Arsenic exposure in Hungary, Romania and Slovakia
Anna-Lena
Lindberg
a,
Walter
Goessler
b,
Eugen
Gurzau
c,
Kvetoslava
Koppova
d,
Peter
Rudnai
e,
Rajiv
Kumar
f,
Tony
Fletcher
g,
Giovanni
Leonardi
g,
Katarina
Slotova
d,
Emilia
Gheorghiu
c and
Marie
Vahter
*a
aInstitute of Environmental Medicine, Karolinska Institute, Box 210, SE-171 77 Stockholm, Sweden. E-mail: Marie.Vahter@imm.ki.se; Fax: +46 8 33 70 39; Tel: +46 8 524 875 40
bInstitut für Chemie – Analytische Chemie, Karl-Franzens-Universität, Graz, Austria
cEnvironmental Health Centre, Cluj-Napoca, Romania
dState Health Institute, Banska Bystrica, Slovakia
e‘Jozef Fodor’ National Centre of Public Health, Budapest, Hungary
fDKFZ, German Cancer Research Centre, Heidelberg, Germany
gLondon School of Hygiene & Tropical Medicine, London, UK
First published on 1st December 2005
Abstract
Inorganic arsenic is a potent human carcinogen and toxicant which people are exposed to mainly via drinking water and food. The objective of the present study was to assess current exposure to arsenic via drinking water in three European countries. For this purpose, 520 individuals from four Hungarian, two Slovakian and two Romanian countries were investigated by measuring inorganic arsenic and methylated arsenic metabolites in urine by high performance liquid chromatography with hydride generation and inductively coupled plasma mass spectrometry. Arsenic in drinking water was determined by atomic absorption spectrometry. Significantly higher concentrations of arsenic were found in both the water and the urine samples from the Hungarian counties (median: 11 and 15 μg dm−3, respectively; p < 0.001) than from the Slovakian (median: 0.94 and 4.5 μg dm−3, respectively) and Romanian (median: 0.70 and 2.1 μg dm−3, respectively) counties. A significant correlation was seen between arsenic in water and arsenic in urine (R2 = 0.46). At low water arsenic concentrations, the relative amount of dimethylarsinic acid (DMA) in urine was increased, indicating exposure via food. Also, high body mass index was associated with higher concentrations of arsenic in urine (p = 0.03), mostly in the form of DMA. Smokers had significantly higher urinary arsenic concentrations than non-smokers (p = 0.03). In conclusion, elevated arsenic exposure via drinking water was prevalent in some of the counties. Exposure to arsenic from food, mainly as DMA, and cigarette smoke, mainly as inorganic arsenic, are major determinants of arsenic exposure at very low concentrations of arsenic in drinking water.
Introduction
Inorganic arsenic (iAs) is a potent human carcinogen and toxicant.1 Epidemiological studies have shown associations between arsenic in drinking water and different forms of cancer, skin lesions, vascular diseases and diabetes mellitus.2 People are exposed to arsenic mainly via drinking water and food. Millions of people in many parts of the world are exposed to arsenic in drinking water, often naturally occuring in ground water, exceeding the guideline of 10 μg As dm−3 recommended by the World Health Organization (WHO).3 Arsenic appear in water almost exclusively as arsenite (As(III)) and arsenate (As(V)),2 whereas seafood and seaweed contain mainly organic arsenic compounds, such as arsenobetaine and arsenosugars.4 Other foods may contain both inorganic and organic arsenic species, although usually at relatively low concentrations5 with the exception of rice.6,7 Elevated concentrations of arsenic in food may also be the concequence of irrigation or industrial pollution.8Exposure to iAs is often assessed by the concentrations in water. However, due to variation in water intake, the actual absorbed amount of arsenic is difficult to evaluate based on the water concentration.9 Individual exposure to iAs may be assessed by concentrations of arsenic in urine, the main route of excretion. The metabolism of iAs in the human body involves alternating reduction and oxidative methylation reactions.10 The urinary excretion consists in general of 10–30% iAs, (As(III) and As(V)), 10–20% methylarsonic acid (MA) and 60–80% dimethylarsinic acid (DMA) and these forms are measured for assessment of iAs exposure.10 Experimental studies have shown that arsenosugars in seafood, and edible seaweed and algae are partly metabolized to DMA in the human body, leading to increased concentration of DMA and other arsenic compounds in urine.11–16 This DMA might be misinterpreted as originating from iAs exposure. The contribution from food to the urinary concentration of arsenic metabolites in the general population has not been evaluated so far. In the present work we investigated the current exposure to arsenic via drinking water and food in four Hungarian, two Slovakian, and two Romanian counties by determination of arsenic concentrations in water and arsenic metabolites in urine.
