A comparison of X-ray fluorescence and wet chemical analysis for lead on air filters from different personal samplers used in a secondary lead smelter/solder manufacturer†‡
Martin
Harper
* and
Bruce
Pacolay
Exposure Assessment Branch, Health Effects Laboratory Division, National Institute for Occupational Safety and Health, 1095 Willowdale Rd., MS-3030, Morgantown, WV 26505, USA
First published on 1st July 2005
Abstract
Portable X-ray fluorescence (XRF) technology may provide faster turn-around without compromising accuracy when assessing personal exposures to metals such as lead, but it has only been tested in limited field environments. This study is part of a series, where various types of sampler are used to collect airborne lead in different environments for presentation to a portable XRF analyzer. In this case personal samples were taken at a manufacturer of solder alloys consisting mainly of lead and tin, using the closed-face 37 mm cassette (CFC), the 37 mm GSP or “cone” sampler, the 25 mm Institute of Occupational Medicine (IOM) inhalable sampler, the 25 mm button sampler, and the open-face 25 mm cassette. Mixed cellulose–ester filters were used in all samplers. Following XRF analysis the samples were extracted with acid and analyzed by inductively coupled plasma optical emission spectroscopy (ICP). The internal surfaces of CFC’s and 25 mm open-face cassettes were also wiped, and the wipes analyzed for lead to assess wall-losses in these two samplers. Analysis of all elements present is useful to ascertain contributions to matrix interference effects. In addition to lead, other metals such as tin, copper, iron, silver, cadmium and antimony were also detected in some or all of the samples by ICP analysis, but only copper and iron could be determined using the XRF analyzer under test. After the removal of a few outliers, all five samplers gave good correlations (r2 > 0.9) between the two analytical methods over the entire range of found lead mass, which encompassed both the action level and the permissible exposure limit enforced in the USA by the Occupational Safety and Health Administration (OSHA). Linear regression on the results from most samplers gave almost 1 ∶ 1 correlations without additional correction, indicating an absence of matrix effects, particularly from tin, which was the most common element after lead. The average of three XRF readings across filters from the GSP samplers gave the best results with 96.7% of results within ±25% and 100% within ±30% of the associated ICP values. Using the center reading only was almost as good with 90.0% of results within ±25% and 96.7% within ±30% of the associated ICP values, and results can be obtained faster with a single reading. The use of an algorithm developed by OSHA for three readings from the CFC filter samples gave the next best results with 93.3% of XRF results within ±25% of the corresponding ICP values. However, analysis of wipes from the interior of the cassettes indicated a substantial loss of sample to the walls, and even larger wall-losses were encountered in the 25 mm open-face cassette. Neither this latter sampler nor the IOM or button sampler met the 95% criterion, even for ±30% accuracy.
Introduction
This study is part of an ongoing large-scale investigation into the use of portable X-ray fluorescence (XRF) analyzers for on-site evaluation of airborne lead samples in the workplace. The results of a pilot project in a small lead scrap smelter and wire-drawing operation have already been published,1 as have the first set of results from this major program, obtained from a leaded bronze melting and casting operation.2 The clinical effects of industrial lead poisoning have been referenced, along with a discussion of the various applicable exposure limit values and sampling equipment, and a discussion of the relationship between air and blood measurements.1 Current protocols require off-site analysis in a laboratory, which may take several days, even weeks. A more rapid response is preferred, especially by health and safety compliance officers who may be under regulatory deadlines, but also by any health and safety managers faced with rapidly changing conditions, such as in the construction industries, or where process changes are being evaluated. XRF technology promises the possibility of analytical results within minutes of the end of sample collection, and, potentially, without compromising accuracy. Bench-top XRF analyzers have been used for determining lead on air filters for many years in the UK.3 However, although currently published portable XRF methodologies from U.S. National Institute for Occupational Safety and Health (NIOSH) and Occupational Safety and Health Administration (OSHA) are generally considered sufficiently accurate for use as screening tools, there is some question as to their ability to be used to support citations based on compliance with legally enforceable limit values.4,5A discussion of the accuracy requirements for sampling and analytical techniques for lead in air has been published.2 Improved accuracy may be possible by presenting the sample differently to the XRF analyzer, through the use of air samplers which provide more uniform sample deposits, and this is the focus of the current investigation. The samplers used in this study are: the 37 mm styrene/acrylonitrile filter cassette with a 4 mm entry inlet (CFC; Omega Specialty Instruments, MA, USA), which is the current U.S. standard method for dust sampling when operated at 2 l min−1; the Institute of Occupational Medicine (IOM; SKC Ltd., UK) sampler, which also operates at 2 l min−1, and which is the only sampler designed specifically to meet the inhalable convention;6 the German GSP (Gesamtstaubprobenahme; Gesellschaft für Schadstoffmesstechnik GmbH, Germany) or “cone” sampler, which has a 9 mm conical entry inlet opening to a 37 mm filter and is operated at a flow rate of 3.5 l min−1; the button sampler from the University of Cincinnati (SKC, Inc., PA, USA) which has a porous hemispherical screen and which uses a 25 mm filter and is operated at 4 l min−1; and a 25 mm version of the CFC (Omega Specialty Instruments, MA, USA), operated in the “open-face” mode at 2 l min−1.
