Aspect ratio dependence on surface enhanced Raman scattering using silver and gold nanorod substrates
Christopher J.
Orendorff
,
Latha
Gearheart†
,
Nikhil R.
Jana‡
and
Catherine J.
Murphy
*
University of South Carolina, Department of Chemistry and Biochemistry, Columbia, SC 29208, USA. E-mail: murphy@mail.chem.sc.edu
First published on 18th October 2005
Abstract
Silver and gold nanorods with aspect ratios from 1 to 16 have been used as substrates for surface enhanced Raman spectroscopy (SERS) in colloidal solution. The nanorod aspect ratio is varied to give different degrees of overlap between the nanorod longitudinal plasmon band and excitation source in order to determine its effect on overall surface enhancement. Results suggest that enhancement factors are a factor of 10–102 greater for substrates that have plasmon band overlap with the excitation source than for substrates whose plasmon bands do not.
1. Introduction
Surface enhanced Raman spectroscopy (SERS) is a powerful analytical tool for obtaining vibrational information for molecules on metallic substrates.1 The SERS phenomenon is often described by traditional enhancement mechanisms; electromagnetic (EM) and chemical (CHEM) enhancement.2–8 EM enhancement stems from the enhancement of the local electromagnetic field incident on an adsorbed molecule at metallic substrates on the 10–200 nm size scale.9–11 The magnitude of EM enhancement is highly dependent on the plasmon absorption of the substrate. CHEM enhancement results from an electronic resonance–charge transfer between a molecule and a metal surface at atomic-scale roughness features (<5 nm), ultimately increasing the polarizability of the molecule and effectively increasing the Raman scattering cross section.12–15 Charge transfer between an adsorbed molecule and the metal surface is also incident wavelength- and substrate-dependent, but it is important to note that it is not necessarily coupled to the surface plasmon of the metal substrate. The largest Raman scattering enhancements, even single molecule SERS, have been described for molecules residing in the fractal space between aggregated colloidal nanoparticles.5,16,17 This is attributed to plasmonic coupling between nanoparticles in close proximity,17–23 which results in huge local electromagnetic field enhancements in these confined junctions or SERS “hot spots”.While the use of colloidal SERS substrates is widespread, studies of substrates with different morphologies are limited, despite their shape-dependent optical properties which make them attractive candidates for SERS.24,25 For example, in addition to having transverse plasmon absorption, gold and silver nanorods have longitudinal plasmon bands that can be tuned from the visible to the near-infrared region by varying the nanorod aspect ratio.24,25 Then, Raman scattering enhancements from these materials can be maximized by plasmon resonance with the excitation source, effectively optimizing contributions from the EM enhancement mechanism for a given aspect ratio.
The use of gold and silver nanorods or wires as SERS substrates has been reported by this and other laboratories. SERS on silver nanowire assemblies have recently been reported by Tao et al.26 giving rise to surface enhancement factors of 105 for hexadecanethiol and 109 for rhodamine 6G. Moskovits and coworkers have studied SERS on similar silver nanowire rafts27 and Aroca et al. have used multilayer films of silver nanowires as SERS substrates.28 Nikoobakht et al.29,30 have reported surface enhancement factors of ∼105 for 2-aminothiophenol on separated and aggregated gold nanorods. For separated gold nanorod SERS experiments, there is no overlap between the plasmon absorption and Raman excitation wavelengths and only a slight overlap for the aggregated nanorod experiments, resulting in primarily CHEM enhancement from the Au{110} surface of these substrates.29,30 Recently, we reported the use of gold nanorods and other nanoparticle morphologies immobilized on self-assembled monolayers on planar substrates.31 While enhancement factors of 108 were estimated for 4-mercaptobenzoic acid monolayers using nanorods in this geometry, nanorod aspect ratio dependence on SERS enhancement factors was not observed because of convoluting effects of nanoparticle optical properties and plasmon coupling between the nanoparticles and the gold surface.31
Those previous reports describe SERS on primarily aggregated nanorods and nanowires, where determining the dependence of SERS on nanoparticle optical properties is difficult due to convoluting plasmon coupling contributions known to vastly improve SERS enhancements.5,16,17 In order to determine EM contributions to SERS from the substrate optical properties alone, one would have to use a more homogeneous experimental system; for example, dilute colloidal solutions of nanorods or well dispersed nanorods on planar substrates.