Experimental
Study population
This study is part of a large case-control study of cancer risks in relation to arsenic exposure via drinking water in Central Europe: “ASHRAM—Arsenic Health Risk Assessment and Molecular Epidemiology”. Study areas were defined as certain counties in Hungary (Bacs, Békés, Csongrad and Jazs-Nagykun-Szolnok), Romania (Bihor and Arad) and Slovakia (Banská Bystrica and Nitra) with known hotspots of arsenic in drinking water. New cases of skin, bladder and kidney cancer, along with hospital controls (N = 537), were invited to participate in the study. Hospital controls, aged 30–79 years old, were general surgery in-patients with appendicities, abdominal hernias, duodenal ulcer or cholelithiasis and orthopaedic and traumatology patients with fractures. A detailed structured interview was conducted spanning the residential and occupational drinking water histories, dietary habits, lifestyle, smoking, and potential confounders for the selected cancers. Informed consent was obtained from the participants. The study was approved by each separate hospital’s ethics committee. In the present study only the controls, 276 men and 261 women, were selected for evaluation of the exposure to arsenic in the above mentioned counties in Hungary, Romania and Slovakia. This was done to eliminate any potential bias that the cancer cases might lead to. Characteristics of the participants are presented in Table 1. In the present study individuals that have smoked more than 100 cigarettes during their lifetime were regarded as life-time smokers, all the other participants were regarded as non-smokers.| Hungary | Romania | Slovakia | ||||||
|---|---|---|---|---|---|---|---|---|
| Bacs | Békés | Csongrad | J-N-Sa | Bihor | Arad | B Bb | Nitra | |
| a Jasz-Nagykun-Szolnok. b Banská Bystrica. | ||||||||
| Gender/N | ||||||||
| Men | 50 | 12 | 33 | 33 | 28 | 46 | 35 | 39 |
| Women | 49 | 11 | 28 | 31 | 28 | 54 | 34 | 29 |
| Age/years | ||||||||
| Mean | 60 | 64 | 62 | 58 | 60 | 61 | 60 | 59 |
| σ | 12 | 9.2 | 11 | 12 | 11 | 13 | 10 | 12 |
| BMI (%) | ||||||||
| <18.5 | 2.0 | 4.4 | 1.7 | 3.1 | 1.8 | 2.0 | 1.5 | 0.0 |
| 18.5–25 | 27 | 22 | 28 | 44 | 36 | 49 | 30 | 37 |
| 26–30 | 39 | 48 | 42 | 38 | 45 | 30 | 36 | 43 |
| >30 | 31 | 26 | 28 | 16 | 18 | 19 | 32 | 20 |
| Non-smokers (%) | 43 | 61 | 56 | 45 | 61 | 64 | 42 | 49 |
| Smokers (%) | 57 | 39 | 44 | 55 | 39 | 36 | 58 | 51 |
Water samples
Water samples were collected in 60 cm3 polyethylene (PE) containers. Prior to sampling the water was flushed and the containers were rinsed twice with the water to be sampled. 0.1 cm3 of analytical grade HNO3 was added to the water collected on the day of sampling to prevent precipitation of iron and coprecipitation of arsenic. The water samples were stored at 4 °C until analysis.Urine samples
Spot urine samples were collected within 6 hours of patient arrival in the hospital. The urine samples were collected in containers routinely used by the hospitals. Hospital staff then transferred the collected urine into three 30 cm3 polyethylene (PE) containers. Immediately thereafter the samples were stored in a freezer at −20 °C or lower. During all the transport samples were kept frozen.Determination of total arsenic concentrations in water