The objective of this study is to examine the performance of a portable XRF analyzer with the samplers described above for use in accordance with the previously published NIOSH Manual of Analytical Methods (NMAM) 77024 in specific field situations, in this case a secondary lead smelter and solder manufacturer. In the manufacturing plant participating in this study lead is encountered in the first place as a primary or trace constituent during the reclamation of solder and other metals from a wide range of industries, and is then used as a primary feedstock for high-quality alloys, solders, and pure metal products. Exposures are found in both the smelting and casting areas of the plant. In the smelting area, metal-bearing materials such as drosses, skims, dusts, ingots, pastes, and spent anodes, are recycled and integrated into solder products. The casting facility has the capability to supply alloy bar sizes (containing various percentages of lead) in the 1.5 to 80 lb range. Tin/lead alloys are the most widely produced of all solders at this facility with lead percentages ranging from 30 to 90. Smelter operators are exposed to lead when tapping furnaces and in other activities directly related to recycling the lead from scrap material. Casting workers are exposed to lead primarily when hand dipping ladles into the liquid alloys and also when pouring bars. The pot temperatures for the liquid alloys are around 600 °C. The workers exposed to lead are in a facility-wide lead program which includes quarterly blood lead monitoring and the use of powered air-purifying respirators (PAPR’s) at all times. This facility also produces wire solder by compression and extrusion of both cold and warmed alloy billets, but exposures in this process were not studied. Lead and tin were the main elements present in the analysis of air filters. Other metals, including copper, iron, silver, cadmium and antimony were present but not in every sample. Some metals (tin, silver, cadmium and antimony) could not be analyzed by the specific XRF analyzer used in this study, but their presence was recorded through ICP analysis.
Experimental
Personal samples were collected from workers in both casting and smelting jobs using the 5 samplers incorporated in this study. Samples were taken for approximately 4–6 h. The useable numbers of samples from each sampler type collected were as follows:32 GSP; 32 CFC; 31 IOM; 31 button; 30 25 mm open-face cassettes. Mixed cellulose–ester filters were used for all samples. The XRF analyzer used (109Cd excitation source, Model XL701, NITON Corporation, Billerica, MA, USA) is the same as that used in the first part of the current program of work,2 and is considered by the manufacturer to be an improvement over the otherwise essentially similar model previously evaluated for the method published as NIOSH NMAM number 7702,4 and used in the pilot project for this study.1 Note that method 7702 does not specify a particular make or model of analyzer should be used, but only one instrument was evaluated in the development of the method. Other instruments are under evaluation.