Halas and coworkers studied the effect of tunable plasmon absorption of gold nanoshells as SERS substrates.32 Large Raman scattering enhancement factors of 109–1010 were observed for 4-mercaptoaniline, however, no significant difference in SERS enhancement factors for shell nanoparticles in and off resonance was observed.32
This report describes the use of silver and gold nanorods with varied aspect ratios as SERS substrates in colloidal solution. The optical properties of these anisotropic nanocrystals have been tailored to have variable degrees of plasmon overlap with the excitation source. As a result, EM contributions to the overall enhancement should vary with the nanorod aspect ratio. The goal is to experimentally interrogate the effect of nanorod plasmon resonance on Raman scattering enhancements in the absence of other convoluting effects, primarily plasmon coupling.
2. Experimental
2.1 Materials and synthetic methods
Silver nitrate, chloroauric acid, sodium borohydride, ascorbic acid, 4-mercaptopyridine, 4-aminothiophenol, and 2,2′-bipyridine were purchased from Aldrich. Cetyltrimethylammonium bromide (CTAB) was purchased from Sigma. All chemicals were used as received. Water used in all synthetic methods was purified (Continental Water Systems) to remove organic, ionic, and bacterial impurities. All glassware was cleaned with aqua regia, thoroughly rinsed with deionized water, and dried prior to use.Silver nanorods with aspect ratios of 3.5 ± 0.7 (length = 43 ± 3, width = 12 ± 2 nm) and 10 ± 5 (length = 214 ± 37, width = 20 ± 4 nm) and gold nanorods with aspect ratios of 1.7 ± 0.2 (length = 41 ± 3, width = 24 ± 3 nm), 4.5 ± 0.2 (length = 57 ± 8, width = 13 ± 2 nm), and 16 ± 5 (length = 372 ± 119, width = 23 ± 4 nm) were prepared via the seed-mediated techniques in aqueous surfactants described previously.33–35 After preparation, solutions of silver and gold nanorods were separated from spheres and excess surfactant by two centrifugation and re-dispersion steps.33–35 Silver spheres 33 ± 7 nm in diameter were prepared according to conventional citrate reduction for comparison.36 Gold spheres of 29 ± 6 nm diameter were prepared using a seeding procedure in aqueous surfactant.37 Excess surfactant was removed from these gold spheres by two successive centrifugation and re-dispersions steps.37 With the primary focus of this work on rod-shaped nanoparticles, both gold and silver spheres will be referred to as aspect ratio 1 nanorods throughout, in order to simplify the discussion.
2.2 SERS solution preparation
After purification from other shapes and excess surfactant, 2 μL of aqueous analytes were added to 20 μL of purified nanoparticles and diluted to 200 μL. The resulting silver atom concentration was calculated to be 2.5 × 10−4 M for aspect ratio 1, 3.5, and 10 nanorods, taking into account the removal of other nanoparticle side products. The final concentration of gold in the same sample volume is 1.1 × 10−5 M for aspect ratio 1, 4.5 × 10−5 M for aspect ratio 2 and 4, and 5 × 10−6 M for aspect ratio 16 nanorods. The final analyte concentrations for 4-aminothiophenol, 4-mercaptopyridine, and 2,2′-bipyridine were 1 × 10−6 M. Aqueous SERS solutions were equilibrated under ambient conditions for 5 min prior to spectral analysis. Since the process of removing excess CTAB from all nanoparticle solutions is the same, and results in the loss of observable CTAB vibrational modes in the SERS spectra, we assume that the local concentration of residual CTAB at the surface of these nanoparticles is similar for all nanoparticle shapes, and any variations in SERS intensity will not be dominated by possible differences in local CTAB concentration. A detailed description of calculating enhancement factors for each of these analytes is provided in the following sections.2.3 Instrumentation