The concentration of iAs in water was determined with an atomic absorption spectrometer (Varian 110, Palo Alto, CA) equipped with the vapor generation system VGA 77. The spectrometer was operated at 193.7 nm with a slit width of 0.5 nm. The lamp current was set 10 mA. The air flow was 3.5 dm3 min−1 and the acetylene flow was 1.5 dm3 min−1. NaOH (pellets), HCl (37%), HNO3 (65%), KI (p.a.), NaBH4 and ascorbic acid (all analytical grade) were purchased from Merck (Darmstadt, Germany). 45 minutes prior to the arsenic determinations, 5 cm3 of the water samples, or water samples diluted to 5 cm3, and the standards were mixed with 2.5 cm3 HCL and 2.5 cm3 of an aqueous 5% KI/ascorbic acid (m/v) for reduction of As(V). Hydride generation (HG) was preformed with 0.6% NaBH4 dissolved in 0.5% NaOH and 5 mol dm−3 HCl. The hydrides were then transported to a heated quartz cell and the atomic absorption of the analyte was measured. The NIST SRM 1643d (trace elements in water) was used for quality control. With this method a determination limit of 0.2 μg dm−3 was obtained.Determination of arsenic compounds with HPLC-HG-ICPMS
For the determination of the arsenic metabolites in urine an inductively coupled plasma mass spectrometer (ICPMS; HP 4500 or Agilent 7500cs, Agilent Technologies, Waldbronn, Germany) equipped with an ISIS and an HG accessory together with an Agilent 1100 chromatographic system equipped with solvent degasser, autosampler, and a thermostatted column were used.17 The ICPMS was operated at 1500 W with a reflected power <5 W. The cooling gas flow was 15 dm3 min−1, the auxiliary gas flow 1 dm3 min−1, the carrier gas 0.35 dm3 min−1, and the make up gas 0.75 dm3 min−1. The instrument was optimised daily to give a maximum signal for arsenic in the mobile phase. The HG conditions were: 3 mol dm−3 HCl and 0.7% NaBH4. The flow rates were 0.5 cm3 min−1 for NaBH4, 0.3 cm3 min−1 for HCl, and 1.5 cm3 min−1 for the HPLC system. For the separation of As(III), DMA, MA, and As(V) a Hamilton PRP-X100 anion-exchange column (4.6 mm × 250 mm) was used. A 20 mmol dm−3 ammonium phosphate buffer pH 5.0 with 5% MeOH (v/v) was used as mobile phase. The column temperature was 40 °C and 20 mm3 of the standard/sample was injected. With the ICPMS m/z 75 and 77 were monitored. All the chemicals used, NaOH, HCl (37%), NaBH4, aqueous ammonia and formic acid, were purchased from Merck (Darmstadt, Germany) at the highest purity available. Standard solutions containing 1000 mg As dm−3 each from the following compounds were prepared with Milli-Q water (18.2 MOhm cm): arsenous acid [As(III) as NaAsO2] and arsenic acid [As(V) as Na2HAsO4·7H2O] were purchased from Merck (Darmstadt, Germany); sodium dimethylarsinate trihydrate (DMA) was purchased from Fluka (Buchs, Switzerland). Methylarsonic acid (MA) was synthesised from As2O3 with CH3I in NaOH (Meyer’s reaction).18 The recrystallized final product had a melting point of 159 °C. Before the analysis the frozen urine samples were brought to room temperature. After vigorous shaking approximately 2 cm3 of the urine was filtered through a 0.22 μm nylon filter (CAMEO 25 NS) and filled into the autosampler vials of the HPLC-system. In an aliquot of the urine samples glucose and the pH was determined with Combur-4-N-Test®, (Roche Austria GmbH, Vienna, Austria) test strips. The arsenic concentrations in urine were adjusted to the average specific gravity in the population (1.017 g cm−3; Leica TS 400 Refractometer, Leica Microsystems Inc., Buffalo, NY, USA). For quality control NIEHS (Ref. Urine 18), NIST 1640 (reference water), and spiked urine samples also used for round robin exercises in this project were employed. With this method the following determination limits were obtained 0.1 μg As dm−3 As(III), DMA, MA, and 0.5 μg As dm−3 As(V).Statistical analyses
