Behavior of di(2-ethylhexyl) phthalate discharged from domestic waste water into aquatic environment
Erini
Yuwatini
,
Noriko
Hata
* and
Shigeru
Taguchi
*
Department of Environmental Biology and Chemistry, Faculty of Science, Toyama University, Gofuku 3190, Toyama, 930-8555, Japan
First published on 11th November 2005
Abstract
The behavior of di(2-ethylhexyl) phthalate (DEHP) discharged from domestic waste water into river water, sediment and submerged aquatic vegetation was investigated. The concentrations of DEHP were found to be between 8–25 μg L−1 in river water, 1000–2000 μg kg−1 in sediment and less than 20–2000 μg kg−1 in submerged aquatic vegetation. The experiments performed in laboratory were on the biodegradation of DEHP in water and sediment, and also adsorption equilibrium of DEHP between water and sediment. The results obtained from the investigations made it clear that the high enrichment of DEHP from water to sediment was caused from not only its high adsorptive potential but also slow degradation in sediment.
Introduction
The amount and variety of man-made organic chemicals are increasing with increasing needs for them. It is important to understand the behavior of those chemicals released into environment as pollutant in order to evaluate the effect of those chemicals on the ecosystem.Di(2-ethylhexyl) phthalate (DEHP) is one of phthalic acid esters that have been widely used in our life as plasticizer in artificial leather, electric cable covering, and film sheets etc.1 DEHP is one of suspected endocrine disrupting chemicals2 and further investigation is required. In this study, the focus is to evaluate the behavior of DEHP as an organic man-made chemical emitted into aquatic environment. A large amount of phthalic acid esters is leached from plastics dumped in municipal landfill sites.3 Aquatic environment such as river is polluted with DEHP discharged from domestic waste water.4 Behavior of DEHP discharged from sewage treatment plant has been reported.5 Interaction between sediment particle and DEHP in simulated estuary was also reported.6 The details of the behavior of DEHP, however, released from domestic waste water into river water has not been reported.
In this study, the distribution of DEHP in river water, sediment and submerged aquatic vegetation was investigated. Essential investigations of biodegradation both in river water and sediment, and also adsorption of DEHP on sediment were performed in laboratory to ensure its behavior in aquatic environment.
Experimental
Investigation location and sampling
Furu River (3.2 km in length, 6–10 m in width, the amount of flow was more than 1.6 m3 s−1 in summer and 0.3 to 1.6 m3 s−1 from autumn to spring) in Toyama City, Japan was chosen as an investigation location because domestic waste water flows into the river. Water and sediment were sampled every month from May 2001 until November 2002 at several points from inlet to outlet of the river. Submerged aquatic vegetation (Potamogeton Octandrus Poir sp.) at the domestic waste water discharge point was sampled from May to August both in 2001 and 2002. At the domestic waste water discharge point in Furu River, only Potamogeton Octandrus Poir sp. was found. At another point in Furu River, several kinds of submerged aquatic vegetation are found in addition to the Potamogeton Octandrus Poir sp. The map of investigation location is shown in Fig. 1. | ||
| Fig. 1 Map of the location of investigation. | ||
The river water samples were taken into dark bottle and kept at cool box during transportation to the laboratory. In laboratory, water samples were filtered through glass fiber filter and kept at 4 °C before analysis. Sediment samples were taken into stainless steel container. In the laboratory, samples were centrifuged to squeeze water from the sediment and dried at 110 °C for 2 hours. The dried sediment sample was sieved (16 mesh) and kept in a glass bottle. The submerged aquatic vegetation collected was drained from water and dried at 110 °C for 2 hours. The dried submerged aquatic vegetation sample was cut and milled in small size, and kept in a glass bottle. All the bottles, glass fiber filters and stainless steel containers were rinsed with acetone before use to avoid contamination.
Reagents and apparatus
DEHP standard solution obtained from Tokyo Chemical Industry, Japan was diluted with methanol. A 0.1 M 4-trifluoromethylaniline (ABTF, Tokyo Chemical Industry) solution was prepared by dilution with 2 M hydrochloric acid. A 0.005 M dodecylbenzenesulfonate (DBS) solution was prepared by dissolving DBS sodium salt (Tokyo Chemical Industry, Japan) with distilled water prepared without contact to any plastics materials. 2-Methoxyethanol, acetone and hexane were of analytical grade.A glass fiber filter (GC 50, 0.5 μm pore size, 47 mm in diameter) purchased from Advantec Toyo, Japan was used to filter the water samples and to prepare sediment samples. Oasis HLB cartridge from Waters, USA was used to clean up the extract from sediment sample. Acetonitrile and potassium chloride from Wako Chemicals were used as mobile phase in HPLC for DEHP determination.