Surface-enhanced Raman spectra were collected using 25 mW of 632.8 nm radiation from a HeNe laser and a Detection Limit Solution 633 Raman system. Integration times are provided in the figure captions. Transmission electron microscopy (TEM) images were acquired using either a JEOL-100CXII or Hitachi H-8000 microscope operated at 100 or 200 kV, respectively. Electronic absorption spectra were acquired using a CARY 500 Scan UV-Vis-NIR spectrometer. Particle size measurements of nanorod samples were made by static light scattering in aqueous solutions using a Zeta PALS ζ potential analyzer (Brookhaven).3. Results and discussion
3.1 Properties of silver and gold nanorods
Plasmon absorption spectra of silver and gold nanorods in aqueous solution are shown in Fig. 1. Aspect ratio 1 silver nanorods (spheres) have only one principal plasmon band at 420 nm. Silver nanorods with 3.5 and 10 aspect ratios have both a transverse plasmon band, also observed at ∼420 nm, and longitudinal plasmon bands at 535 and 615 nm, respectively. Aspect ratio 1 gold nanorods (spheres) exhibit absorption at 520 nm, and aspect ratios 2 and 4 have additional longitudinal bands at 650 and 850 nm, respectively. Aspect ratio 16 gold nanorods show transverse absorption at 520 nm, but longitudinal plasmon absorption >1200 nm. The vertical dashed line in Fig. 1a and 1b represents the excitation wavelength for the SERS experiments at 632.8 nm. It is important to note that the longitudinal plasmon bands for aspect ratio 10 silver nanorods and aspect ratio 4 gold nanorods have the greatest overlap with the excitation source of these nanorod substrates.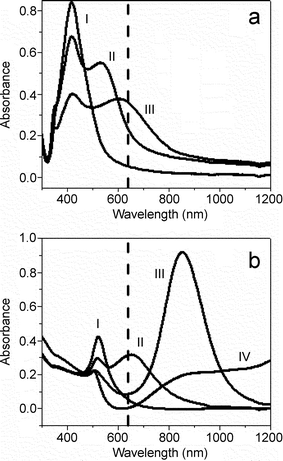 | ||
| Fig. 1 Absorption spectra of (a) silver nanorods with aspect ratios 1 (trace I), 3.5 (trace II), and 10 (trace III) and (b) gold nanorods with aspect ratios 1 (trace I), 1.7 (trace II), 4.5 (trace III), and 16 (trace IV). The vertical dashed line represents the excitation wavelength for SERS measurements at 632.8 nm. | ||
Fig. 2 shows TEMs of nanorods. Aspect ratio 3.5 silver nanorods (∼95%) were homogeneous in size and often formed ordered, self-assembled layers when dried, as shown in Fig. 2b. However, the aspect ratio 10 silver nanorods were polydisperse (10 ± 5), and significant amounts of platelets and spheres (50 ± 10 nm, ∼50%) were still present (Fig. 2c). Gold nanorods with aspect ratios 2 and 16 are generally free from other shapes, but more polydisperse than aspect ratio 4 nanorods. It is important to note that for aspect ratio 10 rods with the presence of significant numbers of other shapes in the SERS samples, the resulting SERS spectra will be a convolution of those for analytes adsorbed to nanorods and to the fraction of other shapes. Since EM enhancement of aspect ratio 10 rods is expected to be greater than that for nanospheres, resulting SERS enhancement factors for these samples could be an underestimate relative to samples containing pure nanorods.