Statistica 7.1 for Windows (StatSoft. Inc., Tulsa, OK, USA) was used to perform the statistical analyses. Arsenic concentrations in water and urine were not normally distributed. When linear regression was performed between arsenic in water and urine the water concentrations were transformed to 0.5 power and the urinary arsenic concentrations were ln-transformed to meet the requirement of equal variance and normal distribution of residuals. The Spearman correlation test (rs) was used when testing for associations between variables. Non-parametric tests, the Mann–Whitney U test and the Kruskal–Wallis test were used when testing for differences between groups. Values of p < 0.05 were used for statistical significance.Results
Urine from 276 men and 261 women as well as 530 water samples from their main, currently used water sources were analyzed for arsenic. In all countries there were about equal numbers of men and women among the participants, except in the county of Nitra where 40% of the participants were women. The participants were 60 years old, on average, with area means ranging from 58 years in Jasz-Nagykun-Szolnok to 64 years in Békés (Table 1). Concentrations of arsenic in water and urine (iAs, MA, DMA) by country and county are shown in Table 2 and Table 3, respectively. There were significantly higher concentrations of arsenic in both water and urine in the Hungarian study areas compared to the Romanian and Slovakian areas (p < 0.001).| Hungary | Romania | Slovakia | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bacs | Békés | Csongrad | J-N-Sa | Bihor | Arad | B Bb | Nitra | ||
| a Jasz-Nagykun-Szolnok. b Banská Bystrica. c U-As is the sum of iAs, MA and DMA in urine. d iAs is the sum of As(III) and As(V). | |||||||||
| N | 93 | 21 | 59 | 60 | 55 | 98 | 69 | 65 | |
| U-Asc | Median | 12 | 16 | 32 | 11 | 2.1 | 2.1 | 4.2 | 5.0 |
| 10th perc. | 3.7 | 9.5 | 11 | 4.0 | 0.63 | 0.82 | 1.3 | 2.0 | |
| 90th perc. | 31 | 41 | 71 | 30 | 7.1 | 7.5 | 13 | 11 | |
| Max. | 84 | 140 | 113 | 94 | 21 | 38 | 33 | 83 | |
| DMA(V) | Median | 8.3 | 12 | 22 | 7.7 | 1.6 | 1.5 | 3.0 | 3.8 |
| 10th perc. | 2.4 | 6.3 | 6.9 | 2.9 | 0.47 | 0.79 | 1.2 | 1.6 | |
| 90th perc. | 21 | 34 | 47 | 21 | 6.2 | 5.1 | 10 | 9.5 | |
| Max. | 66 | 98 | 81 | 70 | 20 | 28 | 31 | 81 | |
| MA(V) | Median | 1.8 | 2.7 | 5.0 | 1.7 | 0.27 | 0.40 | 0.60 | 0.68 |
| 10th perc. | 0.52 | 1.6 | 1.7 | 0.51 | 0 | 0 | 0.10 | 0.12 | |
| 90th perc. | 6.7 | 7.2 | 16 | 7.5 | 0.92 | 0.72 | 1.9 | 1.6 | |
| Max. | 21 | 33 | 26 | 23 | 2.4 | 7.4 | 5.3 | 3.0 | |
| iAsd | Median | 1.1 | 1.2 | 3.5 | 1.1 | 0.23 | 0.16 | 0.23 | 0.29 |
| 10th perc. | 0.23 | 0.59 | 0.77 | 0.28 | 0 | 0 | 0 | 0 | |
| 90th perc. | 3.5 | 3.6 | 7.7 | 3.5 | 0.92 | 0.72 | 0.91 | 0.70 | |
| Max. | 5.9 | 9.1 | 62 | 10 | 2.5 | 2.6 | 1.6 | 1.2 | |
We found a significant correlation between arsenic in drinking water and urine, although with a large variation (linear regression: ln [U-As] = 0.88 + 0.44 ; R2
= 0.46; p < 0.001; Fig. 1). The intercept in the regression equation between water and urine concentrations of arsenic indicates a background level of 2.5 μg dm−3 in urine. The relative amount of DMA was significantly higher below the background level (mean: 80 ± 15%; median: 80%; N
= 127) than above (mean: 73 ± 12%; median: 74%; N
= 393; p < 0.001). There were 26 individuals, all from the same town in Csongrad county, drinking water from the same well (39 μg dm−3). They showed a large variation in urinary excretion of arsenic (range: 13–113 μg dm−3). Excluding these individuals did not change the correlation between arsenic in water and urine. Neither did exclusion of the three individuals with the highest water concentrations.