A centrifugal separator, SCT 5BB (Hitachi, Japan) maximum speed 5000 rpm, was used for water and sediment sample preparation. Filtration apparatus, KG-47 (Advantec Toyo, Japan) was applied for sample filtration joined with vacuum pump. An ultrasonic radiation equipment, SU-30, 120 W of output, Sibata, Japan was utilized for DEHP extraction from sediment sample. A rotary evaporator (Büchi, Switzerland) was used to concentrate the samples solution extracted from sediment sample. DEHP determination was performed by high performance liquid chromatography with octadecyl silica column of Tosoh TSK Gel (ODS-80TS, 4.6 mm × 250 mm, Japan), column heater, U-620 (Sugai, Japan) and an intelligent HPLC pump, PU-980 (Jasco, Japan) conected to multi wavelength detector, MD-1515 (Jasco).
Analytical procedures for the determination of DEHP
All the centrifuge tubes were rinsed with acetone before using to avoid contamination. The detection limit of DEHP, defined as 3 times the standard deviation of the blank signals, was 0.07 μg L−1 (n = 3). In this HPLC condition, the chromatogram peak was not detected less than 0.6 μg L−1 of DEHP concentration and the peak of blank solution was not detected.
Biodegradation experiments for DEHP in laboratory
Adsorption experiment for DEHP in laboratory
The adsorption behavior of DEHP onto sediment was investigated by placing 0.5 g of dried sediment into several centrifuge tubes containing 50 mL of DEHP aqueous solution. All the tubes were shaken at 25 °C, with 70 stroke min−1 and concentrations of DEHP in solution and sediment were determined at suitable shaking time interval. Sterilization of all the centrifuge tubes was performed in drying oven at 110 °C for 1 hour. Distilled water was also irradiated by UV (185 nm + 254 nm) before using. DEHP adsorbed on the inner wall of the tube was also determined at the end of the experiment.Results and discussion
Distribution of DEHP in the aquatic environment
The monthly variation of the concentration of DEHP in Furu River water, sediment and submerged aquatic vegetation at the domestic waste water discharged basin from May 2001 to November 2002 is shown in Fig. 2. The DEHP discharged into the river was distributed to sediment and also submerged aquatic vegetation. DEHP concentrations ranged between 8 and 25 μg L−1 in river water. From 1000 to 2000 μg kg−1 of DEHP was found in sludgy dark sediment. The particle size of the sediments collected from Furu River in April 2001 to January 2002 except July 2001 were 1.0–0.6 mm: 31 ± 8%, 0.6–0.3 mm: 40 ± 7%, less than 0.3 mm: 29 ± 11%. Most of the sediment was sand. The oxidable carbon in the sediment was 0.8–0.95% (w/w). DEHP concentration in submerged aquatic vegetation (Potamogeton Octandrus Poir sp.) was found to be less than 20 to 2000 μg kg−1.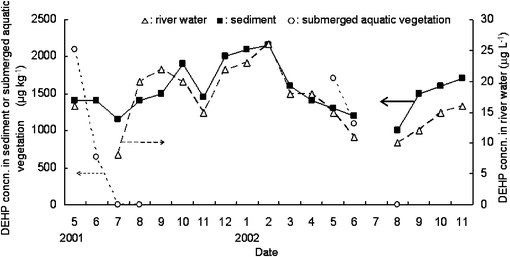 | ||
| Fig. 2 Seasonal change of DEHP concentration at domestic waste water basin of Furu River. Samples were taken from May 2001 to November 2002. | ||
Furu River flows into the main stream, Jinzu River. Then, Jinzu River and Itachi River were also investigated bimonthly from June to October 2002. Itachi River passes through sewered town areas and ends at the confluence with the Jinzu River. The DEHP concentrations in Jinzu and Itachi River waters were generally less than 0.6 μg L−1. Only in October 2002 in Itachi River water DEHP was the concentration found to be 1.3 μg L−1. DEHP concentration at domestic waste water discharged basin in Furu River was higher than the one found in the other rivers described above. This is probably due to the non-treatment of waste water coming from sources such as households and a gasoline station that flows into the Furu River. The range of DEHP concentration in Furu River water was similar to some freshwaters in Italy4 (0.3–31.2 μg L−1) and in Taiwanese rivers11 (less than 1.0–18.5 μg L−1).
DEHP concentration in Furu River sediment was higher than Jinzu River (less than 20–280 μg kg−1) and Itachi River (190–280 μg kg−1). The result was also higher than the one found in Italian freshwater sediments4 (6–490 μg kg−1) but lower than the case of river sediments in Taiwan11 (500–23 900 μg kg−1). High DEHP concentration was reported in lagoon sludge and activated sludge12 as 28.7 and 6.3 mg kg−1, respectively and also in dry compost from sludge13 in the range of 38–43 mg kg−1.
