0.054 | 0.016 | 0.186 | 0.690 | |
| 30 | 0.137 | 0.064 | 0.015 | 0.216 | 0.748 | |
| 40 | 0.141 | 0.065 | 0.014 | 0.219 | 0.681 | |
| 50 | 0.144 | 0.070 | 0.014 | 0.228 | 0.665 | |
| 553 (6.42 MPa) | 1 | 0.081 | 0.032 | 0.020 | 0.134 | 0.549 |
| 3 | 0.077 | 0.049 | 0.015 | 0.141 | 0.685 | |
| 5 | 0.085 | 0.059 | 0.015 | 0.159 | 0.687 | |
| 7 | 0.081 | 0.064 | 0.014 | 0.159 | 0.585 | |
| 10 | 0.076 | 0.067 | 0.013 | 0.156 | 0.546 | |
| 15 | 0.063 | 0.070 | 0.014 | 0.146 | 0.598 | |
| 20 | 0.058 | 0.073 | 0.011 | 0.141 | 0.527 | |
| 30 | 0.046 | 0.071 | 0.0094 | 0.126 | 0.539 | |
| 40 | 0.044 | 0.079 | 0.0097 | 0.133 | 0.534 | |
| 50 | 0.040 | 0.075 | 0.009 | 0.125 | 0.468 | |
The soluble protein composition in the aqueous phase was analyzed using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Many protein bands obtained include those at: 14, 29, 36, 45, and 66 kDa. Increasing both reaction temperature and time cause protein bands to decrease. The disappearance of these bands indicates that the protein molecules were decomposed to water-soluble low molecular weight organic compounds.
Organic acids and phosphoric acid productions in the aqueous phase
Fig. 6 shows the yields of the organic acids produced in the aqueous phase at a reaction time of 5 min, plotted against the reaction temperature. Pyroglutamic acid was predominant among organic acids and illustrated a peak at around 573 K (0.07 kg per kg dry wastes). This means that pyroglutamic acid is produced by the hydrolysis reaction of proteins. The yield of phosphoric acid showed a peak at around 493 K (0.02 kg per kg dry wastes) and then gradually decreased and reached a plateau above 573 K. On the other hand, acetic acid increased with an increase in temperature to 653 K (0.013 kg per kg dry wastes). The yield of formic and lactic acids was negligibly small. | ||
| Fig. 6 Effect of temperature course on the yields of organic acids and phosphoric acid for a reaction time of 5 min in the aqueous phase. | ||
Fig. 7 shows the time course of the yields of organic acids at 553 K. The yields of pyroglutamic, acetic and lactic acids increased and reached a plateau above 10–20 min of 0.068, 0.0054 and 0.0031 kg per kg dry wastes, respectively. This means that the hydrolysis reaction is fast. The yield of phosphoric acid gradually decreased with time.
 | ||
| Fig. 7 Effect of reaction time course on the yields of organic acids and phosphoric acid for a reaction temperature of 553 K (6.42 MPa) in the aqueous phase. | ||
Amino acids production in the aqueous phase
Fig. 8 shows the effect of temperature on amino acid yields at a reaction time of 5 min. Among amino acids, glycine was the most abundant, which dramatically decreased from 443 K (0.043 kg per kg dry wastes) with rising temperature. Fig. 8a illustrates amino acid yields which decreased with temperature from 443 K due to the decomposition to other organic compounds. As shown in Fig. 8b, the yield of amino acids exhibited a peak around 473–513 K, which suggests that those amino acids were produced from protein decomposition. The second largest yield among amino acids was alanine, which increased to a peak at around 513 K (0.013 kg per kg dry wastes) and then dramatically decreased. At 653 K almost no amino acids could be observed due to the complete destruction of organic compounds at this supercritical area.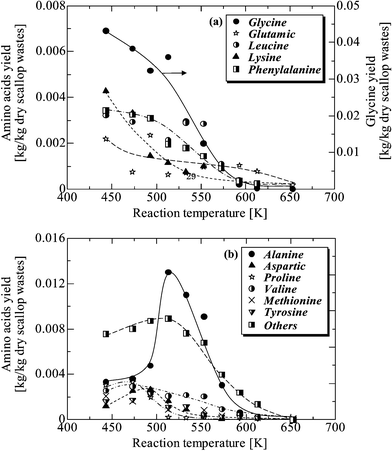 | ||
| Fig. 8 Effect of temperature course on the yields of amino acids for a reaction time of 5 min in the aqueous phase. | ||
The time course of amino acids at 513 K (the temperature of maximum total yield of amino acids in Fig. 5) was further studied and the results are represented in Table 3. With the exception of glycine, valine, glutamic and argentine, the other amino acids gradually increased with time up to 50 min. Glycine with the highest yield among amino acids decreased from 1 min (0.048 kg per kg dry waste), and then started to increase after 10 min and reached a plateau (0.040 kg per kg dry waste) after 30 min. Valine also showed similar behavior in the first 10 min and then gradually increased to 50 min (0.009 kg per kg dry waste). The decrease of these two amino acids in the period of the first 10 min may be due to their hydrophobic property, which hinders them being released from the internal part of the protein during water hydrolysis. The yield of glutamic acid demonstrated a gradual fall from 1 min (0.0044 kg per kg dry waste) due to the dehydration to pyroglutamic acid. On the other hand, the yields of alanine, cystine and leucine showed values of 0.031, 0.013 and 0.014 kg per kg dry waste at a reaction time of 50 min, respectively.