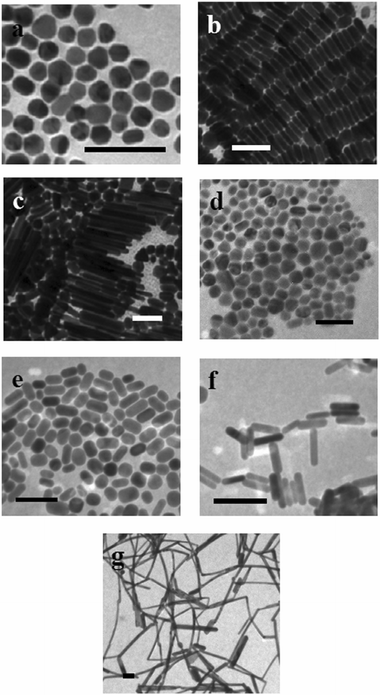 | ||
| Fig. 2 Transmission electron microscopy images of silver (a–c) and gold (d–g) nanorods with aspect ratios (a) 1, (b) 3.5, (c) 10, (d) 1, (e) 1.7, (f) 4.5, and (g) 16. The scale bars represent 100 nm for each image. | ||
3.2 Effect of nanorod aspect ratio on SERS
In order to understand the mechanism of enhancement for molecules adsorbed to these silver and gold nanorods and to deconvolute plasmon coupling from particle aggregation and plasmon resonance effects, SERS experiments were done in aqueous solutions under dilute conditions. This is essential since nanoparticle aggregation effects on the SERS intensity give rise to surface enhancement factors of up to ∼1014 and would greatly outweigh the nanorod aspect ratio effects we hope to investigate.5 Particle sizes measured for gold and silver nanorod samples in solution by light scattering are listed in Table 1. For all samples, the particle sizes are close to the average of the length and width of the nanorods measured by TEM, as reported previously.38 For all nanoparticle samples, no large aggregates of particles were observed. These results suggest that these conditions are dilute enough such that no nanorod aggregation can be detected in these SERS samples. Therefore, resulting SERS spectra should not be convoluted with aggregation and plasmon coupling effects.| Nanoparticle | Average particle sizeb/nm | Dimensionsc/nm |
|---|---|---|
| a Aspect ratio 1.0 = spheres. b Measured by light scattering. c Measured from TEM images (l = length, w = width). | ||
| Aspect ratio 10 silver nanorods | 90 ± 17 | l = 214 ± 37, w = 20 ± 4 |
| Aspect ratio 3.5 silver nanorods | 31 ± 7 | l = 43 ± 3, w = 12 ± 2 |
| Aspect ratio 1.0 silver nanorodsa | 34 ± 5 | Diameter = 33 ± 7 |
| Aspect ratio 1.7 gold nanorods | 34 ± 6 | l = 41 ± 3, w = 24 ± 3 |
| Aspect ratio 4.5 gold nanorods | 38 ± 6 | l = 57 ± 8, w = 13 ± 2 |
| Aspect ratio 16 gold nanorods | 105 ± 24 | l = 372 ± 119, w = 23 ± 4 |
| Aspect ratio 1.0 gold nanorodsa | 31 ± 3 | Diameter = 29 ± 6 |
SERS spectra of 2,2′-bipyridine, 4-aminothiophenol, and 4-mercaptopyridine adsorbed to aspect ratio 1, 3.5, and 10 silver nanorods between 700 and 1400 cm−1 are shown in Fig. 3. SERS spectra of these same analytes on aspect ratio 1, 1.7, 4.5, and 16 gold nanorods are shown in Fig. 4. Peak frequencies and assignments for each of these analytes on gold and silver substrates are given in Table 2. It is important to note that in aqueous solution nanorods are randomly oriented and these SERS spectra are representative of all possible nanorod orientations averaged over the entire acquisition time. The ideal geometry would be to fix the nanorod orientation with the long axis parallel to the excitation source polarization, in order to have maximum longitudinal plasmon overlap. However, colloidal solution samples are used to eliminate nanoparticle aggregation effects. SERS spectra for these analytes are comparable to those acquired previously for these analytes adsorbed to colloidal substrates.39–41 Qualitatively, the vibrational mode intensity and signal-to-noise are generally better for analytes adsorbed to silver than to gold nanoparticles, in fact, no vibrational modes are observed for 2,2′-bipyridine on any gold substrate or for 4-aminothiophenol on aspect ratio 16 gold nanorods. Moreover, the signal-to-noise is generally better for analytes on aspect ratio 10 nanorods than other silver nanorods and for molecules adsorbed to aspect ratio 1 and 1.7 nanorods than aspect ratio 4.5 or 16 nanorods. Even though all of these nanorods are capped with CTAB,33–35 no characteristic vibrational modes for CTAB are observed in these spectra. This is likely to be due to the fact that the ν(C–C), ν(C–N), or δ(C–H) CTAB modes are significantly weaker than the δ(C–H) or ν(C–C)ring modes of these aromatic analytes and are simply below the detection limit of the spectrometer. The dependence of the nanorod aspect ratio on surface enhancement is quantified by calculating surface enhancement factors for the analytes on each substrate.
