Estrogenicity of pyrethroid insecticide metabolites
Anna R.
McCarthy
a,
Barbara M.
Thomson
b,
Ian C.
Shaw
*ab and
Andrew D.
Abell
a
aDepartment of Chemistry, University of Canterbury, Private Bag 4800, Christchurch, New Zealand
bInstitute of Environmental Science and Research Ltd., P.O. Box 29 181, Christchurch 8004, New Zealand
First published on 14th December 2005
Abstract
There is concern that insecticides are able to mimic the action of 17β-estradiol by interaction with the human estrogen receptor. Pyrethroids are commonly used insecticides and several have been assessed for potential endocrine disrupting activity by various methods. It has been noted that some metabolites of pyrethroids, in particular, permethrin and cypermethrin, have chemical structures that are more likely to interact with the cellular estrogen receptor than the parent pyrethroid. For this study permethrin and cypermethrin metabolites 3-(4-hydroxy-3-phenoxy)benzyl alcohol, 3-(4-hydroxy-3-phenoxy)benzoic acid, and N-3-(phenoxybenzoyl)glycine were synthesised, and together with the commercially available 3-phenoxybenzyl alcohol, 3-phenoxybenzaldehyde, and 3-phenoxybenzoic acid, were studied in a recombinant yeast assay expressing human estrogen receptors (YES). Three metabolites, 3-phenoxybenzyl alcohol, 3-(4-hydroxy-3-phenoxy)benzyl alcohol, and 3-phenoxybenzaldehyde, showed estrogenic activity of approximately 105 less than that of 17β-estradiol. No activity was observed in the yeast assay for 3-phenoxybenzoic acid, 3-(4-hydroxy-3-phenoxy)benzoic acid, and N-3-(phenoxybenzoyl)glycine. The results from this study show that pyrethroid metabolites are capable of interacting with the human estrogen receptor, and so might present a risk to human health and environmental well being. The impact would be expected to be small, but still add to the overall environmental xenoestrogen load.
Introduction
There is great concern about synthetic chemicals that have undesirable effects on the environment and people. A wide range of natural and synthetic chemicals that have an effect on the endocrine systems of animals have been identified.1 The main effect of these chemicals is through interactions with steroid hormone receptors, but other mechanisms of action exist. These include hormone synthesis inhibition, alteration of hormone transport or metabolism, as well as the activation of receptor-mediated processes via, for example, receptor phosphorylation.1 Recently, much research has focused on using in-vitro assays to identify chemicals that mimic the action of 17β-estradiol which acts by binding to the human estrogen receptor (hER), initiating a receptor conformational change and increasing the receptor’s affinity for a specific DNA region that regulates protein synthesis. Chemical mimics of 17β-estradiol (xenoestrogens) can also bind to this receptor and cause unwanted environmental effects. In-vitro assays provide a useful tool for predicting chemicals in the environment that might have such an effect through structure–activity relationship studies.Concern has arisen recently that some insecticides may possess the ability to mimic the action of 17β-estradiol through interaction with estrogen receptors, including the hER. An example is the association between the concentration of DDT and its metabolites in Lake Apopka, Florida, and developmental abnormalities in the lake alligators.2 There is evidence to indicate that exposure to pyrethroids may also cause effects that are linked to hER mediated responses.3 Synthetic pyrethroids are commonly used insecticides around the world being the 4th major group used after organochlorines, organophosphates, and carbamates.4 Pyrethroids are used in forestry, on vegetable and fruit crops, wheat and barley.5 Many of these are synthetic analogues of the naturally occurring pyrethrins found in the flowers of Chrysanthemum cinerariaefolium. They are highly toxic to insects, and the level of toxicity greatly decreases in the order of amphibia, fish, mammals and birds. They were regarded as safe because of their favourable insect ∶ mammalian toxicity ratio. Significant toxicity to fish cast doubt on their apparently low environmental impact.
Increased awareness about the environmental effects of xenoestrogens has led to the assay of pyrethroid insecticides for potential estrogenic activity by several different methods.3,6–12 This limited work shows highly variable results in the activity of pyrethroids in receptor binding assays, gene expression assays, or varying cell proliferation assays. The pyrethroid insecticides are readily metabolised and environmentally degraded. Cypermethrin and permethrin are two common pyrethroids that have similar mammalian metabolic pathways, resulting in metabolites with chemical similarity to 17β-estradiol (Fig. 1).
