The ability of biological and organic synthetic materials to accumulate atmospheric particulates containing copper, lead, nickel and strontium†
Mafalda S.
Baptista
*a,
M.
Teresa Vasconcelos
ab,
João
Paulo Cabral
ac,
M.
Carmo Freitas
d and
Adriano M. G.
Pacheco
e
aCIIMAR, Rua dos Bragas, 289, 4050-123, Porto, Portugal. E-mail: mafaldasbaptista@sapo.pt
bChemistry Department, Faculty of Sciences, University of Porto, Rua do Campo Alegre, 687, 4169-071, Porto, Portugal
cBotany Department, Faculty of Sciences, University of Porto, Rua do Campo Alegre, 1191, 4150-181, Porto, Portugal
dITN, E.N. 10, 2686-953, Sacavém, Portugal
eCVRM-IST, Avenida Rovisco Pais, 1, 1049-001, Lisboa, Portugal
First published on 7th October 2005
Abstract
This work was aimed at finding materials that could be used as alternatives to lichens as air quality monitors since the high natural variability and the large amount of lichen collected are two drawbacks of the use of these organisms. Lichen Flavoparmelia caperata (L.) Hale was exposed in three different forms (transplant, detached from the substratum and as a biomass—ground and homogeneized) and compared to the planetree bark (Platanus hybrida Brot.), exposed as a biomass, and two organic synthetic materials (Chelex® 100 resin and cellulose acetate). Materials were exposed for two months in the winter, spring and summer, at three Portuguese coastal cities. The results showed that the airborne accumulations of Cu, Ni, Pb and Sr were partially dependent on the meteorological conditions but mainly dependent on the nature of the exposed material. The standard deviations of the synthetic materials or homogenized biomass were the same or greater than lichen transplants or detached. The accumulation by biological materials, of the four studied elements, was comparable to the lichen transplant accumulation. The replacement of the traditional transplants by the biomass was not considered advantageous, since their preparation is time-consuming. Therefore lichens remain the most suitable in biomonitoring studies. The exposure of detached lichen allows the accurate measurement of the exposed area/volume so it can be useful to relate atmospheric deposition rates with the lichen metal content. The synthetic materials accumulated Cu and Ni and should only be used as an alternative to traditional transplants when these are the elements of interest.
Introduction
Several lichen species have been used as air quality biomonitors in recent years. In many biomonitoring studies lichens are collected from an area with cleaner air, exposed to a more polluted area and then analysed for metal accumulation.1 The yellowing of the lichen thalli is an indicator of damage provoked by atmospheric pollutants2 and therefore the chlorophyll measurement is a common procedure of evaluating thallus vitality over a long period of exposure.3 In addition, ion loss from the cell interior of lichens, particularly K+, has been used as an indicator of the state of membrane integrity.4 More recently tree bark has been used as an alternative to biomonitoring with lichens.5 Biosorption refers to passive or physico–chemical attachment of the sorbate to a biomass, thus excluding metabolic or active uptake processes.6 Algal biomass (dry and ground algae) can bind, in aqueous solutions, high amounts of metal ions7,8 and lichens can sequestrate metals from solutions.4,9 These