It is interesting that the DEHP in the submerged aquatic vegetation (Potamogeton Octandrus Poir sp.) was detected only in May and June in 2001. And in other seasons DEHP was not detected in the submerged aquatic vegetation. The same result was obtained in 2002. The reason of this result is not clear so far. The adsorption of DEHP on seaweed biomass14 in the range of 5.68–6.54 mg g−1 was reported by laboratory experiment.
The concentrations in water and sediment showed almost same seasonal change of high concentration in autumn, winter and early spring. This change could be related to the seasonal changes of the amount of water flowing into the river. As shown in Fig. 3, from late spring to summer, irrigation water from rice field flowed into Furu River and the flow came up to more than 1.63 m3 s−1, and in other seasons the flow came down to 0.28–1.63 m3 s−1. On the other hand, the amount of pollutant from domestic waste water might not change so drastically by seasons. Therefore the seasonal changes of DEHP concentration was caused by the change in flow of river. The concentrations of DEHP in sediment against those in water are plotted in Fig. 4 using all the data in Fig. 2. A correlation (r2 = 0.72) between DEHP concentration in water and in sediment was found where the slope was about 68. This result suggests that the DEHP concentration in sediment was more than 68 times higher than the one in water.
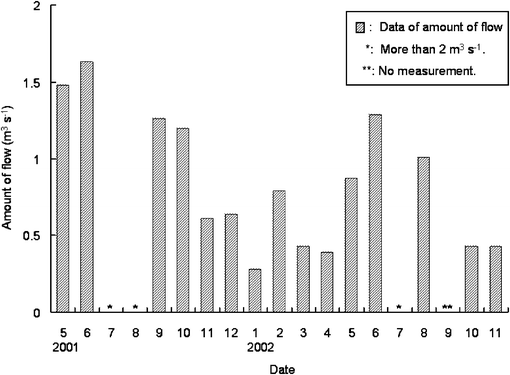 | ||
| Fig. 3 Seasonal change of flow in Furu River. | ||
 | ||
| Fig. 4 Correlation between DEHP concentrations in river water and sediment at domestic waste water basin. Samples were taken from May 2001 to November 2002. | ||
Fig. 5 shows that DEHP concentrations in water and sediment against the distance from the point of domestic waste water discharged. It is clear that the concentrations decreased drastically with increasing distance. DEHP concentration in river water was less than detection limits (0.6 μg L−1) at the 400 m downstream from the waste water discharged point, while DEHP concentration in sediment at the 1300 m downstream was almost same as at 10 m upstream from domestic waste water basin. The decrease of the DEHP concentration was not essentially caused by dilution with waste water discharged at the downstream because of small amount of flow (less than 0.4 m3 s−1). Other reasons of biodegradation in water and adsorption onto sediment during flowing down the river will be focused in the next section.
 | ||
| Fig. 5 Concentration of DEHP in water and sediment. Distance of zero m describes domestic waste water discharged point. Samples were collected at April 2002. | ||
Biodegradation of DEHP in river water and sediment
Biodegradation in water and sediment, and adsorption from water to sediment should be investigated to confirm the decrease of DEHP concentration at the downstream from the domestic waste water discharge point. The degradation and adsorption behaviors of DEHP were investigated in laboratory. The changes of the concentration of spiked DEHP without and with UV irradiation as a control of river water prior spiked with DEHP at 25 °C is shown in Figs. 6(a) and (b). Without UV irradiation, the concentration of DEHP was decreased with increasing the incubation time as shown in Fig. 6(a). On the other hand, with UV irradiation showed very small decrease of the DEHP concentration as shown in Fig. 6(b). The UV irradiation probably reduced the activity of microorganisms in river water. Moreover, in experiment with and without UV radiation, DEHP in water samples was determined in the same way as the determination of DEHP in river water. However, almost no decrease of concentration was found in the experiment with UV irradiation. This result shows that DEHP degradation did not occur under the conditions due to the photodegradation or hydrolysis. DEHP adsorbed on inner glass was also not detected. Therefore, the decrease of concentration without UV irradiation was caused by microorganisms in river water. | ||
| Fig. 6 Degradation of DEHP in river water. Incubation was carried out at 25 °C. DEHP was spiked into river water (52.3 μg L−1) after UV irradiation. | ||
The decrease of the concentration is given as an equation of first-order reaction rate,
| −dCt/dt = kC0 or ln(Ct/C0) = −kt, |
The adsorption of DEHP on the inner wall of glass was not detected during the degradation experiment in sediment. The t1/2 in sediment was also determined by the same manner as in river water to be about 2 weeks as shown in Fig. 7. It is clear that DEHP degradation in sediment is very slow compared to that in river water. This result was almost same as t1/2 (= 14.8 days) of DEHP biodegradation in sediment found by Yuan et al.11
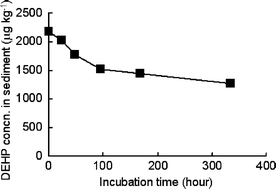 | ||
| Fig. 7 Degradation of DEHP in sediment. Incubation was carried out at 25 °C without any DEHP spiking. Sediment was taken from Furu River in January 2002. | ||
It only takes one hour from the point of domestic waste water discharged to the end point of the river under usual flow of the river. Therefore, probably biodegradation did not contribute significantly on the removal of DEHP in Furu River water. Then, the adsorption of DEHP from water to sediment was investigated by experiment to ensure the decrease of the DEHP concentration at the down stream.