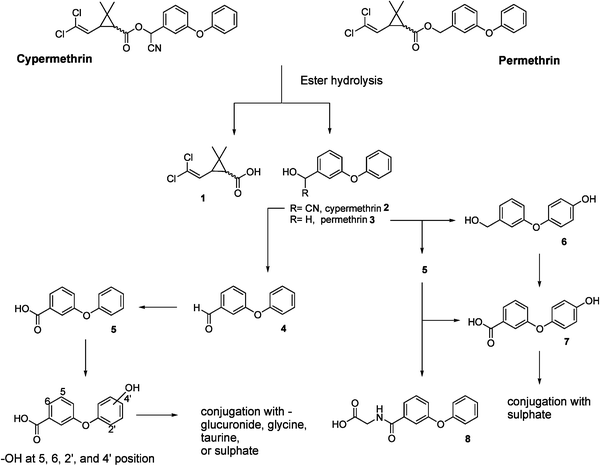 | ||
| Fig. 1 Metabolism of cypermethrin and permethrin13 | ||
The pathways of metabolism and degradation in insects, fish, birds and mammalian animals are well understood.4,13 The metabolism of permethrin and cypermethrin follow a common pathway; the ester of the parent insecticide is enzymatically cleaved by an oxidase for the cis disubstituted cyclopropane of permethrin, and an esterase for the trans. Both isomers of cypermethrin are hydrolysed by an esterase. The resulting cyclopropyl acids in both series are then further metabolised and conjugated with glucuronide or glycine moieties to aid in excretion. The cyanohydrin 2 and the aromatic alcohol 3 are oxidised and hydroxylated in different positions as shown in Fig. 1. These hydroxylated compounds are conjugated with glucuronide, glycine, taurine or sulfate groups. This can occur at either the carboxyl or hydroxyl groups.
Although the parent compounds show little obvious structural resemblance to 17β-estradiol, and therefore have limited estrogenicity, the metabolites are potentially more estrogenic based on structure–activity relationships.14 Only one paper to date has examined the ability of three permethrin metabolites to activate the estrogen receptor.10 In this study the cyclopropyl acid (Fig. 1, 1) was inactive, whereas 3-phenoxybenzyl alcohol 3 was weakly estrogenic (105 fold less potent than 17β-estradiol). 3-Phenoxybenzoic acid 5 was also analysed, and found to behave as an antiestrogen by blocking the action of estradiol in an anti-estrogen screen. The cyclopropyl acid 1 also showed anti-estrogenic behaviour. However metabolites 6 and 7, which are potentially more estrogenic than 1, 3, and 5 were not investigated. Known structure–activity relationships (SAR),14 suggest that the 3-(4-hydroxy-3-phenoxy)benzyl alcohol metabolite 6 of cypermethrin would be more active in the yeast assay than 3, due to the presence of the aromatic hydroxyl functionality which is known to be favoured for receptor binding. 3-(4-Hydroxy-3-phenoxy)benzoic acid 7 (metabolite of both permethrin and cypermethrin) also contains an aromatic hydroxyl, suggesting a potentially greater estrogenicity than 5 also.
In this paper we report the synthesis and estrogenicity of other cypermethrin and permethrin metabolites. We used a yeast based assay (YES) to screen these metabolites for estrogen activity.15 The assay detects chemicals that bind to the hER-α, which is expressed in the yeast, and subsequent gene expression. However it does not discriminate between agonists and antagonists of the receptor. Compounds 3-(4-hydroxy-3-phenoxy)benzyl alcohol 6, 3-(4-hydroxy-3-phenoxy)benzoic acid 7, and N-3-(phenoxybenzoyl)glycine 8 were synthesised, while 3-phenoxybenzyl alcohol 3, 3-phenoxybenzaldehyde 4, and 3-phenoxybenzoic acid 5 were purchased from Sigma-Aldrich. A glycine conjugate of 5, N-3-(phenoxybenzoyl)glycine 8 is biosynthesised in the final step of metabolism. Conjugation with a glycine molecule increases the molecular length of the metabolite. Therefore metabolite 8 was included in the assay, with 3, 4, 5, 6, and 7, as a final step in investigating the relationship between the molecular dimensions of metabolites and their estrogenic activity. The activity of metabolites 4, 6, 7, and 8 in the YES has not previously been reported. This has clear implications for their possible impact on the environment and the consumer.