results suggested to us that lichen or tree bark biomass, exposed to the atmosphere, could bind metals in the same manner as intact lichens or tree bark. Harvesting lichens from the ecosystem can affect the population since they grow slowly. If organic synthetic materials could perform as well as biomonitors, when exposed to the atmosphere, it would obviate the need to harvest lichens. Chelex® 100 resin is a reactive, insoluble, synthetic polymer10 and its affinity for binding metallic cations in aqueous solutions is recognised.11 Cellulose acetate is a synthetic polymer whose chemical binding to metallic cations is weak so this material was initially included in this study as a field control. If the natural variability of biological samples could be reduced, the results obtained with biomonitoring studies would be interpreted more accurately. We hypothesized that standardized organic synthetic materials or ground and homogenized lichen or tree bark would accumulate metals with less natural variability than the intact lichen. Therefore we compared the behaviour of the lichen Flavoparmelia caperata with that of the tree bark (Platanus hybrida) and with that of organic synthetic materials (Chelex® 100 resin and cellulose acetate). The lichen was exposed to the atmosphere either intact or as biomass (lichen that was dried, ground and sieved); the tree bark was exposed as biomass. To the best of our knowledge, this is the first time that biomass or these organic synthetic materials have been used in atmospheric exposure studies.Materials and methods
Sampling site
Three coastal Portuguese cities (Viana do Castelo, Porto and Sines) were chosen, the first two in the northwest of the country and the third in the southwest (Fig. 1a). The samples were exposed in the grounds of meteorological stations (Viana do Castelo and Sines) or Faculty of Sciences (Porto). Both a lichen survey in Portugal12 and the latest national report on air quality13 indicated a high content of some pollution-derived elements in these cities.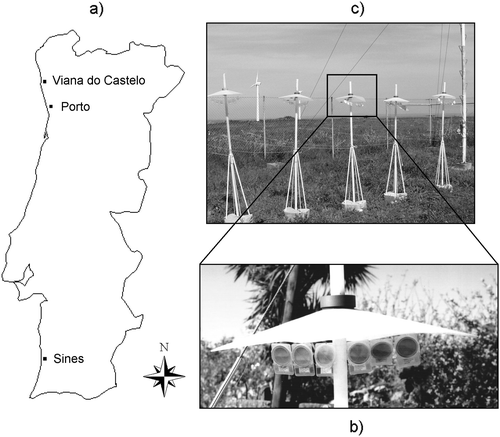 | ||
| Fig. 1 Map of mainland Portugal (north/east: Spain; south/west: Atlantic Ocean) showing (a) the location of the sampling cities, (b) the plastic petrislides and (c) the protection from direct atmospheric deposition. | ||
Sample exposure
Organic synthetic materials (cellulose acetate (Sigma) and Chelex® 100 resin (Bio-Rad), in the sodium form) and biological materials (lichen Flavoparmelia caperata (L.) Hale and tree bark Platanus hybrida Brot.) were exposed in parallel. The lichen was collected from pine tree bark (1.5 m from the soil). Both lichen and tree bark were collected in an area of the Porto district which has a low level of atmospheric pollution. Lichen samples were exposed (1) as they were collected (lichen transplant) and (2) removed from their substratum. In the latter case, visible extraneous leaf, soil and mossy material was removed from the thalli under a binocular microscope (thalli with fungus or discoloured sections were discarded) followed by two washing cycles in deionised water with agitation, for 5 min. Part of this material was cut into rectangular pieces (detached lichen) and part was ground (Teflon nitrogen cooled mill) after freeze-drying (lichen biomass). The biomass preparation process ended with sieving through a 125 μm nylon sieve. The tree bark biomass (approximately 2 mm thickness) was prepared in the same way. The organic synthetic materials were not subjected to any pre-treatment or conditioning. Two-month exposures were set up in the winter (from November 2002 to January 2003—only in Porto), in the spring (from March to May 2003) and in the summer (from June to July 2003). Significant weather factors are shown in Table 1 (data obtained from the Portuguese Meteorological Institute).