When in the presence of the strong hydrogen bonds, the weak hydrogen bonds gain an importance†
Agata
Białońska
* and
Zbigniew
Ciunik
Faculty of Chemistry, University of Wrocław, F. Joliot-Curie 14, 50-383 Wrocław, Poland. E-mail: bialonsk@wcheto.chem.uni.wroc.pl; Fax: +47 71 375 7239; Tel: +48 7173 75
First published on 9th December 2005
Abstract
The crystal structures of brucinium hydrogen N-benzoyl-D-aspartate tetrahydrate (1), brucinium N-benzoyl-D-asparaginate trihydrate (2), brucinium N-tert-butoxycarbonyl-L-alaninate 2-propanol solvate hydrate (3) and brucinium N-tert-butoxycarbonyl-L-prolinate ethanol solvate hydrate (4) were determined. In all crystals under investigation, the brucinium cations form corrugated layers with anions and solvent molecules between them. Our investigations concerning the nature of the brucine layers show that such self-assemblies provide optimally appropriate surfaces for interactions in the hydrophilic environment from which the crystals of brucinium salts were usually grown. In an effort to obtain the most appropriate surfaces, weak hydrogen bonds gain in importance. This suggestion seems to be confirmed by the presence of the same pattern of the weak hydrogen bonds that one can observe in many crystals containing brucine (molecular/ionic).
Introduction
Brucine remains one of the most frequently used resolving agents for the separation of racemic acids.1 However, up to now, the mechanism of chiral discrimination prevalent during crystallization of the diastereomers has not been clarified fully due to its high complexity.2–4 Structural data obtained by the systematic study of diastereomeric salt formations are useful for the rationalization of optical resolution and help in the experimental design of the most important parameters.5–11Specific self-assemblies of resolving agent can be a key in chiral discrimination.12 Gould and Walkinshaw directed attention to the tendency for conserving a packing arrangement of brucine monolayers consisting of corrugated sheets capable of great versatility in forming complexes with wider variety of compounds.5 In a recent paper, we described racemic resolutions of N-protected alanine derivatives by co-crystallization with brucine and strychnine; there we concluded that the donor/acceptor properties of surfaces of resolving agents self-assemblies are determined by the donor/acceptor capabilities of the resolved compound.13 In a similar way, donor/acceptor properties of surfaces of brucine self-assemblies depend on the donor/acceptor capabilities of solvents from which brucine is crystallized.14 It is worth emphasizing here that despite influence of the solvent or resolved compound at the brucine self-assembly, there are but a few of such self-assemblies known which contain brucine in the crystalline state.
Similar motifs of brucine self-assemblies were found in our studies of crystals of brucinium hydrogen N-benzoyl-D-aspartate tetrahydrate (1) and brucinium N-benzoyl-D-asparaginate trihydrate (2), as well as in the crystals of brucinium N-tert-butoxycarbonyl-L-alaninate 2-propanol solvate hydrate (3) and brucinium N-tert-butoxycarbonyl-L-prolinate ethanol solvate hydrate (4) (Scheme 1). Detailed analysis of this motif allowed us to recognize unexpected relationships: among them, relationships between solvation capabilities of guest molecules and the kind of brucine self-assembly. Also, the relationship between the number of weak hydrogen bonds and the number of suitable brucine self-assemblies.