Materials and methods
Materials
All chemicals required for the assay were purchased from Sigma-Aldrich. Metabolites 3, 4, and 5 were also purchased from Sigma-Aldrich. Three metabolites, 6, 7, and 8 were synthesised as reported here. CPRG was obtained from Roche (Auckland, NZ).Synthesis of metabolites
3-(4-Hydroxy-3-phenoxy)benzyl alcohol 6, 3-(4-hydroxy-3-phenoxy)benzoic acid 7, and N-(3-phenoxybenzoyl)glycine 8 (Fig. 3) were synthesised as detailed below. The structure of each compound was confirmed by 1H and 13C NMR.3-(4-Methoxyphenoxy)benzoic acid 10
The aryl ester 916 (153 mg, 0.59 mmol) was refluxed in a 1 ∶ 1 mixture of THF ∶ H2O (5 ml) with KOH (132 mg, 2.36 mmol) for 4 h. After cooling to rt, the solution was acidified with 10% aqueous HCl, and extracted 3× with ethyl acetate. The combined organic phases were dried over MgSO4, and the solvent was concentrated in vacuo, to give 10 as a white solid (130 mg) in 90% yield.1H NMR (acetone-d6, 500 MHz) δ 3.99 (s, 3H, OCH3), 7.17 (d, 2H, J = 9.3 Hz, ArH), 7.24 (d, 2H, J = 9.3 Hz, ArH), 7.39 (d, 1H, J = 10.3 Hz, ArH), 7.66 (t, 1H, J = 7.8 and 15.6 Hz, ArH), 7.73 (s, 1H, ArH), 7.94 (d, 1H, J = 7.8 Hz, ArH).
13C NMR (acetone-d6, 75 MHz) δ 53.76, 113.83, 116.31, 119.91, 120.55, 122.25, 128.7, 130.95, 148.08, 155.34, 157.72, 164.93.
3-(4-Hydroxy-3-phenoxy)benzoic acid 7
The aryl ether 10 (70 mg, 0.29 mmol) was dissolved in a 1 : 1 mixture of 48% HBr/AcOH (5 ml) and heated at reflux for 7 h. The mixture was cooled to rt, and the solvent concentrated in vacuo. The orange residue was dissolved in ether and washed with three portions of 1 N aqueous KOH. The aqueous washings were combined, acidified with 6 N aqueous HCl, and extracted 3× with ether. The organic phase was dried with MgSO4 and concentrated in vacuo to give 7 as a cream solid (44 mg) in 68% yield.1H NMR (acetone-d6, 500 MHz) δ 7.02 (d, 2H, J = 9.3 Hz, ArH), 7.08 (d, 2H, J = 8.8 Hz, ArH), 7.31 (d, 1H, J = 11.7 Hz, ArH), 7.58 (t, 1H, J = 16.1 and 8.3 Hz ArH), 7.64 (s, 1H, ArH), 7.83 (d, 1H, J = 10.3 Hz, ArH), 8.56 (s(br), 1H, OH).
13C NMR (acetone-d6, 75 MHz) δ 114.61, 115.59, 119.54, 119.78, 121.5, 128.06, 130.31, 146.55, 152.43, 157.4, 164.67.