| Month | City | Mean temperature/°C | Mean relative humidity (%) | Total rainfall/mm |
|---|---|---|---|---|
| November | Porto | 13.3 | 82 | 307.3 |
| December | Porto | 12.5 | 83 | 292.4 |
| March | Viana | 13.3 | 85 | 142.6 |
| Porto | 13.8 | 73 | 110.1 | |
| Sines | 13.8 | 87 | 21.1 | |
| April | Viana | 14.1 | 78 | 76.6 |
| Porto | 14.3 | 71 | 37.5 | |
| Sines | 14.7 | 83 | 2.1 | |
| June | Viana | 19.7 | 77 | 76.6 |
| Porto | 19.3 | 71 | 37.5 | |
| Sines | 20.1 | 73 | 2.1 | |
| July | Viana | 19.3 | 81 | 60.2 |
| Porto | 18.9 | 73 | 34.0 | |
| Sines | 19.9 | 74 | 2.1 | |
The lichen transplants were exposed in a nylon bag (2 mm porosity). For the granulated materials a device to keep them exposed to the atmosphere without significant losses was required. This device consisted of plastic petrislide (Millipore) and polyamide linens (Fig. 1b). The upper part of the cover of the petrislide was removed and discarded, thus obtaining a ring. The granulated material was exposed on the support of the petrislide wrapped in polyamide linen (61 μm porosity). The ring was used to fix the material and linen to the support of the petrislide. The detached lichen was exposed as the granulated materials. The time of exposure was not longer than two months to avoid heavy loss of granulated material. Preliminary studies have showed the optimum amount of material to expose: 100 mg for detached lichen; 250 mg for lichen biomass; 400 mg for tree bark biomass; 400 mg for cellulose acetate and 2 g for the resin Chelex® 100. Both materials in the petrislide and lichen bags were exposed protected from direct atmospheric deposition as shown in Fig. 1c and described in a previous work.14 The granulated materials and detached lichen were exposed in triplicate. The lichen transplants were exposed as one single piece (approximately 8 cm2) and replicates were taken according to the amount of lichen obtained after cleaning.
Assessment of lichen vitality
Samples of the lichen transplant, detached and biomass were exposed in Porto as described before and removed weekly, throughout the two month exposure periods. The lichen transplant was then removed from the substratum and washed in deionised water with agitation, for 5 min. Each sample was divided into two portions, one for the analysis of photosynthetic pigments and the other for potassium. From each portion three sub-samples were taken.| Ca = 12.19A665 − 3.45A649 |
| Cb = 21.99A649 − 5.32A665 |
Quantification of metals