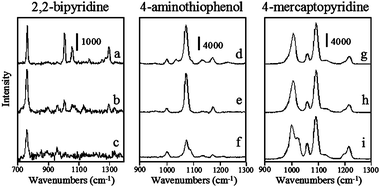 | ||
| Fig. 3 Surface enhanced Raman spectra of 2,2′-bipyridine, 4-aminothiophenol, and 4-mercaptopyridine at 10−6 M using silver nanorods with aspect ratios (a,d, and g) 10, (b,e, and h) 3.5, and (c, f, and i) 1. Acquisition times are (a) 30 s, (b) 60 s, (c) 120 s, (d) 30 s, (e) 60 s, (f) 60 s, (g) 10 s, (h) 10 s, and (i) 60 s. | ||
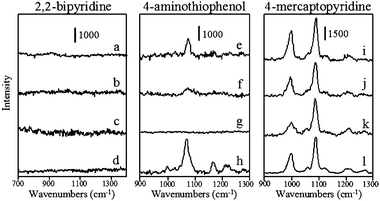 | ||
| Fig. 4 Surface enhanced Raman spectra of 2,2′-bipyridine, 4-aminothiophenol, and 4-mercaptopyridine at 10−6 M using gold nanorods with aspect ratios (a,e, and i) 1.7, (b,f, and j) 4.5, (c, g, and k) 16 and (d, h, and l) 1. Acquisition times are 120 s for all spectra. | ||
| Peak frequency/cm−1 | Assignmentbc | |||||
|---|---|---|---|---|---|---|
| 4-ATP on gold NRse | 4-ATP on silver NRs | 4-mpyr on gold NRs | 4-mpyr on silver NRs | 2,2′-bipy on gold NRs | 2,2′-bipy on silver NRs | |
| a 4-ATP = 4-aminothiophenol, 4-mpyr = 4-mercaptopyridine, 2,2′-bipy = 2,2′-bipyridine. b Assignments from refs. 37–39. c δ = bend or deformation; ν = stretch; ring = ring breathing mode; ip = in-plane mode. d Not observed. e NRs = nanorods. | ||||||
| d | 760 | δ ip(C–C) | ||||
| 1000 | δ(C–H) | |||||
| 1002 | 1006 | ν(C–C)ring | ||||
| d | 1004 | ν(C–C)ring | ||||
| d | 1056 | δ ip(C–H) | ||||
| 1058 | 1058 | δ(C–H) | ||||
| 1071 | 1073 | ν(C–C)ring | ||||
| 1087 | 1091 | ν(C–C)ring | ||||
| d | 1165 | δ ip(C–H) | ||||
| 1167 | 1174 | δ ip(C–H) | ||||
| 1211 | 1217 | δ(C–H), δ(N–H) | ||||
| d | 1298 | ν(C–C), ν(C–N) | ||||
Estimated surface enhancement factors (EF) for each of these substrates are shown in Table 3. EFs were determined using the following expression:29
| EF = [ISERS]/[IRaman] × [Mbulk]/[Mads] | (1) |
| Analyte/vibrational mode | ||||
|---|---|---|---|---|
| Substrate | 4-Mercaptopyridine/ν(C–C) | 4-Aminothiophenol/ν(C–S) | 2,2′-Bipyridine/ν(C–C) | 2,2′-Bipyridine/δip(C–H) |
| a Aspect ratio 1.0 = spheres. b No observed vibrational modes. | ||||
| Aspect ratio 10 silver nanorods | 2.3 ± 0.1 × 107 | 2.3 ± 0.05 × 106 | 8.5 ± 0.07 × 105 | 1.1 ± 0.03 × 106 |
| Aspect ratio 3.5 silver nanorods | 2.5 ± 0.2 × 106 | 2.7 ± 0.1 × 105 | 2.1 ± 0.2 × 104 | 8.3 ± 0.08 × 104 |
| Aspect ratio 1.0 silver nanorodsa | 4.8 ± 0.5 × 106 | 3.9 ± 0.3 × 105 | 2.6 ± 0.2 × 104 | 1.4 ± 0.1 × 105 |
| Aspect ratio 1.7 gold nanorods | 1.4 ± 0.37 × 105 | 2.3 ± 0.07 × 104 | b | b |
| Aspect ratio 4.5 gold nanorods | 6.2 ± 1.84 × 104 | 4.3 ± 0.3 × 103 | b | b |
| Aspect ratio 16 gold nanorods | 1.8 ± 0.2 × 104 | b | b | b |
| Aspect ratio 1.0 gold nanorodsa | 1.2 ± 0.2 × 104 | 2.0 ± 0.4 × 103 | b | b |
In general, enhancement factors for aspect ratio 10 silver nanorods are 101–102 times greater than for other silver nanorods and range from 105–107. Enhancement factors for aspect ratio 1.7 gold nanorods are a factor of 10 greater than for other gold substrates and range from 103–105. This is attributed to greater EM enhancement for the aspect ratio 10 silver nanorods and aspect ratio 1.7 gold nanorods, which have plasmon absorption overlap with the excitation source, relative to the other nanorod substrates, as shown in Fig. 1. As described above, the samples containing aspect ratio 10 silver nanorods also contain a large fraction of spheres and other shapes (∼50%). The EF values calculated for these nanorods are actually an underestimate of the EF anticipated for pure nanorods.