3-(4-Hydroxy-3-phenoxy)benzyl alcohol 6
The acid 7 (82 mg, 0.36 mmol) was dissolved in freshly distilled dry ether (5 ml) and LiAlH4 (33 mg, 0.90 mmol) added at 0 °C. The grey suspension was stirred at rt for 16 h then sat. aqueous NH4Cl was added slowly until bubbling ceased. The mixture was extracted 3× with ethyl acetate. The combined organic phases were washed with water, sat. aqueous NaCl, dried with MgSO4 and concentrated in vacuo to give 6 (19.5 mg) cream solid in 33% yield.1H NMR (acetone-d6, 300 MHz) δ 4.43 (s(br), 1H, OH), 4.70 (s, 2H, CH2), 7.02 (m, 7H, ArH), 7.41 (t, 1H, J = 7.8, 15.6 Hz, ArH), 8.4 (s(br), 1H, OH)
13C NMR (acetone-d6, 75 MHz) δ 62.03, 113.68, 114.11, 114.77, 118.81, 119.58, 127.91, 143.14, 147.64, 152.38, 157.5
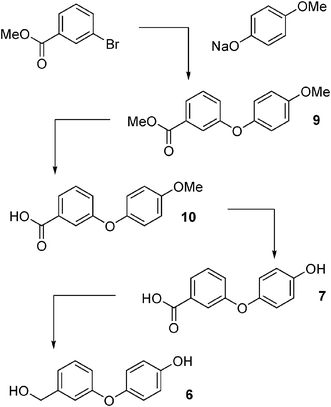 | ||
| Fig. 2 Synthesis of 3-(4-hydroxy-3-phenoxy)benzyl alcohol 6. | ||
N-(3-Phenoxybenzoyl)glycine methyl ester 11
Diisopropylethylamine (0.41 ml, 2.34 mmol) was added to a stirring solution of glycine methyl ester hydrochloride (293 mg, 1 eq.) in dry CH2Cl2 (10 ml). EDCI (448 mg, 1 eq.), HOBT (316 mg, 1 eq.) and 5 (500 mg, 1 eq.) were added in succession, and the resulting clear solution stirred for 16 h. The solution was diluted with ethyl acetate and washed successively with 10% aqueous HCl, 10% aqueous NaHCO3, and water. The organic phase was dried (MgSO4) and the solvent concentrated in vacuo to give 11 as viscous pale yellow oil (596 mg) in 85% yield.1H NMR (CDCl3, 300 MHz) δ 3.77 (s, 3H, OCH3), 4.20 (d, 2H, J = 4.8 Hz, CH2), 6.65 (s(br), 1H, NH), 6.99 (d, 2H, J = 8.8 Hz, ArH), 7.13 (d, 2H, J = 6.8 Hz, ArH), 7.39 (m, 5H, ArH).
13C NMR (CDCl3, 75 MHz) δ 41.53, 52.25, 117.28, 119.02, 121.45, 121.7, 123.65, 129.76, 129.8, 135.29, 156.35, 157.5, 166.88, 170.31.
N-(3-Phenoxybenzoyl)glycine 8
The ester 11 (200 mg, 0.70 mmol) was hydrolysed with a 1 ∶ 1 mixture of THF ∶ H2O (6 ml) with KOH (157 mg, 2.80 mmol) for 4 h as described for 10. Work-up gave 8 as a fluffy white solid (124 mg) in 65% yield.1H NMR (acetone-d6, 300 MHz) δ 4.23 (d, 2H, J = 2.9 Hz, CH2), 7.16 (d, 2H, J = 7.8 Hz, ArH), 7.29 (d, 2H, J = 8.3 Hz, ArH), 7.59 (m, 4H, ArH), 7.81 (d, 1H, J = 7.8 Hz, ArH), 8.13 (s(br), 1H, NH).
13C NMR (acetone-d6, 75 MHz) δ 39.41, 115.8, 117.46, 120.03, 120.4, 122.19, 128.47, 134.66, 155.3, 156.03, 164.7, 168.97.
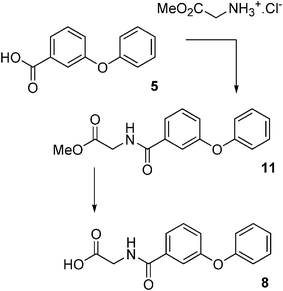 | ||
| Fig. 3 Synthesis of N-3-(phenoxybenzoyl)glycine 8. | ||
Recombinant yeast estrogen screen
Estrogenicity was measured according to the method described by Routledge and Sumpter15 using a recombinant yeast strain which expresses the human estrogen receptor (hER-α). Chemicals that interact with the hER-α produce a response measurable by light absorbance.The yeast strain was developed by integrating the DNA sequence for the hER-α in the chromosome of Saccharomyces cerevisiae. The recombinant yeast cells also contain a plasmid that possesses the reporter gene lac-Z. This gene codes for the expression of β-galactosidase enzyme, which cleaves a dye added to the assay medium. The binding of a compound results in a complex that binds to estrogen responsive elements (ERE) on the plasmid. β-Galactosidase is then expressed which cleaves a dye, chlorophenol red β-galactopyranoside (CPRG), in the yeast growth medium. The dye is normally yellow, but is converted to a red colour by β-galactosidase activity, reflecting the estrogenicity of the test chemical. The absorbance of the red colour is measured at 540 nm.