; R2
= 0.46; p < 0.001; Fig. 1). The intercept in the regression equation between water and urine concentrations of arsenic indicates a background level of 2.5 μg dm−3 in urine. The relative amount of DMA was significantly higher below the background level (mean: 80 ± 15%; median: 80%; N
= 127) than above (mean: 73 ± 12%; median: 74%; N
= 393; p < 0.001). There were 26 individuals, all from the same town in Csongrad county, drinking water from the same well (39 μg dm−3). They showed a large variation in urinary excretion of arsenic (range: 13–113 μg dm−3). Excluding these individuals did not change the correlation between arsenic in water and urine. Neither did exclusion of the three individuals with the highest water concentrations.
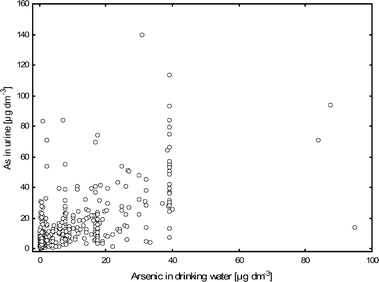 | ||
| Fig. 1 Relationship between urinary arsenic concentrations (sum of iAs, MA and DMA, μg dm−3) and water arsenic concentrations in the studied individuals in Hungary, Romania and Slovakia. | ||
The distribution of arsenic concentrations in water and urine of all individuals by gender, age, body mass index (BMI; calculated as the body weight in kilograms divided by the square of the length in metres) and smoking habits is shown in Table 4. There was no difference in urinary or water arsenic concentrations between the different age groups. Also, high BMI was associated with higher concentrations of arsenic in urine, but not with higher water arsenic concentrations. Smokers had significantly higher concentrations of arsenic in urine, but not in water, than non-smokers.
| N | U-Asa/μg dm−3 | p value | W-As/μg dm−3 | p value | |||
|---|---|---|---|---|---|---|---|
| Median | 90th perc. | Median | 90th perc. | ||||
| a U-As is the sum of iAs, MA and DMA in urine. b 18.5–25 vs. >30, p < 0.01. | |||||||
| Gender | |||||||
| Men | 269 | 6.2 | 40 | 1.6 | 30 | ||
| Women | 251 | 5.8 | 28 | 0.05 | 1.1 | 21 | 0.2 |
| Age/years | |||||||
| <55 | 164 | 6.3 | 30 | 1.0 | 26 | ||
| 55–67 | 189 | 7.1 | 32 | 2.0 | 24 | ||
| >67 | 167 | 5.1 | 37 | 0.5 | 1.0 | 27 | 0.4 |
| BMI/kg m−2 | |||||||
| 18.5–25 | 181 | 4.6 | 31 | 1.0 | 19 | ||
| 26–30 | 204 | 6.5 | 31 | 1.5 | 26 | ||
| >30 | 125 | 7.6b | 33 | 0.03 | 1.7 | 31 | 0.5 |
| Non-smokers | 268 | 5.5 | 30 | 1.5 | 26 | ||
| Smokers | 252 | 6.5 | 37 | 0.03 | 1.3 | 26 | 0.8 |
Discussion
The results of the present study show a wide variation in arsenic exposure in some areas of Europe, previously identified as areas with elevated arsenic in drinking water.19–21 In the Romanian and Slovakian study areas water arsenic concentrations were relatively low with, at the most, 8% of the drinking water concentrations exceeding the European Union drinking water standard of 10 μg As dm−3.22 However, in the Hungarian study areas nearly 70% of the studied individuals drank water with arsenic concentrations exceeding 10 μg dm−3, except in Bacs where it was 30%. Although, the found concentrations are lower than previously reported, indicating that the exposure has decreased in the study areas, many water sources still exceeded 10 μg As dm−3. Thus, further mitigation and follow up measures are needed.As a measure of current individual exposure, we used arsenic concentrations in urine. In accordance with the higher water arsenic concentrations in the Hungarian study areas, the concentrations in urine were generally above 10 μg As dm−3 (overall median: 15 μg As dm−3). In the Romanian study areas the concentrations of arsenic in urine were generally below 7 μg As dm−3 and in the Slovakian study areas below 12 μg