| Reaction time/min | Amino acid yield/kg per kg dry scallop wastes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycine | Alanine | Cystine | Valine | Leucine | Phenylalanine | Hystidine | Lysine | Glutamic | Argenine | Others | |
| 1 | 0.048 | 0.008 | 0.0013 | 0.0046 | 0.0038 | 0.0044 | 0.0046 | 0.0036 | 0.0044 | 0.0072 | 0.017 |
| 3 | 0.044 | 0.012 | 0.0021 | 0.0049 | 0.0041 | 0.0047 | 0.0048 | 0.0038 | 0.0019 | 0.0058 | 0.013 |
| 5 | 0.037 | 0.014 | 0.0034 | 0.0044 | 0.0042 | 0.0043 | 0.0048 | 0.0034 | 0.0010 | 0.0051 | 0.012 |
| 7 | 0.035 | 0.016 | 0.0043 | 0.0044 | 0.0052 | 0.0044 | 0.0051 | 0.0036 | 0.0007 | 0.0043 | 0.011 |
| 10 | 0.033 | 0.018 | 0.0059 | 0.0047 | 0.0062 | 0.0046 | 0.0054 | 0.0033 | 0.0005 | 0.0038 | 0.011 |
| 15 | 0.034 | 0.022 | 0.0085 | 0.0055 | 0.0083 | 0.0053 | 0.0061 | 0.0036 | 0.0004 | 0.0035 | 0.012 |
| 20 | 0.037 | 0.025 | 0.0099 | 0.0066 | 0.0093 | 0.0054 | 0.0058 | 0.0041 | 0.0003 | 0.0026 | 0.010 |
| 30 | 0.039 | 0.030 | 0.0133 | 0.0084 | 0.0123 | 0.0068 | 0.0069 | 0.0049 | 7.7 e-5 | 0.0017 | 0.012 |
| 40 | 0.040 | 0.030 | 0.0128 | 0.0085 | 0.0129 | 0.0069 | 0.0065 | 0.0056 | 7.6 e-5 | 0.0011 | 0.015 |
| 50 | 0.041 | 0.031 | 0.0133 | 0.0086 | 0.0135 | 0.0071 | 0.0065 | 0.0058 | 6.8 e-5 | 0.0004 | 0.016 |
Material balance
Based on 100 tonnes of scallop wastes with water content of 83.8%, the overall mass balance is shown in Fig. 9. The major concern is to find the optimum condition for protein hydrolysis, which mainly goes to amino and organic acid production. | ||
| Fig. 9 Material balance for sub-critical water treatment of scallop viscera wastes. | ||
For the first stage, 513 K and 50 min was selected due to the high yield of organic and amino acids. The solid residual was continuously processed at 553 K and 50 min until no more un-reacted solid remained. A part of the scallop wastes may also be converted to some heavy compounds such as very heavy oils and carbon, which may adhere to the reactor wall. The estimated material balance showed that at least 88% of scallop wastes were converted to valuable materials under the optimum condition.
Besides hydrolysis reaction, separation and purification of the organic compounds in the aqueous phase reveals the central position of this study. The separating of amino and organic acids in single or binary systems has been already reported by one of the authors using adsorption/ion exchange technology.30–34 However, separation of multicomponent organic compounds will be reported in the subsequent stages of this research.
Conclusions
Hydrolysis in sub-critical water has been experimentally proven to be an effective way to convert scallop viscera wastes to valuable materials. Based on our experimental results, glycine was predominant among amino acids and pyroglutamic acid was the most abundant among organic acids. At 513 K and 50 min, the maximum yield of amino acids (0.15 kg per kg dry scallop waste) was obtained, while maximum organic acids were found at 553 K and 40 min (0.08 kg per kg dry scallop waste). Fat and oil produced by this method could be considered as valuable materials because they may be used as an energy source. The material balance based on the analytical results was estimated to be at least 88% of conversion from scallop wastes. This experimental finding leads to a new and greener method for the effective conversion of scallop wastes to useful compounds with significantly lower, and perhaps no, environmental emission. Applications of this technology in the fishery industry could have great potential economically and environmentally.Acknowledgements
A part of this research funding was provided by the Ministry of Education, Culture, Sports, Science and Technology of Japan in the form of the 21st Century COE Program (E-1), entitled “Science and Engineering for Water-Assisted Evolution of Valuable Resources and Energy from Organic Wastes”.The authors would like to thank Mrs Yoshie Hirata, research assistant in the Department of Chemical Engineering, Osaka Prefecture University for her helpful support with some of the analytical work.