After being retrieved the lichen transplant was removed from the substratum and washed in deionised water with agitation, for 5 min. The materials exposed in the petrislides were separated from the linen. All the samples were dried at 80 °C for 24 h, weighed, transferred to Teflon vessels (except Chelex® 100 resin) and digested with 4 ml HNO3/HF (3 ∶ 1). For this purpose, the following microwave program (Milestone MLS-1200 Mega) was used: 5 min at 250 W, 5 min at 400 W and 5 min at 500 W.18 The extraction of the Chelex® 100 resin was carried out by batch method, at room temperature, with HNO3 2 M, for 30 min, with agitation.19 In all cases the obtained solutions were diluted to 10 ml with deionised water and analysed for copper, lead, nickel and strontium, by flame atomic absorption spectrophotometry (spectrophotometer Philips PU 9200X). The accuracy of this procedure was checked by parallel determinations on Reference Material IAEA 336 and statistically identical results were obtained. Standard additions were performed both to the samples and IAEA 336 and the results showed no statistically significant differences to the calibration with aqueous standards, except for Sr. Therefore Sr was determined using the standard addition method. Controls (i.e. materials not exposed to the atmosphere) were digested and analysed in parallel with the samples, in every exposure.Reagents and material
All glassware and plastic containers were soaked in 20% (v/v) HNO3 for 24 h and rinsed several times with deionised water (conductivity <0.1 μS cm−1) before use. The chemicals used were of pro analysis grade or equivalent. Standard solutions for metal analysis were prepared from 1000 mg L−1 stock solutions (BDH (Spectrosol), Panreac and Merck) by weighing with deionised water.Statistical analysis
Results are expressed as the mean value of three independent replicates, except for controls (nine replicates). The limit of detection (lod) was calculated as recommended in the literature.20 A two-tailed t-student test21 was performed to compare the initial and the final metal content in each material, accumulations of airborne metals considered significant for P ≤ 0.05. The weekly quantification of total chlorophyll (a + b) and K was plotted on a time scale and a t-student21 was performed to check whether the slope was significantly different from zero (P ≤ 0.05), thus indicating a change in content throughout the exposure time.Results and discussion
Quantification of photosynthetic pigments and potassium
Only the lichen transplant and the lichen biomass exhibited statistically significant decreases (P ≤ 0.05) in the content of total chlorophyll in the summer (Fig. 2). The observed variations for both K and total chlorophyll concentrations probably reflected the natural variability of the lichens. It is known that lichens accumulate metals extracellularly and often the total metal content can be high without apparent harm because it is the nature and form of the metal that is important.22 Some authors indicate that metal accumulation increases chlorophyll degradation,2 while others state that the concentration of photosynthetic pigments increases with increasing levels of nitrogen- and sulfur-rich pollution.23 Carreras et al.3 concluded that atmospheric pollutants can be responsible for both the pigment degradation and an increase in synthesis. In this study the levels of photopigments did not systematically decrease within the two-month exposure period. Either the levels or the form of metals were not sufficient to cause physiological damage and/or the re-synthesis of these pigments, throughout the two-month exposure period, prevented the levels of photopigments from decreasing significantly. Moreover, it has been suggested that K ions protect the photobiont chlorophyll against degradation and possibly stimulate chlorophyll synthesis;2 indeed the levels of K