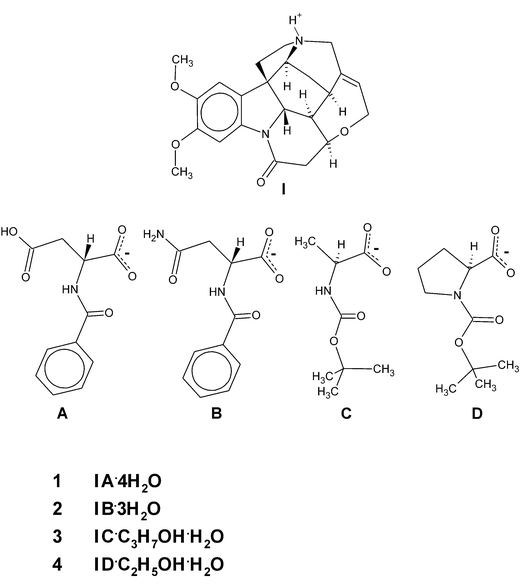 | ||
| Scheme 1 | ||
Results and discussion
Selected views of the crystal structures of 1–4, together with the numbering scheme employed are presented in Fig. 1. Brucinium cations form corrugated monolayer sheets (L1) in the crystals of 1, 2 and 4 and puckered sheets (L2) in crystals of 3. The layers are separated by anions of the amino acid derivative and by solvent molecules (Fig. 2). In all crystals under investigation, cationic and anionic/solvent layers are linked by N–H+⋯O− and O–H⋯O hydrogen bonds. The protonated amine N2 atom, and deprotonated carboxyl group via the O5 atom participate in the ionic N–H+⋯O− hydrogen bonds. The interlayer O–H⋯O hydrogen bond is formed between the water O1W molecule and the carbonyl O4 atom of brucinium cations. The hydrogen N-benzoyl-D-aspartate anion and four water molecules present in crystals of 1, and the disordered N-benzoyl-D-asparaginate anion and three water molecules in the crystal of 2, form extensive hydrogen bonds networks (see Fig. 2a and Fig. 2b). As presented in Fig. 3 and Table 1, the schemes of hydrogen bonds involving anion and water molecules are very similar in crystals of 1 and 2. The hydrogen bonding networks involving the N-tert-butoxycarbonyl-L-alaninate anions and the solvent molecules in the crystal of 3, as well as the N-tert-butoxycarbonyl-L-prolinate anions and the solvent molecules in the crystal of 4, are poor (Table 1). In crystals of 3, the hydroxyl O33 atom of the 2-propanol molecule is a hydrogen bond donor and the amide O7 atom is its acceptor. The amide N3 atom of the anion is the donor of the intramolecular N–H⋯O hydrogen bond while the carboxyl O6 atom is its acceptor. In the crystal of 4, the carboxyl O6 atom of the anion participates in the hydrogen bond and the hydroxyl O34 atom of ethanol molecule is its donor. | ||
| Fig. 1 View of molecular structures of (a) 1, (b) 2, (c) 3 and (d) 4 with atomic numbering. Non-hydrogen atoms are shown as 30% probability thermal ellipsoids. In the crystal structure of 2 anion and water O2W and O3W molecules were found in two positions with an occupancy factor equal to 0.5. | ||
![Molecular packing of (a) 1, (b) 2, (c) 3 and (d) 4. Along [100] direction similar ribbons of brucinium cations construct corrugated (1, 2 and 4) and puckered sheets (3) (consecutive corrugated ribbons of puckered sheet are distinguished by solid and blank line). For clarity hydrogen atoms are omitted.](/image/article/2006/CE/b515365d/b515365d-f2.gif) | ||