Validation of yeast culture
For assay reproducibility it is important to standardise the amount of yeast that was added to each assay run. A cell count was performed to investigate the number of cells in the 24 h yeast culture. The number of yeast cells in the 250 μl of culture added to the assay medium was calculated to be 3.75 × 106 cells correlating to an optical density at 640 nm (OD640) of 2.5. Therefore the OD of all 24 h yeast cultures was read to ensure the amount of yeast added to the assay was consistent.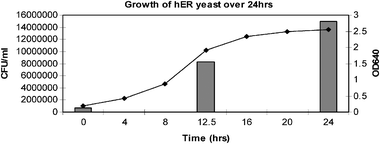 | ||
| Fig. 4 Cell count of the yeast solution after 24 h. | ||
Assay method
All preparations of assay solutions and assay plates were carried out in a laminar flow cabinet. Test chemical solutions of appropriate concentrations in ethanol were serially diluted in ethanol across rows in a 96 well plate. 10 μl of each concentration was transferred to a second row, and allowed to evaporate. 17β-Estradiol was used as a positive control, and ethanol used as a negative. For each assay, growth solution (50 ml) was seeded with 250 μl of a 24 h yeast culture (optical density at 640 nm of 2.5). 200 μl of this assay medium was added to each well, the plates were sealed with autoclave tape and shaken in a fixed wavelength plate reader (BIO-TEK EL312) for 2 min. The plates were incubated for about 48 h at 30 °C, until a colour degradation from red to yellow was observed across the estradiol standard. The red colour denoting estrogenicity was measured at 540 nm and 610 nm. 610 nm measures the turbidity of the medium, hence the growth rate of the yeast. The negative controls appeared as a pale orange colour due to background β-galactosidase activity. The absorbance at 540 nm was therefore corrected by applying; A540 − (A610 − blank A610).Relative activity of test chemicals to 17β-estradiol
Each of the six metabolites 3, 4, 5, 6, 7 and 8 was assayed at least 4 times to assess variation between assays and the consistency of data. For the metabolites that possessed estrogenic activity an EC50 was calculated. This was performed by using the absorbance that gave half the maximum response on the absorbance vs. concentration curve. The corresponding concentration value was then obtained. This was compared to the 17β-estradiol value in terms of a relative potency, where estradiol had a value of 1.Results
Estrogenic activity of pyrethroid metabolites
Table 1 shows the estrogenic activity of the pyrethroid metabolites analysed in this study. Of the six metabolites tested, three showed an estrogenic response in the yeast system. Our results confirmed the weak estrogenicity of 3-phenoxybenzyl alcohol 3 reported by Tyler et al.10 3-Phenoxybenzaldehyde 4, and 3-(4-hydroxy-phenoxy)benzyl alcohol 6 showed approximately 105 fold less activity than 17β-estradiol. The other metabolites, 3-phenoxybenzoic acid 5, 3-(4-hydroxy-3-phenoxy)benzoic acid 7, N-3-(phenoxybenzoyl)glycine 8 showed no apparent activity above that of the blank (ethanol and seeded assay medium only). To put these results into context, the activity of these metabolites can be compared to the activity of known xenoestrogens. In a YES run by Schultz et al.17 genistein (a phytoestrogen) had a relative potency of 2.16 × 10−2 and bisphenol A (plasticiser) had a relative potency of 9.14 × 10−3 (relative to the potency of estradiol which was 100).| Pyrethroid metabolite | Estrogenic activity EC50/μM | Relative potency (estradiol = 1) |
|---|---|---|
| 3 | 6.67 ± 3.11 | 5 × 10−5 |
| 4 | 4.8 ± 3.42 | 7 × 10−5 |
| 5 | NA | — |
| 6 | 6.75 ± 2.28 | 5 × 10−5 |
| 7 | NA | — |
| 8 | NA | — |
NA denotes that no activity above that of the ethanol blank was observed.
The EC50 value for 17β-estradiol was 0.349 ± 0.16 nM.