Method 7702 as written uses the CFC and 37 mm filters and requires taking three readings (top, bottom and center) with a portable XRF detector, in order to account for non-uniform sample distribution across the filter, and the three readings are then combined according to a formula given in the method. OSHA has published a slightly different algorithm for combining the three readings.5 The XRF analyzer has a 2 cm × 1 cm analysis window and provides readings in μg cm−2. For the CFC filters, the three readings were entered into a spreadsheet and the algorithms from the NIOSH and OSHA methods were used to determine the total mass of lead present on the filters. For the filters from the GSP samplers, three readings were taken according to the procedure for CFC filters, but the results were calculated using the unweighted average of all three readings, or the central reading only, adjusted for the ratio of the XRF window reading in μg cm−2 to the nominal area of the filter covered by the sample deposit (7.5 cm2). For samplers using 25 mm filters (Button and IOM), homogenous deposition was assumed and the results from analyzing the central portion only were also corrected by the ratio of the XRF window to the nominal filter area (3.46 cm2 for the Button sampler, 3.46 or 2.84 cm2 for the IOM, and 3.80 cm2 for the 25 mm open-face cassette). The reason that two areas were used for the IOM sampler is that the value taken from measurements and used in the pilot project (2.84 cm2)1 was not the best match to the data from the bronze foundry studies.2 Measurement of the deposit areas of filters from this solder manufacturer study provided the value that gave the best match to the data in the bronze foundry study (80% of measurements gave 3.46 cm2), which should, therefore, be the most applicable.
Note that the OSHA method calls for the CFC filter support pad to be included with the filter in the filter holder. OSHA suggests that this is the reason for the difference in algorithm between the NIOSH and OSHA methods, although without providing evidence.5 Careful handling is required to remove the filter from the support pad without disturbing the dust deposit on the filter, so keeping the filter and pad together is an attractive option. However, the Mylar film will not in that case sit smoothly over the filter because the filter holder is not deep enough to accommodate the pad. The CFC filters were analyzed without the support pad in this study.
All XRF measurements involved counts accumulated for 240 nominal seconds. This time-period is considered the best trade-off between accuracy and speed of analysis. The filters were removed from the samplers and placed in the appropriately sized filter holder, 25 mm or 37 mm, provided by the manufacturer of the XRF analyzer. The filter holders are made of cardboard, with Mylar film over the filter area. The cardboard holders were placed on a test stage also provided by the manufacturer, so that the filter holder could be placed in the same position(s) each time under the analyzers X-ray beam. The XRF analyzer’s calibration was checked with thin film standards from Micromatter Company (Deer Harbor, WA, USA). Following XRF analysis the filters were analyzed at a laboratory accredited by the American Industrial Hygiene Association (AIHA) by NIOSH Method 7300 (metals by ICP7) modified to include extraction of the Mylar film covering the filters, and described previously.2 Filters from previous AIHA proficiency test rounds were incorporated as quality checks. Media blanks and field blanks were also included.
Statistical analysis was performed in accordance with the rationale and protocol provided in the pilot project paper.1 Some log-normality is associated with the data, but no transformation was used in the development of linear correlations. For some of the graphs presented in the figures, a few data points were removed from the regression where it was considered that their presence might cause an undue bias to the trendline. The decision point used to exclude values was where the XRF measurement differed by more than ±31% from the ICP measurement, except in the case of the CFC filter results, where a slightly higher value was used to offset the obvious positive bias from the NIOSH algorithm. All points have been plotted, unless they were very high values that would cause the graphs to be difficult to read. No statistical tests were performed to determine outliers because linear regression is used only to determine whether a systematic bias might exist between the XRF and reference chemical methods, and it is not used as a measure of accuracy (uncertainty). The robust measure of accuracy which includes all data points, described in the pilot project paper1 was used. This method essentially compares each XRF value directly with its corresponding ICP reference value as to whether it meets or exceeds a pre-determined measure of closeness to that value (e.g. ±20%,8 25%9 or 30%10). This estimate is conservative in that the uncertainty in the reference method is not addressed. Through this method, samplers can be ranked in accordance with the percentage of values falling within the selected limit, and an acceptability criterion can be used (e.g. 95%) to classify samplers as appropriate for specific decision end-points, such as compliance with limit values. As stated, no experimental data points are removed for this calculation.