| Fig. 2 Molecular packing of (a) 1, (b) 2, (c) 3 and (d) 4. Along [100] direction similar ribbons of brucinium cations construct corrugated (1, 2 and 4) and puckered sheets (3) (consecutive corrugated ribbons of puckered sheet are distinguished by solid and blank line). For clarity hydrogen atoms are omitted. | ||
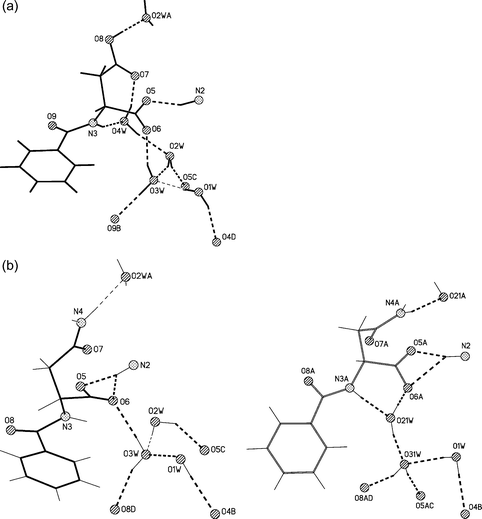 | ||
| Fig. 3 Schemes of the hydrogen bonds (a) for 1 and (b) for 2. In the crystal of 2 disordered anion and water molecules form two alternative hydrogen bonds patterns. Symmetry operators are distinguished by letter of atomic numbering, where for 1: A = x − 1/2, −y + 3/2, −z + 1, B = x + 1/2, −y + 1/2, −z + 1, C = x + 1, y, z and D = −x + 2, y − 1/2, −z + 3/2 and for 2 A = x − 1/2, −y + 1/2, −z + 1, B = −x + 2, y + 1/2, −z + 1/2, C = x + 1, y, z and D = x + 1/2, −y + 3/2, −z + 1. | ||
| D–H | H⋯A | D⋯A | ∠DHA | |
|---|---|---|---|---|
| a 1: (i) x − 1/2, −y + 3/2, −z + 1; (ii) −x + 2, y − 1/2, −z + 3/2; (iii) x + 1, y, z; (iv) x + 1/2, −y + 1/2, −z + 1. b 2: (v) x − 1/2, −y + 1/2, −z + 1; (vi) −x + 2, y + 1/2, −z + 1/2; (vii) x + 1, y, z; (viii) x + 1/2, −y + 3/2, −z + 1. c 3: (ix) −x + 3/2, y + 1/2, −z. d 4: (x) −x + 2, y − 1/2, −z. | ||||
| 1 | ||||
| N2–H2⋯O5 | 0.93 | 1.71 | 2.592(6) | 157.8 |
| O8–H87⋯O2Wi | 0.91 | 1.69 | 2.588(6) | 168.5 |
| N3–H37⋯O4W | 0.88 | 2.11 | 2.948(7) | 159.2 |
| O1W–H11W⋯O3W | 0.87 | 1.96 | 2.817(6) | 173.1 |
| O1W–H12W⋯O4ii | 0.87 | 2.02 | 2.859(6) | 160.1 |
| O2W–H21W⋯O5iii | 0.87 | 1.78 | 2.627(6) | 163.2 |
| O2W–H22W⋯O3W | 0.87 | 1.84 | 2.667(6) | 159.7 |
| O3W–H31W⋯O6 | 0.85 | 1.82 | 2.637(6) | 159.2 |
| O3W–H32W⋯O9iv | 0.86 | 1.85 | 2.705(6) | 175.5 |
| O4W–H41W⋯O7 | 0.87 | 1.96 | 2.803(7) | 161.1 |
| O4W–H42W⋯O2W | 0.87 | 1.96 | 2.826(7) | 169.6 |
| 2 | ||||
| N2–H2⋯O5A | 0.93 | 1.67 | 2.588(5) | 169.1 |
| N2–H2⋯O5 | 0.93 | 1.75 | 2.671(3) | 168.7 |
| N4–H47A⋯O2Wv | 0.88 | 2.02 | 2.899(7) | 175.6 |
| N3A–H37A⋯O21W | 0.88 | 2.03 | 2.902(7) | 173.3 |
| N4A–H47C⋯O21Wv | 0.88 | 2.09 | 2.941(6) | 161.4 |
| O1W–H11W⋯O4vi | 0.86 | 1.97 | 2.829(3) | 176.6 |
| O1W–H12W⋯O3W | 0.85 | 1.74 | 2.508(6) | 148.8 |
| O1W–H12W⋯O31W | 0.85 | 2.20 | 3.036(5) | 166.5 |
| O2W–H21W⋯O5vii | 0.89 | 1.82 | 2.562(6) | 139.3 |
| O2W–H22W⋯O3W | 0.89 | 1.71 | 2.600(7) | 177.0 |
| O3W–H31W⋯O6 | 0.86 | 1.97 | 2.827(7) | 171.1 |
| O3W–H32W⋯O8viii | 0.89 | 1.93 | 2.787(6) | 159.7 |
| O21W–H23W⋯O6A | 0.85 | 1.68 | 2.495(7) | 160.1 |
| O21W–H24W⋯O31W | 0.87 | 1.90 | 2.746(6) | 163.0 |
| O31W–H33W⋯O5Avii | 0.89 | 1.99 | 2.869(7) | 170.3 |
| O31W–H34W⋯O8Aviii | 0.89 | 1.93 | 2.780(5) | 161.0 |
| 3 | ||||
| N2–H2⋯O5 | 0.93 | 1.68 | 2.584(3) | 162.6 |
| N3–H3⋯O6 | 0.88 | 2.46 | 2.756(3) | 100.3 |