did not decline significantly below initial levels during the exposure periods. The loss of K from the cell interior has been used as indication of membrane integrity based on the fact that most of the K is located intracellularly.24 The K concentration should remain constant in the lichen transplants and the detached lichen if there was no lost of K from within the cells or if part of the intracellular lost K was being compensated with uptakes from rainwater. Garty et al.25 found no correlation between concentration of K in the lichen thalli and the concentration of Cu, Pb or Sr which suggested to them that an additional source of K-rich dust must have been present in the air. Since there are no differences between the initial amounts of K in the three lichen forms (biomass, detached or transplants) we can state that the grinding did not lower the levels of K. We concluded that any differences found in the metal accumulation of the three forms should be attributed to differences in the binding capacity and not to physiological damages.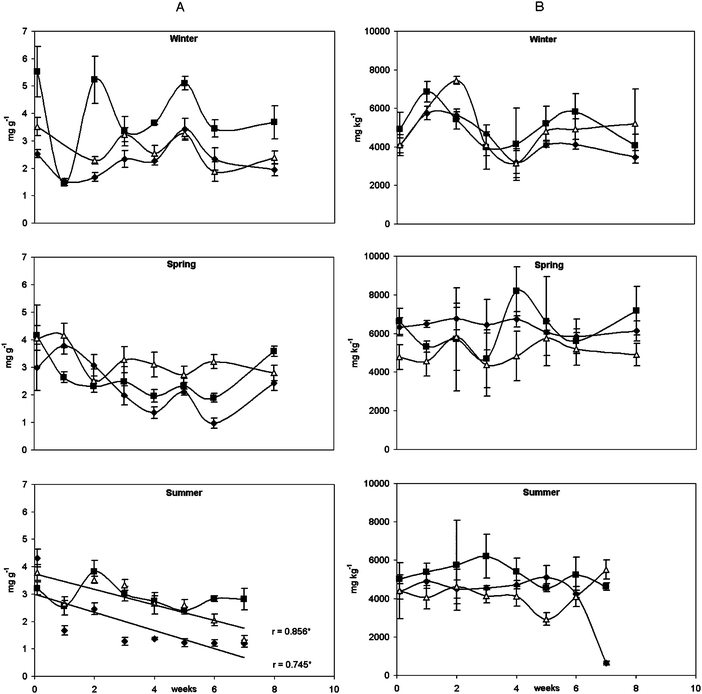 | ||
| Fig. 2 Weekly quantification of (A) total chlorophyll (a + b) and (B) total K, in lichen biomass (●), lichen detached (■) and lichen transplanted (△) exposed in the city of Porto. Symbols are means and bars are the standard deviations of three sub-samples; *significant decrease for P ≤ 0.05. | ||
Weather influence on metal uptake
The climatic conditions are known to be of great importance to the metal content of lichen species.26Table 1 shows differences in the mean temperature and total rainfall between spring and summer, for each city. The total rainfall decreased from north to south, in each season. In spite of the gradient we could not find a metal accumulation pattern that related with the season or city of exposure. The standard deviations result after the exposure were not higher than the standard deviations of the control, indicating that inter-exposure variability was higher than intra-exposure (Table 2). Increases in the accumulation of dissolved metals during rainy weather have been reported for lichens1 due to the fact that rain dissolves and transports particles and also facilitates the metal uptake since lichens have good ability to retain water.27 However, others state that periods of heavy rain can leach metals from the lichen rather than aid accumulation.24 We found different metal accumulation patterns suggesting that the role played by the climatic and meteorological conditions was important but not the main factor driving the accumulation of airborne metals. The protection from direct rain and the linen and petrislides used may have contributed to a lack of correlation with the environmental conditions.| Season | City | Lichen transplant mean ± sda | Lichen detached mean ± sd | Lichen biomass mean ± sd | Tree bark biomass mean ± sd | Cellulose acetate mean ± sd | Chelex® 100 resin mean ± sd | |
|---|---|---|---|---|---|---|---|---|
| a sd—standard deviation (n = 3 for samples and n = 9 for controls). b Values in bold represent a significant increase (P ≤ 0.05). c lod—limit of detection. | ||||||||
| Cu | ||||||||
| Control | 13.8 ± 3.5 | 13.8 ± 3.5 | 14.5 ± 3.3 | 5.9 ± 1.1 | <0.4 (lod)c | 0.7 ± 0.1 | ||
| Winter | Porto | 33.5 b ± 0.2 | 18.0 ± 2.7 | 11.3 ± 0.3 | 5.7 ± 0.3 | 4.1 ± 0.7 | 2.1 ± 0.4 | |
| Spring | Viana | 15.5 ± 1.3 | 15.4 ± 0.7 | 13.1 ± 0.5 | 5.3 ± 0.7 | 2.3 ± 1.1 | 1.2 ± 0.2 | |