The exteriors of the CFC and 25 mm open-face sample cassettes were carefully wiped with a “wet wipe”, typically a Ghost Wipe (Environmental Express, SC, USA), to remove external contamination before opening, following the OSHA procedure, and the wet wipe discarded. After removing the filter, the cassettes were re-assembled, and the open-face cassettes were capped, and stored until the end of field collection. In the laboratory, these cassettes were re-opened, and, using a fresh pair of gloves each time, a new Ghost Wipe was opened and folded a few times to make a very small square and then used to wipe the internal surface of the cassette. It was then folded again so as to make sure the exposed side was protected, placed in a 50 ml centrifuge tube and sent for analysis. Field blank filter cassettes were also wiped in this manner. Few protocols using 37 mm and 25 mm plastic cassettes require an estimation of sample collected on the interior of the cassettes when the analysis is by chemical means, even though several protocols require this to be accounted for when gravimetric analysis is used. Therefore, the results given below are for the filter analyses only.
Results and discussion
Lead was the metal present in the largest quantities on the filters, with tin being a close second. For the CFC samples, this represents concentration ranges of 10 to 471 μg m−3 lead (mean: 133 μg m−3, geometric mean: 84 μg m−3) and concentration ranges of 11 to 313 μg m−3 tin (mean: 95 μg m−3, geometric mean: 63 μg m−3). These two elements were detectable by ICP analysis on all filters. For reference, the OSHA Action Level for lead is 30 μg m−3, while the Permissible Exposure Limit (PEL) is set at 50 μg m−3. Tin was observed in all samples up to about 300 mg m−3. Iron, copper and silver were found in much smaller quantities, being below detection limits for the ICP on some CFC filters. Maximum concentrations observed were 69 μg m−3 iron, 57 μg m−3 copper and 29 μg m−3 silver. Cadmium was also present in about 60% of samples. Generally, the results were low, with the geometric mean of samples where cadmium was detected being 2.3 μg m−3, and with only one sample exceeding 10 μg m−3. Antimony was also found in trace amounts, but in fewer than 50% of samples. Of these elements, only iron and copper were able to be analyzed by the XRF used. Comparison of XRF results with ICP analysis showed a similar negative bias in the analysis of copper by XRF that had been noted previously.2Field blanks of both 37 mm and 25 mm filters generally gave non-detectable ICP results for lead, with a single high value of 3 μg/filter on each size. Field blank filter cassettes wipes gave ICP results between non-detectable and 2 μg/wipe. The lower ICP mass cut-off limits for data inclusion (5 μg/filter for 25 mm filters and 10 μg/filter for 37 mm filters) used in the prior studies1,2 were generally used in this study, except that a value of 7 μg/filter was used for GSP 37 mm filters. Samples below these limits included 2 GSP, 2 CFC, and 3 IOM samples, but none of the button or 25 mm open-face cassette samples, resulting in usable sample numbers of 30 GSP, 30 CFC, 28 IOM, 31 button and 30 25 mm open-face cassettes. As noted above, in order to obtain the most representative graphical correlations between the XRF values and the corresponding ICP analyses, results judged to be outliers deviating strongly from the trendline were removed from the calculation, as also were two very high values that were not considered outliers, but which would still have skewed the trendlines. However, all of these values were included in the calculation of accuracy. The number of results considered outliers were GSP: 1 (center reading), 0 (average reading); CFC 4 (NIOSH algorithm), 0 (OSHA algorithm); IOM: 5 (3.46 cm2), 3 (2.84 cm2); button: 0, but one very high non-outlier value was also removed; 25 mm open-face cassette: 2, and one very high non-outlier value was removed. A summary of all statistics for bias and accuracy is given in Table 1. All trendlines were forced through the origin. The slopes showed little bias from unity compared to the negative bias seen with many of the corresponding graphs from the bronze foundry.2 The GSP sampler and 25 mm open-face cassettes had the smallest change in slope from that study to this (6% and 7%, respectively), and the other samplers showed a greater change (16% for CFC OSHA, IOM and button samplers).