| O33–H33⋯O7 | 0.74 | 2.09 | 2.821(3) | 170.4 |
| O1W–H11W⋯O4ix | 0.99 | 1.86 | 2.843(3) | 170.3 |
| O1W–H12W⋯O6 | 0.83 | 1.93 | 2.765(3) | 175.0 |
| 4 | ||||
| N2–H2⋯O5 | 0.93 | 1.73 | 2.626(3) | 160.3 |
| O34–H34⋯O6 | 0.90 | 1.86 | 2.759(4) | 173.6 |
| O1W–H11W⋯O5 | 0.94 | 1.90 | 2.804(3) | 161.6 |
| O1W–H12W⋯O4x | 0.84 | 2.10 | 2.933(3) | 172.5 |
As mentioned above, brucinium cations form the L1 layers in crystals of 1, 2 and 4, and L2 self-assemblies in crystal of 3 (see Fig. 2). More detailed analysis reveals some similarities in the arrangement of brucinium cations forming layers L1 and L2. In both L1 and L2 self-assemblies, brucinium ions, related by two-fold screw axis symmetry, form corrugated ribbons along [010] directions. Neighboring corrugated ribbons are related by translation vectors in the crystal of 1, 2 and 4 and by two-fold axes symmetry in crystals of 3.
The surfaces of the resulting layers, L1 and L2, in all crystals under our investigation differ by their corrugation (Fig. 4f and 4g) but retain similar donor/acceptor properties, revealing their capabilities of formation the hydrogen bonds with anions by protonated N2 atoms. A similar observation applies to the interaction of water molecules with the carbonyl O4 atom.
 | ||
| Fig. 4 (a) The weak C–H⋯O and C–H⋯π hydrogen bonds stabilizing brucine corrugated ribbon of (b) layer L1, (c) layer L2 and (h) ribbon separated by guest molecules. (d) Brucine layered L1, (e) L2 and h) ribbons Rs self-assembly. Surfaces of (f) brucine layered L1 and (g) L2 self-assemblies with water molecules in the holes.42 | ||
We wondered why different brucine packing modes share similar donor/acceptor capabilities at the layer's surfaces. To the best of our knowledge, there are thirty-four known crystal structures (including the four reported herein) which contain brucine (molecular/ionic) and for which atomic coordinates are available.5,13–35 Among them, we selected those twenty-four (70.6%) crystal structures for which molecules or cations of brucine form corrugated ribbons similar to those presented in Fig. 4. Among the twenty-four selected structures, ribbons Rs, separated by a co-crystallizing compound, were found in seven crystals of those structures (see Fig. 4h). In thirteen crystal structures, brucine layers L1 were present while layers L2 were observed for four crystal structures (see Fig. 4d and 4e). This analysis clearly shows that brucine tends, in general, to form corrugated ribbons and the brucine ribbons reveal a tendency to form L1 layers. The tendency of brucine to form L2 layers is minor.