It is also interesting to note the variations in EF values among the different analytes, where the thiol molecules give larger surface enhancements than 2,2′-bipyridine on all substrates. Recently, Alvarez-Puebla et al.42 studied the effect of nanoparticle surface charge (ζ potential) on SERS enhancement. The authors observed larger SERS enhancement for analytes that are electrostatically attracted to nanoparticle substrates than those samples without electrostatic interactions.42 In our case, adsorption of negatively charged thiols should be facilitated on CTAB-protected nanoparticles with a positive ζ potential, leading to greater overall enhancement for thiol analytes over 2,2′-bipyridine. However, this is a limited data set and more molecules of varying analyte functionality need to be tested in order to fully describe these observations.
With limited experimental reports on SERS using substrates with tunable plasmon resonance,32 and no reports on the aspect ratio dependence on SERS using nanorods, observed differences in EF values for nanorod substrates can be compared to previous theoretical or experimental studies of wavelength-dependent SERS on spherical metallic substrates.8,9,43–46 In these theoretical calculations, the plasmon bands of the substrates are fixed and the incident wavelengths are variable. However, these can be used as models for wavelength-dependent EM contributions to enhancement using gold and silver nanorods. According to Xu et al.,8 the magnitude of EM enhancement, MEM, is approximated by
| MEM = [EL (ωI)/EI (ωI)]4 | (2) |
| EL (ωI) = EI (ωI) + Eind(ωI) | (3) |
| Eind α (ω/c)2 (ε2 − ε1) | (4) |
Using the model for analyte molecules adsorbed to the surface of 30 nm diameter silver nanoparticles, maximum calculated MEM values are ∼104 for incident photon energies at the plasmon resonant wavelength (∼400 nm), while minimum MEM values are ∼102 for incident wavelengths of 600–1200 nm.8 For gold nanoparticles, calculated MEM values reach a maximum of ∼103 for excitation energies at the plasmon resonant wavelength (∼520 nm), and are <10 at 400 nm, and ∼102 for incident wavelengths of 700–1200 nm.8 Based on these theoretical calculations we can estimate that EF values for silver and gold substrates with plasmon bands in resonance with the incident radiation should be 101−102 times greater than those without overlapping bands. While this simple approximation does not consider nanoparticle shape effects known to affect localized electromagnetic fields,46 this theoretical model for wavelength-dependent EM enhancement is consistent with our observations for experimentally determined enhancement factors for gold and silver nanorods.