| Sampler | Slope | r 2 (outliers or other points not used) | % Confidence at specified % accuracy | ||
|---|---|---|---|---|---|
| 20% | 25% | 30% | |||
| CFC OSHA | 1.14 | 0.98 (0) | 83.3 | 93.3 | 96.7 |
| CFC NIOSH | 1.26 | 0.98 (3) | 46.7 | 53.3 | 70.0 |
| GSP average | 0.97 | 0.98 (0) | 90.0 | 96.7 | 100 |
| GSP center | 1.05 | 0.96 (1) | 86.7 | 90.0 | 96.7 |
| IOM 3.46 cm2 | 1.10 | 0.99 (5) | 60.7 | 75.0 | 78.6 |
| IOM 2.84 cm2 | 0.95 | 0.96 (3) | 71.4 | 78.6 | 89.3 |
| Button | 0.89 | 0.99 (1) | 74.2 | 83.9 | 93.5 |
| 25 mm | 0.98 | 0.94 (2) | 76.7 | 80.0 | 93.3 |
GSP
ICP values for lead ranged up to 670 μg/filter and are plotted in Fig. 1 against both the average of the three (top, bottom, and center) XRF readings and the central XRF reading only. For the average of all three readings the slope of the correlation was 0.97 with a correlation co-efficient of 0.98 (no outliers). All (100%) of the XRF results were within 30% of the corresponding ICP results. 96.7% of the XRF results were within ±25% of the corresponding ICP results. Since this meets the requirement for the OSHA construction standard,8 the more restrictive criterion for the general industry standard9 can also be considered: 90% of XRF results were within ±20% of the corresponding ICP values. For the center reading only the slope of the correlation was 1.05 with a correlation coefficient of 0.96 (1 outlier). 96.7% of XRF results were within ±30%, and 90% were within ±25% of the corresponding ICP results. As in the bronze foundry study, the center reading is biased higher than the average, indicating a slightly greater deposition towards the center of the filter. Wipe samples were not taken from the GSP samplers. | ||
| Fig. 1 Plot of ICP vs. XRF results for the GSP sampler (N = 30) calculated using the average of three readings (dashed line) and the central reading only (solid line). One of the plotted values for the central reading only was omitted in the regression calculation. | ||
CFC
ICP values for lead ranged up to 320 μg/filter and are plotted in Fig. 2 against the results from the three (top, bottom and center) XRF readings processed through both the OSHA and NIOSH algorithms. For the OSHA algorithm the slope of the correlation was 1.14 with a correlation coefficient of 0.98 (all points included). 96.7% of the XRF results were within ±30%, and 93.3% of XRF results were within ±25% of the corresponding ICP results. For the NIOSH algorithm the slope of the correlation was 1.25 with a correlation coefficient of 0.99 after the exclusion of 4 points judged outliers. Only 70.0% of the XRF results by the NIOSH algorithm were within ±30%, and 53.3% of XRF results were within ±25%, of the corresponding ICP results, with a high positive bias. Wiping the interior of the cassettes revealed substantial quantities of lead. No sample interior was lead-free, and only 10% of samples had less than 10% of the filter value on the corresponding interior wipe. In more than half the samples, greater than 25% of the filter value was found on the wipe, reaching a maximum of 74%. The average sample loss was 38% of the filter catch or 27.5% of the total (filter plus walls). No relationship could be seen between percentage wall loss and loading. | ||
| Fig. 2 Plot of ICP vs. XRF results for the CFC sampler (N = 30) calculated using both the NIOSH (solid line) and OSHA (dashed line) algorithms. Three values plotted for the NIOSH algorithm were omitted in the regression calculation. | ||
IOM
ICP values for lead ranged up to 360 μg/filter, except for one value of 790 μg/filter which was considered an outlier, and also was not plotted in Fig. 3 for clarity of the rest of the data. The data are presented for both filter areas which have been proposed for this sampler. For the 3.46 cm2 filter area that was measured on the filters taken in this study, and that best fit the data from the bronze foundry study2 the slope of the correlation was 1.1 with a correlation co-efficient of 0.99 after the removal of 5 points judged outliers. 78.6% of XRF results were within ±25% of the corresponding ICP results. For the 2.84 mm2 filter area that was measured on samples from the bullet casting study,1 and which best fit the data in that study, the slope of the correlation was 0.95 with a correlation co-efficient of 0.96 after the removal of 3 outliers. 75.0% of XRF results were within ±25% of the corresponding ICP results. Neither area value gave results that bettered 95%, even at the ±30% criterion. Wipe samples were not taken from the IOM samplers. | ||
| Fig. 3 Plot of ICP vs. XRF results for the IOM sampler (N = 28) using both proposed nominal filter deposit areas: 3.46 cm2 (solid line) and 2.84 cm2 (dashed line). One extreme value (790 μg/filter by ICP) is not plotted. It was also considered an outlier and was omitted in the regression calculation. In addition, four other values for the 3.46 cm2 sample deposit area and 3 other values for the 2.84 cm2 sample deposit area were also considered outliers; they are plotted, but were omitted from the regression calculation. | ||