As could be expected, in all selected crystal structures, the tertiary amine N2 atoms of brucine, in either neutral or ionic form, participate in strong hydrogen bond formation with co-crystallizing guest molecules/anions, if such are present. The carbonyl O4 atom of brucine participates in either strong or weak hydrogen bonds formation, depending on the kind of brucine self-assembly (ribbon Rs, layers L1 or L2). Strong O–H⋯O4 hydrogen bonds with solvent molecules are observed in most crystals in which brucine molecules, or brucinium cations, form layered L1 or L2 packing arrays (see Fig. 4f and 4g). Crystals of brucine, brucine (-)-1-(o-bromophenyl)-1-phenyl-2-propynol and brucinium L-glycerate 4.5 hydrate are the only known exceptions to this observation. There, the carbonyl O4 atom participates in hydrogen bonding in which the brucine molecule, or co-crystallizing guest, is the donor. In crystals containing brucine ribbons Rs, at most one solvent molecule, per brucinium ion, is present. In that case, the carbonyl O4 does not have to be involved in hydrogen bonding with solvent molecules, even if such solvent molecules are present. It can, therefore, be concluded that depending on the degree of solvation of brucinium salts, the brucinium cations form an intriguing variety of architectural ensembles in the crystalline state. Thus, one may conclude that, depending on guest molecules' susceptibility to be solvated, various brucine self-assemblies may be present in crystals. This observation requires further investigation in order to confirm our current observations, especially important would be to explore the possibility that polymorphous or pseudopolymorphous forms exist.
Next, we have done an analysis of weak intermolecular contacts within the brucine self-assemblies: all known crystals of brucine, brucine solvates, brucine complexes and brucinium salts belong to the triclinic, monoclinic and orthorhombic systems. In most cases, molecules/ions of brucine are related by translation vectors or by two-fold, or two-fold screw, axes of symmetry. The translation vector, two-fold and two-fold screw axes symmetry generating acceptor (A) atoms of the C–H⋯A hydrogen bond are distinguished by (↑), (2) and (21) following the A symbol, respectively. We found that independently of layer L1, layer L2 or ribbon Rs, in each crystal structure examined, brucine corrugated ribbons are stabilized by weak C16–H16⋯O2 (21), C17–H17⋯O3 (21) (O2, O3 – methoxyl), C23–H23⋯O1 (↑) (O1 – ether) and C10–H10⋯π (arene) (21) hydrogen bonds. For more details, see Fig. 4a and the supplementary material.† As presented in Fig. 4b and 4c, the neighbor brucine ribbons are linked by weak C19–H19⋯O2 (21), C18–H18⋯O3 (21) and C11–H11⋯π (21) hydrogen bonds in the crystals containing brucine layers L1, and by the weak C23–H23⋯π (2) hydrogen bonds in the crystals containing brucine layers L2. In those crystals containing ribbons Rs, the brucine arene ring participates in intermolecular interaction with co-crystallizing compounds.
The geometry of selected C–H⋯O and C–H⋯π hydrogen bonds was analyzed (see supplementary material†), which allowed us to find some useful relationships: in most cases of layers L1 the C10–H10⋯π (21) hydrogen bonds are shorter than the C11–H11⋯π (21) hydrogen bonds. A similar relationship is observed between C10–H10⋯π (21) and C23–H23⋯π (2) hydrogen bonds and between C10–H10⋯π (21) and C–H⋯π (co-crystallizing compound being a donor) in the case of layers L2 and ribbons Rs, respectively. Another relationship concerns the offset of C–H⋯π hydrogen bonds36 as can be expected, the offset has lower values for the C10–H10⋯π (21) hydrogen bonds than for other C–H⋯π hydrogen bonds, and increases when the H⋯π distance increases.
Among C–H⋯O intermolecular contacts, the shortest H⋯O length found in crystals of brucine for the C19–H19⋯O2 hydrogen bonds was 2.29 Å, with an average value of about 2.5 Å. For other C–H⋯O intermolecular interactions, the H⋯O distance is usually greater than 2.5 Å.