4. Conclusions
Surface enhanced Raman scattering on silver and gold nanorod substrates is presented. SERS experiments are done in aqueous solutions under dilute conditions in order to study the effect of nanorod plasmon resonance, by controlling the nanorod aspect ratio, in the absence of other contributing effects such as plasmon coupling between particles. In general, aspect ratio 10 silver nanorods and aspect ratio 1.7 gold nanorods give 10–102 greater SERS enhancements than nanorods of other aspect ratios. Larger SERS enhancements on these substrates that have plasmon resonance with the excitation source are attributed to greater contributions from the EM enhancement mechanism and are consistent with theoretical calculations for wavelength-dependent EM contributions.Acknowledgements
We thank the National Science Foundation and the University of South Carolina for funding.References
- M. Moskovits, Rev. Mod. Phys., 1985, 57, 783 CrossRef CAS.
- A. Campion and P. Kambhampati, Chem. Soc. Rev., 1998, 27, 241 RSC.
- K. Kneipp, H. Kneipp, I. Itzkan, R. R. Dasari and M. S. Feld, Chem. Rev., 1999, 99, 2957 CrossRef CAS.
- J. L. Yao, G. P. Pan, K. H. Xue, D. Y. Wu, B. Ren, D. M. Sun, J. Tang, X. Xu and Z. Q. Tian, Pure Appl. Chem., 2000, 72, 221 CrossRef CAS.
- J. Jiang, N. Bosnick, M. Maillard and L. Brus, J. Phys. Chem. B, 2003, 107, 9964 CrossRef CAS.
- J. A. Creighton, in Spectroscopy of Surfaces, Wiley, Chichester, 1988 Search PubMed.
- M. Kerker, Appl. Opt., 1991, 30, 4699 Search PubMed.
- H. X. Xu, J. Aizpurua, M. Kall and P. Apell, Phys. Rev. E, 2000, 62, 4318 CrossRef CAS.
- A. D. McFarland, M. A. Young, J. A. Dieringer and R. P. Van Duyne, J. Phys. Chem. B, 2005, 109, 11279 CrossRef CAS.
- P. Etchegoin, L. F. Cohen, H. Hartigan, R. J. C. Brown, M. J. T. Milton and J. C. Gallop, J. Chem. Phys., 2003, 119, 5281 CrossRef CAS.
- G. C. Schatz and R. P. Van Duyne, in Handbook of Vibrational Spectroscopy, John Wiley and Sons, Ltd., Chichester, 2002 Search PubMed.
- A. Otto, I. Mrozek and C. Pettenkofer, Surf. Sci., 1990, 238, 192 CrossRef.
- S. G. Schultz, M. Janik-Czachor and R. P. Van Duyne, Surf. Sci., 1984, 104, 419.
- A. Otto, T. Bornemann, U. Erkturk, I. Mrozek and C. Pettenkorer, Surf. Sci., 1989, 210, 363 CrossRef CAS.
- D. L. Jeanmaire and R. P. Van Duyne, J. Electroanal. Chem., 1977, 84, 1 CrossRef.
- H. X. Xu, E. J. Bjerneld, M. Kall and L. Borjesson, Phys. Rev. Lett., 1999, 83, 4357 CrossRef CAS.
- A. M. Michaels, J. Jiang and L. Brus, J. Phys. Chem. B, 2000, 104, 11965 CrossRef CAS.
- F. J.-G. Vidal and J. B. Pendry, Phys. Rev. Lett., 1996, 77, 1163 CrossRef CAS.
- D.-S. Wang and M. Kerker, Phys. Rev. B, 1981, 24, 1777 CrossRef CAS.
- V. A. Markel, V. M. Shalaev, P. Zhang, W. Huynh, L. Tay, T. L. Haslett and M. Moskovits, Phys. Rev. B, 1999, 59, 10903 CrossRef CAS.