Button
ICP values for lead ranged up to 720 μg/filter, except for one value of 2300 μg/filter which was not considered an outlier but was removed from Fig. 4 to give clarity to the rest of the data. One other value was considered an outlier but was not removed from the figure. After these two points were removed, the slope of the correlation was 0.89 with a correlation coefficient of 0.99. 93.5% of the XRF results were within ±30% and 83.9% of XRF results were within ±25% of the corresponding ICP results. It is interesting that the slope has a greater negative bias than any of the other samplers, and it should be compared with the need for a 20% upward correction factor in the bronze foundry study,2 while only 10% was necessary for the other samplers in that study. Wipe samples were not taken from the button samplers.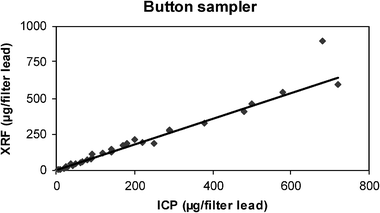 | ||
| Fig. 4 Plot of ICP vs. XRF results for the button sampler (N = 31). One extreme value (2300 μg/filter by ICP) was not considered an outlier, but was not plotted and was omitted from the regression calculation. | ||
25 mm open-face cassette
ICP values for lead generally ranged up to 250 μg/filter. One value of 1500 μg/filter was not considered an outlier but was removed from Fig. 5 to give clarity to the rest of the data, Two other values were considered outliers but were not removed from the figure. After the removal of these three points, the slope of the correlation was 0.98 with a correlation co-efficient of 0.94. 93.3% of the XRF results were within ±30% and 80.0% of XRF results were within ±25% of the corresponding ICP results. Wiping the interior of the cassettes revealed an even greater loss of lead to the walls than was observed for CFC samples, in many cases exceeding the levels found on the filters. No cassette interior was lead-free, with only one sample having less than 10% of the corresponding filter value on the interior wipe. In 80% of the samples, greater than 25% of the filter value was found on the wipe, and greater than 50% of the filter value was found on the wipe in almost half the samples. The average sample loss was 66% of the filter catch or 40% of the total (filter plus walls). No relationship was seen between the percentage wall loss and loading.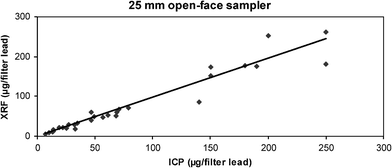 | ||
| Fig. 5 Plot of ICP vs. XRF results for the 25 mm open-face cassette sampler (N = 30). One extreme value (1500 μg/filter by ICP) was not considered an outlier, but was not plotted and was omitted from the regression calculation along with two values that are plotted, but which were considered outliers. | ||
Limits of quantitation
The lower cut-off limits for data inclusion in these studies might be considered as limits of quantitation (LOQ) based on the cumulative analysis of field samples. In Fig. 6 (representing all 37 mm filter samples combined from the bronze foundry study and this study) and Fig. 7 (representing all 25 mm filter samples combined from the two studies) are plotted all samples in the range of ICP values up to 30 μg/filter. A trend of increasing relative error with decreasing ICP value may be present, although it is not clear, but, in any case, the relative error does not exceed that noted for analysis over the total range of analyses. For the values in the range from 10 to 30 μg/filter for 37 mm filters the percentage of XRF analyses within ±25% of the corresponding ICP values is 94%. For values between 5 and 15 μg/filter for 25 mm filters the percentage of XRF analyses within ±25% of the corresponding ICP values is 85%, while for values between 15 μg and 30 μg/filter, the percentage of XRF results within ±25% of the corresponding ICP results increases to 93%. 15 μg/filter is also the level at which the analysis of loaded 25 mm filters in the HSL round-robin11 became unacceptably poor. Therefore, 37 mm filters may have a lower LOQ than 25 mm filters, perhaps because of the longer XRF analysis time involved for the triplicate determinations. | ||
| Fig. 6 Plot of ICP results vs. relative percent error of the corresponding XRF results for values up to 30 μg per filter (ICP analysis) for 37 mm filter samples from this study combined with those from the bronze foundry study. A few values falling outside of the limits of the graph are not shown for clarity. | ||
 | ||
| Fig. 7 Plot of ICP results vs. relative percent error of the corresponding XRF results for values up to 30 μg per filter (ICP analysis) for 25 mm filter samples from this study combined with those from the bronze foundry study. A few values falling outside of the limits of the graph are not shown for clarity. | ||
Conclusions