As far as we can see, the nature of the relationships that are observed for the C–H⋯π hydrogen bonds is not observed either for the C–H⋯O hydrogen bonds or between C–H⋯O and C–H⋯π hydrogen bonds. One that should be mentioned here is that, independently of the co-crystallizing guest, few brucine donors recognize available acceptors that form weak C–H⋯O and C–H⋯π hydrogen bonds prevailing in similar self-assemblies observed in many crystals. The above statistical and geometrical analyses demonstrate the importance of the C–H⋯π hydrogen bonds. Among the two kinds of possible ribbons—one with shorter C–H⋯O and longer C–H⋯π hydrogen bonds, and the other with longer C–H⋯O and shorter C–H⋯π hydrogen bonds—the latter is much more favourable.
Hydrophilic medium, usually preferred for crystallization of brucinium salts, provides for many strong interactions. Brucine is barely soluble in water; thus, in such medium, brucine molecules should easy recognize each other because it reduces a number of hydrophobic surfaces, thus forming ribbons such as Rs or more complicated self-assemblies. Layers L1 and L2 supply hydrophilic sites for interaction with solvent molecules, as well as with the co-crystallizing compound. Then, the weak C–H⋯O and C–H⋯π hydrogen bonds gain an importance in molecular recognition. (We tried to unsuccessfully crystallize brucine and brucinium salts, for example, from tetrachloromethane.)
Finally, a number of crystals containing layers L1, layers L2, or ribbons Rs, is consistent with the number of the weak hydrogen bonds stabilizing suitable brucine self-assemblies. Both, methoxyl O2 and O3 atoms, arene rings and ether O1 atoms of the brucine molecule/ion are involved in hydrogen bonds that lead to corrugated ribbon formation. It was found that this is a common feature present in twenty-four structures. Neighbor ribbons are linked via C–H⋯O and/or C–H⋯π interactions. Both, methoxyl O2 and O3 atoms, and arene rings, and occasionally O1 atoms, are involved in these hydrogen bonds resulting in the formation of layer L1, while only the arene ring is involved in hydrogen bonds resulting in layer L2. Among seventeen crystal structures, the L1/L2 ratio is approximately thirteen to four (3.25). The relatively large number of examples of crystal structures with brucine forming ribbons Rs can be explained by the tendency of brucine, and some co-crystallizing compounds, to form weak hydrogen bonds.
Comparison of layers L1 and L2 indicates that the latter is stabilized by relatively small amounts of weak hydrogen bonds and provides surfaces, which are more corrugated and have more hydrophobic sites. Despite this, layers L2 are observed in some crystals. Why? There could be a lot of reasonable answers. We found one of them: layer L2 is more compact than the layer L1. The distance between ‘first’ and ‘third’ ribbon is, on average, equal to 14.13 and 15.80 Å in the layers L2 and L1, respectively. This observation is consistent with Kitaigorodskii considerations37 that for a given molecule, the actual crystal structure corresponds to one which is the most densely packed. On the other hand, the relatively small number of crystals containing brucine layers L2, illustrates the importance of the weak hydrogen bonds. Layer L2, which is more compact than layer L1, is, nonetheless less favourable due to a lower stabilization by weak hydrogen bonds.