Discussion
Cypermethrin and permethrin hydrolyse under enzymatic or high UV conditions to products 2 or 3, respectively (Fig. 1). Metabolite 3 has estrogenic activity (Table 1), and both are very likely to be further metabolised by hepatic first pass metabolism following ingestion with food in humans, or environmental exposure in animals. Our results from this work show that the Phase I first pass metabolites 4 (for cypermethrin) and 6 (for permethrin), respectively, are both weakly estrogenic. Following oral ingestion of the estrogenic environmental degradation product the first pass Phase I metabolites are also estrogenic so increasing the risk of xenoestrogenic pharmacological effects on the human consumer or exposed animals.From known SAR studies, it was hypothesised that 6 would display greater activity than 3 due to the presence of two hydroxyl groups at either end of the molecule (see earlier for a reasoning). This structural arrangement is well known to be preferred for binding to the estrogen receptor, as is the presence of phenolic rings.14 However, based on the assay results reported here, the activity of 6 was of the same order of magnitude as that of 3. The presence of a phenol in 6 did not have an added effect on activity and hence binding. It is noted that the SAR,14 from which our predictions were made, are based on receptor binding assays and computational calculations, whereas this yeast assay measures the effects of gene expression, further down the effect pathway than receptor binding alone. This is a likely reason as to why we see a difference in our predicted activity compared to the observed, and also reiterates the fact that determining absolute SAR for xenoestrogens is complex and dependent on the type of assay utilised as well as the mechanism of action. The yeast assay measures an estrogenic response further down the effect pathway, but only the response of a single gene in a genetically modified cell system, unlike the complex system of an animal or human. It is for this reason that this assay is considered a simple screen to prioritise candidates that might require further biological testing.
There are two main sources of the metabolites shown to be estrogenic in this study. Microbial enzymes that can hydrolyse the parent insecticide to produce the metabolites are widely found in the environment (e.g. in the bacteria of aquatic silts). Therefore the metabolites are more likely to be found in the environment than the parent compound. They might then form residues in food crops and so be ingested with food.18 The other source of these metabolites is internal mammalian metabolism, 5 and 7 have been detected in human blood and urine.19–21 These compounds are end products of cypermethrin and permethrin metabolism after ingestion. Phase I enzyme reactions produce the estrogenic metabolites 3, 4, and 6via hydroxylation of the inactive parent. Subsequent Phase II enzyme reactions are responsible for conjugating molecules to metabolites to lower toxicity and aid in excretion. Phase I enzymes are mostly localised on the endoplasmic reticulum, while phase II enzymes are found in the cytoplasm.22 Thus the estrogenic insecticide metabolites produced from phase I reactions are then available for conjugation by phase II enzymes or interaction with hER in the cell. Therefore Phase II metabolism could lower the concentration of estrogenic metabolites, thus decreasing possible interaction with the hER. Conjugation with glycine inactivates the metabolite (as shown by the non-activity of 8).
The synthetic pyrethroid insecticides were designed to be relatively unstable to minimise their toxicological impact on both the environment and humans. For this reason food residues are infrequent and very low even though their usage is relatively high.23 The major environmental degradation products of the pyrethroids (Fig. 1) are not normally measured as part of pesticide residues surveillance programmes and therefore there are few, if any data, from which exposure estimates can be made. The finding that several of these breakdown products are estrogenic brings into question their impact on humans. This risk assessment cannot be carried out without food residues data. However it is likely that the breakdown products do occur as residues in food since pyrethroid insecticides are applied directly to crops, e.g. tomatoes.18 Pyrethroid degradation occurs on/in the crop and so the breakdown products are likely to remain as residues. This reasoning suggests that there will be an impact on human health, but the magnitude of the impact cannot be assessed without detailed residue data.
In conclusion, three metabolites 3, 4, and 6 have been shown to be weakly estrogenic in a recombinant yeast assay. This would suggest that a large amount of metabolite would have to be ingested or produced internally to observe any estrogenic effect. Pyrethroids are also metabolised rapidly, via renal excretion with a half life of about 6 h.20 Upon evidence to date our opinion is that these insecticide metabolites are not contributing significantly to the xenoestrogenic impact on humans, but might be more significant to animals in the environment.