This study provided an opportunity to test the comparative analysis of samples from the field by portable XRF against a laboratory chemical technique (ICP) in the absence of significant interferences (the excitation energy for tin being too high to allow absorbance of the lead fluorescence emission, and other elements being present only at relatively low levels). It should be compared to the bronze foundry study where potentially interfering elements (copper, zinc and iron) were present in similar proportions as lead, and where a negative bias in the regression slope of approximately 10% was observed for lead with most samplers, except the button sampler that had a 20% negative bias.2 No bias of negative 10% was observed for any of the samplers in this study, with the exception of the button sampler. The constant difference between the button samplers and the others used in this study may indicate a further interference simply from the thickness of the sample deposit, as the button sampler, with its high flow rate and small filter area, gives the greatest depth of sample for a given sampling time.The GSP sampler using an average of the three XRF measurements met the EU and at least one of the OSHA requirements for a compliance method, without correction. Instruments, such as the one used here, that employ sealed radioactive sources as the source of X-rays have the disadvantage that the source decays over time. This is corrected in the unit by increasing the time taken for readings. A 240 s nominal count reading can stretch to become many minutes before the source is considered in need of replacement, so that a result from triplicate filter readings can take up to a half-hour. A single reading of the center portion of the filter only, which takes less time and would therefore be preferred, was not as accurate as the triple readings. Nevertheless, single readings could be used to identify which filters had lead masses close to the compliance values and would be candidates for more thorough analysis. When the LOQ for 37 mm filters is combined with the relatively high flow-rate, this sampler provides greater sensitivity (at 7 μg/filter the LOQ concentration is 4.2 μg m−3, below one-tenth the OSHA PEL) than many other samplers and pump failure was rarely encountered with this sampler in this or any other studies by the authors. This sampler is also generally considered to meet the inhalable convention. Wall losses have been shown in one study to be low for all but the largest particles,12 but this has not been tested in these studies. Since the GSP sampler also performed well without the need for a correction in the bronze foundry study,2 which involved probable matrix interferences from the presence of other metals, it is likely to be recommended as an appropriate sampler to use with on-site XRF analysis.
The CFC sampler using the OSHA algorithm also performed well, assuming only the filter deposit is counted as the sample. The slightly high bias (slope of 1.14) could easily be corrected by a minor change in the algorithm. However, analysis of wipes from the interior of the cassettes indicated a substantial loss of sample to the walls, confirming the work of others.13–15 CFC samplers used for gravimetric determinations often contain an internal, weighable, capsule to ensure all collected particles are included, but this capsule is rarely included in metals determinations because of the difficulty of digesting the capsule. Thus the wall losses are not considered part of the sample. The NIOSH algorithm continued to show a high positive bias in line with previous studies.1,2,16
The IOM sampler can give reasonably good results for the filter sample provided the exact area of the filter catch is known. Since it appears to perhaps vary by site, possibly because of differences in the particle size distribution, it must be measured on each occasion. It is not, however, possible to accurately measure losses to the IOM cassette wall using an on-site XRF analyzer, and these are not considered losses but are meant to be an integral part of the sample. However, other studies have indicated that such losses may be smaller than expected.1,14 Nevertheless, the IOM sampler does not appear to offer any advantage over the GSP sampler if both are considered inhalable samplers. The button sampler did not perform particularly well in this study, and also had the largest bias when used in the bronze foundry. Pumps frequently faulted after only short periods of time in these dusty atmospheres when the button sampler was used because of the high pressure drop across a heavily-loaded, 25 mm diameter, 0.8 μm pore-size, filter at 4 l min−1, and this is a significant practical disadvantage. The 25 mm open-face cassette performed the best of the 25 mm filter samplers in this study, provided the filter catch only is assessed, and it also performed well in the bronze foundry although a correction was needed for best results.2 However, the wall losses found in this study are extraordinarily high, in many cases amounting to more than half the sample. It is also not considered an inhalable sampler.