Conclusions
The crystal structures of brucinium hydrogen N-benzoyl-D-aspartate tetrahydrate (1), brucinium N-benzoyl-D-asparaginate trihydrate (2), brucinium N-tert-butoxycarbonyl-L-alaninate 2-propanol solvate hydrate (3) and brucinium N-tert-butoxycarbonyl-L-prolinate ethanol solvate hydrate (4) were determined. In the crystals of 1, 2 and 4, brucinium cations form the corrugated layers L1 while puckered layers L2 are present in the crystal of 3. Independently of layers L1 or L2, they are extensibly separated or contain poorly hydrogen bonded anions and solvent molecules. Among strong hydrogen bonds, the ones linking both cationic and anionic/solvent layers are similar in the crystals of 1–4. Analysis of these interlayered hydrogen bonds in all known crystals containing brucine layers L1 and L2, reveals a tendency of the tertiary amine nitrogen atom and the carbonyl oxygen atom of brucinium cation to form hydrogen bonds with anions and solvent molecules, respectively. Layers L1 and L2 are constructed by similar ribbons; and yet, in crystals containing brucine ribbons Rs, which are separated by co-crystallizing guest anions, the carbonyl oxygen atom of brucinium cation seems to reveal a rather minor tendency to form hydrogen bond with solvent molecules. In this light, various brucine self-assemblies (layers or the ribbons) seem to result in inducing guest molecule to form strong bonds with solvent molecules or weak ones with brucine. Brucine molecules, forming layered self-assemblies, provide probably the most appropriate surfaces for hydrophilic environment and, therefore, give rise to the process occurring in hydrophilic solution, from which the crystals of brucinium salts were usually grown. Weak hydrogen bonds gain an importance because they stabilize brucine self-assemblies which provide most appropriate surfaces. Indeed, the same pattern of the weak hydrogen bonds is present in many crystals containing brucine (molecular/ionic).Experimental
The crystals of 1 and 2 were obtained during racemic resolution of N-benzoyl-DL-amino acids from water/ethanol solution containing equimolar amount of brucine (commercially available) and N-benzoyl-DL-aspartic acid or N-benzoyl-DL-asparagine,38 respectively. The crystals of 3 and 4 were grown from solution containing equimolar amount of brucine and N-tert-butoxycarbonyl-L-amino acid (alanine or proline, respectively).38 While the crystals of 4 were grown from water/ethanol solution, crystals of 3 were obtained from water/2-propanol solution. Crystallization of 1–4 were carried out in room temperature by slow solvent evaporation.X-ray data were collected at 100 K using an Oxford Cryosystem device on a Kuma KM4CCD κ-axis diffractometer with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Data reduction and analysis were carried out with the CrysAlice ‘RED’ program.39 The space groups were determined using the XPREP program. Structures were solved by direct methods using the XS program and refined using all F2 data, as implemented by the XL program.40 Non-hydrogen atoms were refined with anisotropic displacement parameters. The occupancy factor for the disordered N-benzoyl-D-asparaginate anion and the water O2W and O3W molecules in the crystal structure of 2 were refined and fixed with a 0.5 value. H atoms bonded to C atoms in the brucinium cation were included in their idealized positions and treated as riding in the subsequent refinement, with C–H distances in the range 0.95–1.00 Å and with Uiso(H) = 1.2Ueq(C). The remaining H atoms were located in difference density maps and refined with isotropic displacement parameters; in the final cycle of refinement, they were made to ride on their parent atoms. The Friedel pairs were merged before the final refinement. The absolute structures were chosen on the basis of the known absolute configuration of brucine.41
Crystal data for 1
C23H27N2O4+ C11H10NO5−·4H2O, M = 703.73, colourless plate, crystal dimensions 0.25 × 0.25 × 0.12 mm, orthorhombic, space group P212121, a = 8.2334(14), b = 12.283(2), c = 33.625(5) Å, V = 3400.6(9) Å3, Z = 4, Dc = 1.375 Mg m−3, T = 100(2) K, R = 0.078, wR = 0.164 (2955 reflections with I > 2σ(I)) for 451 variables.Crystal data for 2
C23H27N2O4+ C11H11N2O4−·3H2O, M = 684.73, colourless needle, crystal dimensions 0.20 × 0.06 × 0.06 mm, orthorhombic, space group P212121, a = 7.8711(8), b = 12.4649(12), c = 33.044(3) Å, V = 3242.0(6) Å3, Z = 4, Dc = 1.403 Mg m−3, T = 100(2) K, R = 0.058, wR = 0.072 (2109 reflections with I > 2σ(I)) for 598 variables.Crystal data for 3
C23H27N2O4+ C8H14NO4−·C3H7OH·H2O, M = 661.78, colourless plate, crystal dimensions 0.20 × 0.20 × 0.20 mm, monoclinic, space group C2, a = 13.611(2), b = 12.374(2), c = 20.458(3) Å, β = 93.146(18)°, V = 3440.2(10) Å3, Z = 4, Dc = 1.278 Mg m−3, T = 100(2) K, R = 0.041, wR = 0.069 (2644 reflections with I > 2σ(I)) for 424 variables.Crystal data for 4
C23H27N2O4+ C10H16NO4−·C2H5OH·H2O, M = 673.79, colourless plate, crystal dimensions 0.30 × 0.30 × 0.15 mm, monoclinic, space group P21, a = 7.5255(10), b = 12.0556(15), c = 18.597(2) Å, β = 96.238(10)°, V = 1677.2(4) Å3, Z = 2, Dc = 1.334 Mg m−3, T = 100(2) K, R = 0.039, wR = 0.086 (3002 reflections with I > 2σ(I)) for 433 variables.Acknowledgements
We are indebted to Professor Ivan Bernal for valuable discussion and kind help during paper preparation. This work was supported in part by the Polish Committee for Scientific Research, Grant No. 2532/W/WCH/2004–2005.References
- J. Jacques, A. Collet and S. H. Wilen, Enantiomers, Racemates and Resolutions, Krieger Publishing Company, Malabar, FL, 1991 Search PubMed.