Adsorption of DEHP on sediment
The adsorption equilibrium of DEHP between water and sediment is expressed by distribution constant (Kd) defined as:| Kd = (DEHP)s/[DEHP]aq |
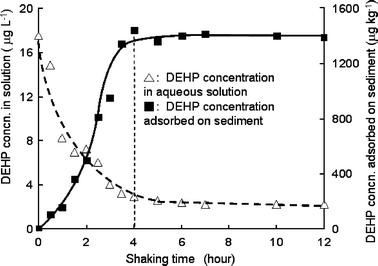 | ||
| Fig. 8 Distribution of DEHP between aqueous solution and sediment. DEHP was spiked into distilled water (17.5 μg L−1) after UV irradiation. Vertical broken line describes the time at where the equilibrium was reached. | ||
There was a difference, however, between the ratio of real DEHP concentrations in river to in sediment and the equilibrium experimental results in laboratory. The ratio of DEHP concentration in real sediment to river water was ca. 340 L kg−1 from the relationship in Fig. 4, that was smaller than that in laboratory experiment (Kd = 560 L kg−1). Probably the distribution equilibrium for DEHP was not attained for the real aquatic environment.
As a conclusion we assumed that the tendency of high DEHP concentration in sediment was caused by its high adsorptive potential and also slow degradation in sediment.
References
- The Chemical Daily, 14303 no Kagaku Shohin (in Japanese, 14303 sorts of Chemical Products), The Chemical Daily, Tokyo, Japan, 2003, p. 1145.
- Ministry of the Environment of Japan (MoE), Risk assessment strategy for suspected endocrine disrupters, Strategic Program on Environmental Endocrine Disrupters, SPEED ’98, 2000 Search PubMed.
- M. J. Bauer, R. Herrmann, A. Martin and H. Zellmann, Water Sci. Technol., 1998, 38, 185–192 CrossRef CAS.
- M. Vitali, M. Guidotti and G. Macilenti, Environ. Int., 1997, 23, 337–347 CrossRef CAS.
- S. K. Marttinen, R. H. Kettunen, K. Sormunen and J. A. Rintala, Water Res., 2003, 37, 1385–1393 CrossRef CAS.
- J. L. Zhou and Y. P. Liu, Mar. Chem., 2000, 71, 165–176 CrossRef CAS.
- N. Hata, E. Yuwatini, K. Ando, M. Yamada, I. Kasahara and S. Taguchi, Anal. Sci., 2004, 20, 149–152 CAS.
- M. A. Sánchez-Monedero, A. Roig, C. Martínez-Pardo, J. Cegarra and C. Paredes, Biores. Technol., 1996, 57, 291–295 Search PubMed.
- S. Iwaguchi, K. Matsumura, Y. Tokuoka, S. Wakui and N. Kawashima, Colloids Surf., B, 2002, 25, 299–304 CrossRef.
- S. Iwaguchi, T. Mochizai, M. Hattori, M. Takahashi and N. Kawashima, Colloids Surf., B, 2000, 18, 113–118 CrossRef CAS.
- S. Y. Yuan, C. Liu, C. S. Liao and B. V. Chang, Chemosphere, 2002, 49, 1295–1299 CrossRef CAS.
- S. Amir, M. Hafidi, G. Merlina, H. Hamdi, A. Jouraiphy, M. El Gharous and J. C. Revel, Process Biochem., 2005, 40, 2183–2190 CrossRef CAS.
- B. Bago, Y. Martin, G. Mejia, F. Broto-Puig, J. Diaz-Ferrero, M. Agut and L. Comellas, Chemosphere, 2005, 59, 1191–1195 CrossRef CAS.
- H. W. Chan, T. C. Lau, P. O. Ang, M. Wu and P. K. Wong, J. Appl. Phycol., 2004, 16, 263–274 Search PubMed.
- J. J. Ellington and T. L. Floyd, Environmental Research Brief US EPA/600/S-96/006, 1996 Search PubMed.
| This journal is © The Royal Society of Chemistry 2006 |