References
- N. Yoshii, S. Miura and S. Okazaki, Chem. Phys. Lett., 2001, 345(1–2), 195 CrossRef CAS.
- D. Laria, J. Marti and E. Guardia, J. Am. Chem. Soc., 2004, 126(7), 2125 CrossRef CAS.
- K. Tomita and Y. Oshima, Ind. Eng. Chem. Res., 2004, 43(10), 2345 CrossRef CAS.
- T. Yagasaki, K. Iwahashi, S. Saito and I. Ohmine, J. Chem. Phys., 2005, 122(14), Art No. 144504.
- M. Dyoulgerov, Ocean Coastal Manage., 1998, 39(3), 265 Search PubMed.
- Protocol to the Convention on the Prevention of Marine Pollution by Dumping of Wastes and Other Matter, www.londonconvention.org/documents/meetings/consultative/26th/15.pdf, accessed on August 30, 2005.
- T. M. Hayward, I. M. Svishchev and R. C. Makhija, J. Supercrit. Fluids, 2003, 27, 275 CrossRef CAS.
- F. Vogel, K. A. Smith, J. W. Tester and W. A. Peters, AIChE J., 2002, 48(8), 1827 CrossRef CAS.
- M. Goto, T. Nada, A. Kodama and T. Hirose, Ind. Eng. Chem. Res., 1999, 38(5), 1863 CrossRef CAS.
- H. Yoshida, Y. Takahashi and M. Terashima, Biotechnol. Prog., 1999, 15(6), 1090 CrossRef CAS.
- H. Yoshida, Y. Takahashi and M. Terashima, Jpn. Soc. Waste Manag. Experts, 2001, 12, 163 Search PubMed.
- H. Yoshida, Y. Takahashi and M. Terashima, J. Chem. Eng. Jpn., 2003, 36(4), 441 Search PubMed.
- H. Yoshida and O. Tavakoli, J. Chem. Eng. Jpn., 2004, 37(2), 253 Search PubMed.
- O. Tavakoli and H. Yoshida, Environ. Sci. Technol., 2005, 39(7), 2357 CrossRef CAS.
- H. Yoshida and T. Nakahashi, Proceedings of the 10th APCChE Congress, November, 2004, 3P-03-026 Search PubMed.
- H. Yoshida and Y. Katayama, Proceedings of the 10th APCChE Congress, November, 2004, 3P-03-025 Search PubMed.
- H. Daimon, K. Kang, N. Sato and K. Fujie, J. Chem. Eng. Jpn., 2001, 34(9), 1091 Search PubMed.
- A. T. Quitain, M. Faisal, K. Kang, H. Daimon and K. Fujie, J. Hazard. Mater., 2002, 93(2), 209 CrossRef CAS.
- K. Y. Kang and B. S. Chun, Korean J. Chem. Eng., 2004, 21(6), 1147 Search PubMed.
- M. Goto, R. Obuchi, T. Hiroshi, T. Sakaki and M. Shibata, Bioresour. Technol., 2004, 93(3), 279 CrossRef CAS.
- A. Shanableh, Water Res., 2000, 34, 945 CrossRef CAS.
- B. M. Kabyemela, T. Adschiri, R. M. Malaluau and K. Arai, Ind. Eng. Chem. Res., 1999, 38, 2888 CrossRef CAS.
- M. Sasaki, Z. Fang, Y. Fukushima, T. Adschiri and K. Arai, Ind. Eng. Chem. Res., 2000, 39, 2883 CrossRef CAS.
- M. Sasaki, T. Adschiri and K. Arai, AIChE J., 2004, 50(1), 192 CrossRef CAS.
- N. Sato, A. T. Quitain, K. Kang, H. Daimon and K. Fujie, Ind. Eng. Chem. Res., 2004, 43(13), 3217 CrossRef CAS.
- J. D. Taylor, J. I. Steinfeld and J. W. Tester, Ind. Eng. Chem. Res., 2001, 40, 67 CrossRef CAS.
- B. Myrnes and A. Johansen, Prep. Biochem., 1994, 24(1), 69 Search PubMed.
- I. Sarashina and K. Endo, Am. Mineral., 1998, 83, 1510 CAS.
- J. N. C. Whyte, N. Bourne and N. G. Ginther, J. Exp. Mar. Biol. Ecol., 1991, 149(1), 67 CrossRef.
- H. Yoshida, O. Okamoto, H. Jitsukawa and T. Shigeta, Fundam. Adsorpt., 2002, 7, 520 Search PubMed.
- H. Yoshida and W. A. Galinada, AIChE J., 2002, 48(10), 2193 CrossRef CAS.
- W. Takatsuji and H. Yoshida, AIChE J., 1998, 44(5), 1216 CrossRef CAS.
- W. Takatsuji and H. Yoshida, AIChE J., 1998, 37(4), 1300 CAS.
- H. Yoshida, Y. Saiki and E. Kamio, Proceedings of the 10th APCChE Congress, November, 2004, 3P-12-056 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