As dm−3, with averages of 2–5 μg As dm−3. The present study is the first, to our knowledge, to speciate the urinary arsenic metabolites at these low concentrations. The intercept in the regression equation between arsenic concentrations in urine and water (water = 0 μg As dm−3) indicates a background level of about 2.5 μg As dm−3 in urine. Previously it has been reported that people who are not exposed to elevated arsenic levels via water and seafood in general have concentrations between 5–10 μg As dm−3 in urine.23–27 A probable explanation for this discrepancy is that the analytical method in this study has lower determination limits than methods previously used. Most likely, the background exposure comes mainly from food, since arsenic levels in air are generally low and of minor importance in comparison to oral exposure.21 A further support is that individuals with urinary concentrations below the background level of 2.5 μg As dm−3 had higher relative amounts of DMA in their urine and lower relative amounts of MA or iAs. One part of arsenic in food is in the form of DMA or arsenosugars, which partly are metabolized to DMA in the body.11–16 Yet another support of arsenic exposure from food is that individuals with a higher BMI had higher urinary arsenic concentrations, mostly in the form of DMA (mean% DMA was 62, 75, 74 and 77% for BMI < 18.5, 18.5–25, 26–30 and >30 kg m−2, respectively). It seems likely that individuals with a higher BMI eat more and therefore are exposed to higher amounts of arsenic, mainly as DMA, via food. There was no difference in water arsenic concentrations between individuals with different BMI.
Some common food items (bread, rice, milk, pork meat, chicken meat, cabbage and potatoes) from the Slovakian study area were collected and analyzed for total arsenic concentrations (unpublished data). Rice contained the highest concentration of arsenic (mean = 158 ng As g−1, N = 12), which is in accordance with previous studies (averages between 100–950 ng As g−1).7 The major part of the arsenic in rice seems to be iAs and DMA.7 Also, potatoes (33 ng As g−1, N = 12) and poultry meat (mean = 28 ng As g−1, N = 12) contained considerable amount of arsenic, although arsenobetaine accounted for more than 80% in the poultry meat. This arsenobetaine may originate from fishmeal, which is commonly used as protein source in feed for poultry and pig.28 When the potatoes were peeled the concentrations of arsenic were lower (mean = 2.3 ng As g−1, N = 9), probably due to the presence of arsenic in soil particles on the potato peel.
A significant correlation was found between arsenic concentrations in water and urine, as seen in several previous studies.9,26,29,30 The ratios between arsenic concentrations in urine and water were close to 1 ∶ 1 in the Hungarian study areas, in line with previous studies on people exposed to water concentrations exceeding 50 μg As dm−3.26,29–32 However, the ratios were 3 ∶ 1 and 5 ∶ 1, respectively, in the Romanian and Slovakian study areas, where the water arsenic concentrations were much lower. There was a wide variation in the urine concentrations at one and the same water concentration (Fig. 1), which probably can be explained by a large inter-individual variation in the amount of local water consumed and the consumption of other waters and beverages. Furthermore, differences in excretion might also be due to metabolism which has been shown to influence the retention of arsenic in the body.33 However, further studies are needed to clarify this assumption.