| Porto | 29.9 ± 0.6 | 26.2 ± 1.9 | 16.0 ± 0.6 | 8.0 ± 2.0 | 3.5 ± 0.2 | 1.7 ± 0.1 | ||
| Sines | 16.6 ± 0.6 | 21.7 ± 3.2 | 19.7 ± 2.5 | 7.0 ± 0.9 | 1.5 ± 0.9 | 1.2 ± 0.2 | ||
| Summer | Viana | 28.1 ± 2.5 | 22.2 ± 4.5 | 19.6 ± 0.9 | 6.9 ± 1.9 | 1.3 ± 0.4 | 1.0 ± 0.1 | |
| Porto | 32.6 ± 4.1 | 16.5 ± 4.2 | 19.2 ± 0.7 | 8.9 ± 0.7 | 1.7 ± 0.3 | 1.5 ± 0.4 | ||
| Sines | 12.5 ± 2.4 | 16.7 ± 2.1 | 18.7 ± 1.3 | 4.2 ± 0.9 | <0.4 (lod) | 1.5 ± 0.3 | ||
| Ni | ||||||||
| Control | 10.0 ± 2.4 | 10.0 ± 2.4 | 6.5 ± 1.6 | 1.5 ± 0.4 | <1.1 (lod) | 1.3 ± 0.3 | ||
| Winter | Porto | 5.5 ± 0.1 | 25.2 ± 7.3 | 45.4 ± 8.5 | 6.7 ± 0.2 | 3.2 ± 0.2 | 1.5 ± 0.1 | |
| Spring | Viana | 12.3 ± 0.5 | 19.7 ± 4.2 | 4.9 ± 1.0 | 4.8 ± 1.5 | 4.9 ± 2.6 | 2.5 ± 0.4 | |
| Porto | 14.3 ± 1.2 | 14.7 ± 3.1 | 4.2 ± 0.4 | 6.9 ± 2.7 | 2.6 ± 0.3 | 2.1 ± 0.1 | ||
| Sines | 6.4 ± 0.1 | 19.8 ± 2.1 | 14.7 ± 4.2 | 8.3 ± 1.9 | 2.8 ± 0.1 | 6.3 ± 2.9 | ||
| Summer | Viana | 17.2 ± 4.0 | 14.5 ± 0.9 | 4.9 ± 0.8 | 2.5 ± 0.7 | 1.6 ± 0.4 | 2.2 ± 0.5 | |
| Porto | 18.7 ± 2.5 | 15.8 ± 1.8 | 3.5 ± 0.5 | 3.9 ± 0.9 | 1.7 ± 0.1 | 1.7 ± 0.3 | ||
| Sines | 12.6 ± 1.9 | 18.9 ± 0.6 | 7.1 ± 0.8 | 4.9 ± 0.4 | 1.6 ± 0.2 | 3.0 ± 0.4 | ||
| Pb | ||||||||
| Control | 11.6 ± 2.2 | 11.6 ± 2.2 | 9.4 ± 3.7 | <2.2 (lod) | <2.2 (lod) | 1.5 ± 0.3 | ||
| Winter | Porto | 16.2 ± 0.3 | 39.7 ± 2.8 | 9.9 ± 0.5 | 13.3 ± 3.4 | <2.2 (lod) | 1.3 ± 0.7 | |
| Spring | Viana | 12.3 ± 0.8 | 26.8 ± 5. 7 | 7.3 ± 2.8 | 9.3 ± 1.6 | <2.2 (lod) | <1.1 (lod) | |
| Porto | 19.3 ± 2.2 | 19.1 ± 8.0 | 7.2 ± 0.2 | <2.2 (lod) | <2.2 (lod) | 1.7 ± 0.4 | ||
| Sines | 16.2 ± 1.3 | <8.9 (lod) | 14.3 ± 4.5 | <2.2 (lod) | <2.2 (lod) | 2.4 ± 1.9 | ||
| Summer | Viana | 17.6 ± 3.0 | <8.9 (lod) | 11.7 ± 0.3 | 4.3 ± 0.8 | <2.2 (lod) | 1.2 ± 0.3 | |
| Porto | 31.3 ± 7.5 | 33.5 ± 5.0 | 13.7 ± 2.9 | 4.5 ± 1.3 | <2.2 (lod) | 2.5 ± 0.3 | ||
| Sines | 10.7 ± 1.8 | 26.0 ± 4.0 | 21.8 ± 5.3 | 6.6 ± 1.0 | <2.2 (lod) | 1.7 ± 0.3 | ||
| Sr | ||||||||
| Control | 6.2 ± 1.1 | 6.2 ± 1.1 | 7.1 ± 1.0 | 105 ± 17 | <0.9 (lod) | 12.9 ± 2.0 | ||
| Winter | Porto | 15.1 ± 0.1 | 19.9 ± 3.1 | 11.8 ± 0.5 | 23.5 ± 0.6 | 13.3 ± 1.1 | <1.7 (lod) | |
| Spring | Viana | 6.8 ± 0.1 | 6.7 ± 1.8 | 12.2 ± 4.4 | 102 ± 6 | <0.9 (lod) | 14.4 ± 0.3 | |
| Porto | 6.29 ± 0.02 | 7.8 ± 1.2 | 8.9 ± 0.6 | 101 ± 5 | <0.9 (lod) | 13.2 ± 0.2 | ||
| Sines | 17.0 ± 0.4 | 21.0 ± 4.6 | 15.1 ± 3.0 | 115 ± 7 | 7.9 ± 3.1 | 16.7 ± 2.5 | ||
| Summer | Viana | 114 ± 3 | 5.5 ± 0.9 | 6.4 ± 0.1 | 104 ± 13 | <0.9 (lod) | 13.0 ± 0.3 | |
| Porto | 10.8 ± 0.3 | 11.1 ± 1.9 | 6.4 ± 0.2 | 103 ± 3 | <0.9 (lod) | 15.3 ± 0.7 | ||
| Sines | 15.9 ± 0.7 | 6.3 ± 1.1 | 7.4 ± 0.2 | 109 ± 2 | <0.9 (lod) | 15.7 ± 2.1 | ||
Metal uptake by the different materials
Lichens integrate long-term deposition patterns because the elemental concentration is the result of the equilibrium between uptake and release from and into the surrounding environment.24 The t-student test shows that the response of the materials oscillated from significant accumulation of airborne metals to maintenance of the control levels (Table 2). Other workers3,28,29 report similar findings for lichens, when the time of exposure to the atmosphere is shorter than three months as it is in this study.All the materials accumulated Pb and Sr in a more irregular fashion than Cu or Ni. The synthetic materials exhibited almost no