Acknowledgements
The recombinant yeast was developed by Glaxo Wellcome (UK) and generously gifted by John Sumpter of Brunel University, UK. Thanks to ESR for part-funding A. R. McCarthy’s postgraduate studentship.References
- International Programme on Chemical Safety, Global Assessment of the State-of-the-Science of Endocrine Disruptors, World Health Organisation, 2002 Search PubMed.
- L. J. Guillette Jr, D. B. Pickford, D. A. Crain, A. A. Rooney and H. F. Percival, Gen. Comp. Endocrinol., 1996, 101, 32–42 CrossRef CAS.
- J. Garey and M. S. Wolff, Biochem. Biophys. Res. Commun., 1998, 251, 855 CrossRef CAS.
- T. R. Roberts and D. H. Hutson, in Metabolic Pathways of Agrochemicals Part II- Insecticides and Fungicides, Royal Society of Chemistry, Cambridge, UK, 1999, pp. 579–585, 610–634, and 678–684 Search PubMed.
- The UK Pesticide Guide 2002, ed. R. Whitehead, United Kingdom, 2002, pp. 237–238 and 434–435 Search PubMed.
- H. Chen, J. X. Gang Hu, J. Zhou, H. Xiao and X. Wang, J. Toxicol. Environ. Health, 2002, 65, 1419 CrossRef CAS.
- K. Saito, Y. Tomigahara, N. Ohe, N. Isobe, I. Nakatsura and H. Kaneko, Toxicol. Sci., 2000, 57, 54 CrossRef CAS.
- V. Go, J. Garey, M. S. Wolff and B. G. T. Pogo, Environ. Health Perspect., 1999, 107, 173 CrossRef CAS.
- H. R. Andersen, A. M. Vinggaard, T. H. Rasmussen, I. M. Gjermandsen and E. C. Bonefeld-Jorgensen, Toxicol. Appl. Pharmacol., 2002, 179, 1 CrossRef CAS.
- C. H. Tyler, N. Beresford, M. Van der Woning and J. P. Sumpter, Environ. Toxicol. Chem., 2000, 19, 801 CrossRef CAS.
- T. Kunimatsu, T. Yamada, K. Ose, O. Sunami, Y. Kamita, Y. Okuno, T. Seki and I. Nakatsuka, Reg. Toxicol. Pharm., 2002, 35, 227 Search PubMed.
- I. C. Shaw and J. Chadwick, Principles of Environmental Toxicology, Taylor and Francis Ltd., London, UK 1998 Search PubMed.
- J. P. Leahey, The Pyrethroid Insecticides, Taylor and Francis Ltd., London, UK, 1985 Search PubMed.
- H. Fang, W. Tong, L. M. Shi, R. Blair, R. Perkins, W. Branham, B. S. Hass, Q. Xie, S. L. Dial, C. L. Moland and D. M. Sheehan, Chem. Res. Toxicol., 2001, 14, 280 CrossRef CAS.
- E. J. Routledge and J. P. Sumpter, Environ. Toxicol. Chem., 1996, 15, 241 CAS.
- T. Unai and J. E. Casida, J. Agric. Food Chem., 1977, 25, 979 CrossRef CAS.
- T. W. Schultz, G. D. Sinks and M. T. D. Cronin, Environ. Toxicol., 2002, 17, 14–23 CrossRef CAS.
- H.-M. Lin, I. C. Shaw and J. A. Gerrard, Food Addit. Contam., 2005, 22, 15–22 CrossRef CAS.
- B. H. Woollen, J. R. Marsh, W. J. D. Laird and J. E. Lesser, Xenobiotica, 1992, 22, 983 CrossRef CAS.
- G. Leng, U. Ranft, D. Sugiri, W. Hadnagy, E. Berger-Preib and H. Idel, Int. J. Hyg. Environ. Health, 2003, 206, 85 Search PubMed.
- G. Leng, K. Kuhn and H. Idel, Sci. Total Environ., 1997, 199, 173 CrossRef CAS.
- G. Gibson and P. Skett, Introduction to Drug Metabolism, Blackie Academic and Professional, Glasgow, 1994 Search PubMed.
- F. Low, H. Lin, J. A. Gerrard, P. J. Cressey and I. C. Shaw, Pest. Manage. Sci., 2004, 60, 842 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