Another interesting finding in the present study was that smokers had slightly higher, on average 1 μg As dm−3, arsenic concentrations in urine than non-smokers. In the past, levels of up to 40 mg As kg−1 were detected in US cigarettes, in the form of As2O3, owing to the use of arsenical pesticides.34 A decrease in the use of such pesticides in the tobacco fields has resulted in lower arsenic levels in tobacco, on average 1.5 mg As kg−1.35 Assuming 1 gram tobacco per cigarette, an average of 1.5 μg As per cigarette would give an absorbed amount of approximately 2 μg As per pack of cigarettes.35 This would give rise to 1–2 μg As dm−3 in urine, i.e. similar to our findings.
In conclusion, the present study indicates that the arsenic exposure has decreased in the studied areas, but there are still many water sources exceeding the European Union standard for drinking water. Very few publications are available on arsenic concentrations in ground water in Europe. Recent studies by the Geological Survey of Sweden have found elevated levels of arsenic in ground water from tube wells also in Sweden.36 Therefore, it is motivated to further investigate the arsenic concentrations in drinking water all over Europe. Arsenic contaminated foods, like rice and poultry meat, as well as cigarette smoke are major determinants of urinary arsenic at very low concentrations in drinking water. The background level of 2.5 μg As dm−3 in urine would correspond to an intake of 2–3 μg As per day from food and, in smokers, tobacco smoking. The exposure from food seems to be mainly DMA and/or arsenosugars, which are metabolized to DMA. It is important to bear this in mind when doing risk assessment as these organic arsenic species shows a lower toxic potency, compared to iAs.37 Speciation of arsenic in urine may give valuable information on the sources of arsenic exposure.
Acknowledgements
Financial support provided by EC project No. QLK4-CT-2001-00264 (ASHRAM) is gratefully acknowledged.References
- IARC, Some Drinking-water Disinfectants and Contaminants, including Arsenic, IARC Press, Lyon, 2004 Search PubMed.
- NRC, Arsenic in Drinking Water, National Academy Press, Washington, DC, 1999 Search PubMed.
- WHO, Guidelines for Drinking-Water Quality, Vol 1: Recommendations, World Health Organization, Geneva, 2nd edn, 1998 Search PubMed.
- K. A. Francesconi and D. Kuehnelt, in Environmental Chemistry of Arsenic, ed. W. T. Frankenberger, Marcel Dekker, New York, 2002, pp. 51–94 Search PubMed.
- O. P. Diaz, I. Leyton, O. Munoz, N. Nunez, V. Devesa, M. A. Suner, D. Velez and R. Montoro, J. Agric. Food Chem., 2004, 52, 1773–1779 CrossRef CAS.
- M. D’Amato, G. Forte and S. Caroli, J. AOAC Int., 2004, 87, 238–243 CAS.
- P. N. Williams, A. H. Price, A. Raab, S. A. Hossain, J. Feldmann and A. A. Meharg, Environ. Sci. Technol., 2005, 39, 5531–5540 CrossRef CAS.
- F. X. Han, Y. Su, D. L. Monts, M. J. Plodinec, A. Banin and G. E. Triplett, Naturwissenschaften, 2003, 90, 395–401 CrossRef CAS.
- R. L. Calderon, E. Hudgens, X. C. Le, D. Schreinemachers and D. J. Thomas, Environ. Health Perspect., 1999, 107, 663–667 CrossRef CAS.
- M. Vahter, Toxicology, 2002, 181–182, 211–217 CrossRef CAS.
- J. P. Buchet, J. Pauwels and R. Lauwerys, Environ. Res., 1994, 66, 44–51 CrossRef CAS.
- K. A. Francesconi, R. Tanggaar, C. J. McKenzie and W. Goessler, Clin. Chem., 2002, 48, 92–101 CAS.
- R. Heinrich-Ramm, S. Mindt-Prufert and D. Szadkowski, J. Chromatogr., B, 2002, 778, 263–273 CrossRef CAS.
- X. C. Le, W. R. Cullen and K. J. Reimer, Clin. Chem., 1994, 40, 617–624 CAS.
- M. Ma and X. C. Le, Clin. Chem., 1998, 44, 539–550.