- K.-H. Su, Q.-H. Wei, X. Zhang, J. J. Mock, D. R. Smith and S. Schultz, Nano Lett., 2003, 3, 1087 CrossRef CAS.
- T. Atay, J.-H. Song and A. V. Murmikko, Nano Lett., 2004, 4, 1627 CrossRef CAS.
- D. P. Fromm, A. Sundaramurthy, P. J. Schuck, G. Kino and W. E. Moerner, Nano Lett., 2004, 4, 957 CrossRef CAS.
- J. A. Creighton and D. G. Eadon, J. Chem. Soc., Faraday Trans., 1991, 87, 3881 RSC.
- M. A. El-Sayed, Acc. Chem. Res., 2001, 34, 257 CrossRef CAS.
- A. Tao, F. Kim, C. Hess, J. Goldberger, R. He, Y. Sun, Y. Xia and P. Yang, Nano Lett., 2003, 3, 1229 CrossRef CAS.
- D. H. Jeong, Y. X. Zhang and M. Moskovits, J. Phys. Chem. B, 2004, 108, 12724 CrossRef CAS.
- R. F. Aroca, P. J. G. Goulet, D. S. dos Santos, R. A. Alvarez-Puebla and O. N. Oliverira, Anal. Chem., 2005, 77, 378 CrossRef CAS.
- B. Nikoobakht, J. Wang and M. A. El-Sayed, Chem. Phys. Lett., 2002, 366, 17 CrossRef CAS.
- B. Nikoobakht and M. A. El-Sayed, J. Phys. Chem. A, 2003, 107, 3372 CrossRef CAS.
- C. J. Orendorff, A. Gole, T. K. Sau and C. J. Murphy, Anal. Chem., 2005, 77, 3261 CrossRef CAS.
- J. B. Jackson and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2004, 52, 17930 CrossRef.
- N. R. Jana, L. Gearheart and C. J. Murphy, Chem. Commun., 2001, 617 RSC.
- N. R. Jana, L. Gearheart and C. J. Murphy, J. Phys. Chem. B, 2001, 105, 4065 CrossRef CAS.
- T. K. Sau and C. J. Murphy, Langmuir, 2004, 20, 6414 CrossRef CAS.
- P. C. Lee and D. Meisel, J. Phys. Chem., 1982, 86, 3391 CrossRef CAS.
- N. R. Jana, L. Gearheart and C. J. Murphy, Langmuir, 2001, 17, 6782 CrossRef CAS.
- C. J. Orendorff, P. L. Hankins and C. J. Murphy, Langmuir, 2005, 21, 2022 CrossRef CAS.
- M. Fatamata, J. Phys. Chem., 1995, 99, 11901 CrossRef.
- J. Hu, B. Zhao, W. Xu, B. Li and Y. Fan, Spectrochim. Acta, Part A, 2002, 58, 2827 CrossRef.
- A. L. Kamyshny, V. N. Zakharov, Y. V. Federov, A. E. Galashin and L. A. Aslanov, J. Colloid Interface Sci., 1993, 158, 171 CrossRef CAS.
- R. A. Alvarez-Puebla, E. Arceo, P. J. G. Goulet, J. J. Garrido and R. F. Aroca, J. Phys. Chem. B, 2005, 109, 3787 CrossRef CAS.
- M. Kerker, D.-S. Wang and H. Chew, Appl. Opt., 1980, 19, 4159 CrossRef CAS.
- J. Gersten and A. Nitzan, J. Chem. Phys., 1980, 73, 3023 CrossRef CAS.
- J. J. Gersten and A. Nitzan, Surf. Sci., 1985, 158, 165 CrossRef CAS.
- G. C. Schatz, Acc. Chem. Res., 1984, 17, 370 CrossRef CAS.
Footnotes |
| † Present address: Presbyterian College, Department of Chemistry, 503 South Broad St., Clinton, SC 29325, USA. |
| ‡ Present address: Institute of Bioengineering and Nanotechnology, 51 Science Park Rd., Singapore Science Park II, Singapore, 117586. |
| This journal is © the Owner Societies 2006 |