accumulation of Pb or Sr. The latter finding suggests that metals were present in the atmosphere as particulates and not in the soluble ionic form. Table 2 shows, for Ni in lichen biomass, in Porto, in winter, and for Sr in lichen transplant, in Viana, in summer, accumulations much higher than in any other exposure which were considered non-representative. The relative standard deviations varied between 2 and 50% both for the synthetic and biological materials, being the mean value 20%. The heterogeneous deposition of particulate matter upon the materials is the possible cause of the variability of the results. Our hypothesis that synthetic materials or homogenized biomass would have lower standard deviations than transplant and detached lichens was not confirmed.
The intact lichen (either transplants or detached) exhibited the largest accumulations of Cu, Ni and Pb in the city of Porto while the greatest accumulations of Sr occurred in Sines. Most of the accumulation of Sr is probably due to the proximity of the ocean. A favourable wind regime in Sines may have contributed to the higher content of Sr. It can also be seen that the lichen transplants and detached do not exhibit the same pattern of accumulation in every exposure. No explanation could be found for this lack of correlation in the behaviour of both lichens. The lichen transplant final/initial metal content ratios were equal to the ones of the detached lichen leading to the conclusion that the detached lichen is suitable for biomonitoring.
The lichen biomass accumulated more metals in Sines, particularly during the spring. The tree bark biomass accumulated more Cu in Porto, during the summer, Ni in Sines, during the spring, and Pb in Porto, during the winter. A possible explanation is that the level of hydration of the biomass acts as a selector of the available binding sites. The biomass final/initial metal content ratios were inferior to transplants or detached lichen, except for Ni for tree bark biomass that was higher than in any other material. Thus the hypothesis that the biomass would bind more atmospheric metals was not confirmed.
Both cellulose acetate and resin accumulated more Cu in Porto, during the spring and summer. As for the accumulation of Ni, the resin exhibited a decreasing gradient from south to north and from the summer to winter. The cellulose acetate exhibited the lowest accumulations in the summer, regardless of the city of exposure. The synthetic materials presented the highest final/initial metal content ratios of Cu and could be an alternative to biomonitoring with lichens only when Cu and Ni are the elements of interest.
Acknowledgements
This work was founded by Fundação para a Ciência e a Tecnologia, Portugal (POCTI/38411/CTA/2001 – BIOCAL).References
-
J. Garty, in Protocols in Lichenology: culturing, biochemistry, ecophysiology and use in biomonitoring, ed. I. Kranner, R. Beckett and A. Varma, Springer lab manual, Berlin, 2002, pp. 458–482 Search PubMed
.
- J. Garty, Y. Karary and J. Harel, Environ. Exp. Bot., 1992, 32, 229 CrossRef CAS
.
- H. A. Carreras, E. D. Wannaz, C. A. Perez and M. L. Pignata, Environ. Res., 2005, 97, 50 CrossRef CAS
.
- C. Branquinho, D. H. Brown and F. Catarino, Environ. Exp. Bot., 1997, 38, 165 CrossRef CAS
.
- A. M. G. Pacheco, M. C. Freitas, L. I. C. Barros and R. Figueira, J. Radioanal. Nucl. Chem., 2001, 249(2), 327 CrossRef CAS
.