- R. Raml, W. Goessler, P. Traar, T. Ochi and K. A. Francesconi, Chem. Res. Toxicol., 2005, 18, 1444–1450 CrossRef CAS.
- E. Schmeisser, W. Goessler, N. Kienzl and K. A. Francesconi, Anal. Chem., 2004, 76, 418–423 CrossRef CAS.
- A. J. Quick and R. Adams, J. Am. Chem. Soc., 1922, 44, 805–816 CrossRef CAS.
- H. V. Aposhian, E. S. Gurzau, X. C. Le, A. Gurzau, S. M. Healy, X. Lu, M. Ma, L. Yip, R. A. Zakharyan, R. M. Maiorino, R. C. Dart, M. G. Tircus, D. Gonzalez-Ramirez, D. L. Morgan, D. Avram and M. M. Aposhian, Chem. Res. Toxicol., 2000, 13, 693–697 CrossRef.
- M. Borzsonyi, A. Bereczky, P. Rudnai, M. Csanady and A. Horvath, Arch. Toxicol., 1992, 66, 77–78 CAS.
- WHO, EHC 224, Arsenic and Arsenic Compounds, World Health Organization, Geneva, 2001, 2nd edn Search PubMed.
- European-Commission, European Union Directive on the Quality of Water Intended for Human Consumption (Council Directive 98/83/EC), Brussels, 1998 Search PubMed.
- P. Apostoli, D. Bartoli, L. Alessio and J. P. Buchet, Occup. Environ. Med., 1999, 56, 825–832 CrossRef CAS.
- V. Foa, A. Colombi, M. Maroni, M. Buratti and G. Calzaferri, Sci. Total Environ., 1984, 34, 241–259 CrossRef CAS.
- D. A. Kalman, J. Hughes, G. van Belle, T. Burbacher, D. Bolgiano, K. Coble, N. K. Mottet and L. Polissar, Environ. Health Perspect., 1990, 89, 145–151 CrossRef CAS.
- P. Kurttio, H. Komulainen, E. Hakala, H. Kahelin and J. Pekkanen, Arch. Environ. Contam. Toxicol., 1998, 34, 297–305 CrossRef CAS.
- M. Vahter and B. Lind, Sci. Total Environ., 1986, 54, 1–12 CrossRef CAS.
- A. Lindberg, K. A. Bjornberg, M. Vahter and M. Berglund, Environ. Res., 2004, 96, 28–33 CrossRef CAS.
- H. Ahsan, M. Perrin, A. Rahman, F. Parvez, M. Stute, Y. Zheng, A. H. Milton, P. Brandt-Rauf, A. van Geen and J. Graziano, J. Occup. Environ. Med., 2000, 42, 1195–1201 CrossRef CAS.
- A. L. Hinwood, M. R. Sim, D. Jolley, N. de Klerk, E. B. Bastone, J. Gerostamoulos and O. H. Drummer, Int. J. Environ. Health Res., 2003, 13, 271–284 CAS.
- M. M. Meza, M. J. Kopplin, J. L. Burgess and A. J. Gandolfi, Environ. Res., 2004, 96, 119–126 CrossRef CAS.
- M. Vahter, G. Concha, B. Nermell, R. Nilsson, F. Dulout and A. T. Natarajan, Eur. J. Pharmacol., 1995, 293, 455–462 CrossRef CAS.
- M. Vahter, Sci. Prog., 1999, 82(Pt 1), 69–88 Search PubMed.
- R. H. Holland and A. R. Acevedo, Cancer, 1966, 19, 1248–1250 CAS.
- EPA, Health assessment document for inorganic arsenic. Final report EPA 600/8-83-021F, US Environmental Protection Agency, Environmental Criteria and Assessment Office, Research Triangle Park, NC, 1984 Search PubMed.
- SGU, Geological Survey of Sweden, http://www.sgu.se (in Swedish), accessed 8th November 2005.
- P. Andrewes, D. M. Demarini, K. Funasaka, K. Wallace, V. W. Lai, H. Sun, W. R. Cullen and K. T. Kitchin, Environ. Sci. Technol., 2004, 38, 4140–4148 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