All of these samplers will continue to be used in similar studies in other workplaces where lead is encountered, such as at battery recyclers and in mining.
References
- M. Harper, T. S. Hallmark, M. E. Andrew and A. J. Bird, J. Environ. Monit., 2004, 6, 819–826 RSC.
- M. Harper, B. Pacolay and M. E. Andrew, J. Environ. Monit., 2005, 7, 592–597 RSC.
- Health and Safety Executive: Methods for the Determination of Hazardous Substances. Lead and Inorganic Compounds of Lead in Air, MDHS 7, HSE, London, 1987.
- NIOSH Manual of Analytical Methods 4th edn., ed. P. Eller, Method 7702: Lead by field portable XRF, National Institute for Occupational Safety and Health, Cincinnati, OH, Publication 94-113, 1994 (supplement issued Jan 15, 1998) Search PubMed.
- C. J. Elskamp, OSHA Manual of Analytical Methods, Method OSA1: Lead (Pb) in Workplace Air by NITON 700 Series Field Portable X-Ray Fluorescence (XRF) Analyzer, Occupational Safety and Health Administration, OSHA Salt Lake Technical Center, Salt Lake City, UT, April, 2003. http://www.osha.gov/dts/sltc/methods/onsite/osa1/osa1.html, accessed Feb 29, 2004, but recently withdrawn.
- J. H. Vincent and D. Mark, Ann. Occup. Hyg., 1990, 34, 249–262 CrossRef CAS.
- NIOSH Manual of Analytical Methods, 4th edn., ed. P. Eller, Method 7300 (Issue 2): Elements by ICP, National Institute of Occupational Safety and Health, Cincinnati, OH, Publication 94-113, 1994 Search PubMed.
- U.S. Code of Federal Regulations: 29CFR1926.62(d)(9).
- U.S. Code of Federal Regulations: 29CFR1910.1025(d)(9).
- Workplace Atmospheres—General Requirements for the Performance of Procedures for the Measurement of Chemical Agents, EN 482, Comité Europeén de Normalisation, Brussels, Belgium, 1994 Search PubMed.
- R. D. Foster, XRF analysis of harmful metals collected onto filters as thin layers from workplace air: interlaboratory exercise, paper accepted for submission at the 2005 Denver X-ray Conference, Colorado Springs, CO, August 1–5, 2005 Search PubMed.
- S.-N. Li, D. A. Lundgren and D. Rovell-Rixx, Am. Ind. Hyg. Assoc. J., 2000, 61, 506–516 CrossRef CAS.
- M. Demange, J. C. Gendre, B. Hervé-Basin, B. Carton and A. Peltier, Ann. Occup. Hyg., 1990, 34, 399–403 CrossRef.
- M. Demange, P. Görner, J.-M. Elcabeche and R. Wrobel, Appl. Occup. Environ. Hyg., 2002, 17, 200–208 CrossRef CAS.
- M. Demange, J.-M. Elcabeche and A. Boulet, Can. J. Anal. Sci. Spectrosc., 2003, 48, 362–371 CAS.
- P. L. Drake, N. J. Lawryk, K. Ashley, A. L. Sussell, K. J. Hazlewood and R. Song, J. Hazard. Mater., 2003, 102, 29–38 CrossRef CAS.
Footnotes |
| † Presented at the Fifth International Symposium on Modern Principles of Air Monitoring & Biomonitoring, June 12–16 2005, Norway. |
| ‡ Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health. The mention of any company names or products does not imply an endorsement by NIOSH or the Centers for Disease Control, and nor does it imply that alternative products are unavailable, or unable to be substituted after appropriate evaluation. |
| This journal is © The Royal Society of Chemistry 2006 |