- D. Aderhold, C. J. Williams and R. G. J. Edyvean, Bioresour. Technol., 1996, 58, 1 CrossRef CAS
.
- C. J. Williams and R. G. J. Edyvean, Trans Inst. Chem. Eng., Part B, 1997, 75, 1926 Search PubMed
.
- W. A. Stirk and J. van Staden, Bot. Mar., 2000, 43, 467 Search PubMed
.
- J. P. Cabral, Environ. Exp. Bot., 2003, 49, 237 CrossRef CAS
.
-
A. G. Howard and P. J. Statham, Inorganic Trace Analysis. Philosophy and Practice, John Wiley & Sons Ltd, Chichester, 1993, pp. 46–50 Search PubMed
.
- M. Pesavento, G. Alberti and A. Profumo, Anal. Chim. Acta, 2000, 450(1–2), 309 CrossRef
.
- M. C. Freitas, M. A. Reis, L. C. Alves, H. Th. Wolterbeek, T. Verburg and M. A. Gouveia, J. Radioanal. Nucl. Chem., 1997, 217, 21 CrossRef CAS
.
- Portuguese Environmental Institute web page (http://www.iambiente.pt).
- M. C. Freitas, M. A. Reis, A. P. Marques and H. Th. Wolterbeek, J. Radioanal. Nucl. Chem., 2000, 244, 109 CrossRef CAS
.
-
H. W. Pfeifhofer, R. Willfurth, M. Zorn and I. Kranner, in Protocols in Lichenology: culturing, biochemistry, ecophysiology and use in biomonitoring, ed. I. Kranner, R. Beckett and A. Varma, Springer lab manual, Berlin, 2002, pp. 363–378 Search PubMed
.
- A. R. Wellburn, J. Plant Physiol., 1994, 144, 307 CAS
.
- S. F. Stone, M. C. Freitas, R. M. Parr and R. Zeisler, J. Anal. Chem., 1995, 352, 227 CAS
.
- H. M. C. F. Tavares and M. T. S. D. Vasconcelos, Toxicol. Environ. Chem., 1996, 54, 195 CrossRef CAS
.
- P. Figura and B. McDuffie, Anal. Chem., 1980, 52, 1433 CrossRef CAS
.
- G. L. Long and J. D. Winefordner, Anal. Chem., 1983, 55(7), 712 CrossRef
.
-
J. H. Zar, Biostatistical Analysis, Prentice Hall, Upper Saddle River, New Jersey, 4th edn., 1999, pp. 122–175 Search PubMed
.
-
D. H. S. Richardson, Pollution monitoring with lichens, The Richmond Publishing Co. Ltd, Slough, 1992, pp. 37–38 Search PubMed
.
- H. A. Carreras, G. L. Gudiño and M. L. Pignata, Environ. Pollut., 1998, 103, 317 CrossRef CAS
.
-
C. Branquinho, in Metals in the Environment Analysis by Biodiversity, ed. M. N. V. Prasad, Marcel Dekker Inc., New York, 2001, pp. 117–157 Search PubMed
.
- J. Garty, N. Kloog and Y. Cohen, Arch. Environ. Contam. Toxicol., 1998, 34, 136 CrossRef CAS
.
- H. A. Carreras and M. L. Pignata, Environ. Pollut., 2001, 111, 45 CrossRef CAS
.
-
T. H. Nash III, Lichen Biology, Cambridge University Press, Cambridge, 1996, pp. 1–5 Search PubMed
.
- I. Calliari, G. Caniglia, S. Nardi, A. M. Tollardo and R. Callegaro, X-Ray
Spectrom., 1995, 24, 143 CAS
.
- J. Garty, M. Kauppi and A. Kauppi, J. Environ. Qual., 1996, 25, 265 CAS
.
Footnote |
| † Presented at the Fifth International Symposium on Modern Principles of Air Monitoring & Biomonitoring, June 12–16 2005, Norway. |
| This journal is © The Royal Society of Chemistry 2006 |