- K. Kinbara, K. Oishi, Y. Harada and K. Saigo, Tetrahedron, 2000, 56, 6651 CrossRef CAS.
- K. Kinbara, Y. Kobayashi and K. Saigo, J. Chem. Soc., Perkin Trans. 2, 1998, 1767 RSC.
- E. Kozsda-Kovacs, G. M. Keserii, Z. Bocskei, I. Szilagyi, K. Simon, B. Bertok and E. Fogassy, J. Chem. Soc., Perkin Trans. 2, 2000, 149 RSC.
- R. O. Gould and M. D. Walkinshaw, J. Am. Chem. Soc., 1984, 106, 7840 CrossRef CAS.
- K. Kinbara, Y. Kobayashi and K. Saigo, J. Chem. Soc., Perkin Trans. 2, 2000, 111 RSC.
- K. Nemak, M. Acs, Z. M. Jaszay, D. Kozma and E. Fogassy, Tetrahedron, 1996, 52, 1637 CrossRef CAS.
- R. Yoshioka, H. Hiramatsu, K. Okamura, I. Tsujioka and S. Yamada, J. Chem. Soc., Perkin Trans. 2, 2000, 2121 RSC.
- K. Kinbara, K. Sakai, Y. Hashimoto, H. Nohira and K. Saigo, Tetrahedron: Asymmetry, 1996, 7, 1539 CrossRef CAS.
- M. Pallavicini, C. Bolchi, B. Moroni, E. Valoti and O. Piccolo, Tetrahedron: Asymmetry, 2003, 14, 2247 CrossRef CAS.
- O. Barabas, D. K. Menyhard, Z. Bocskei, K. Simon, I. Kiss-Ajzert, K. Takacs and I. Hermecz, Tetrahedron: Asymmetry, 2000, 11, 4061 CrossRef CAS.
- K. Kinbara, Y. Tagawa and K. Saigo, Tetrahedron: Asymmetry, 2001, 12, 2927 CrossRef CAS.
- A. Białońska and Z. Ciunik, CrystEngComm, 2004, 47, 276 RSC.
- A. Białońska and Z. Ciunik, Acta Crystallogr., Sect. C, 2004, 60, o853 CrossRef.
- J. L. Wright, B. W. Caprathe, D. M. Downing, S. A. Glase, T. G. Heffner, J. C. Jaen, S. Johnson, S. R. Kesten, R. G. MacKenzie, L. T. Meltzer, T. A. Pugsley, S. J. Smith, L. D. Wise and D. J. Wustrow, J. Med. Chem., 1994, 37, 3523 CrossRef CAS.
- K. Krajewski and Z. Ciunik, Pol. J. Chem., 1999, 73, 1687 CAS.
- K. Sada, M. Miyata and K. Yoshikawa, Chem. Commun., 1998, 1763 RSC.
- J. L. Wright, B. W. Caprathe, D. M. Downing, S. A. Glase, T. G. Heffner, J. C. Jaen, S. Johnson, S. R. Kesten, R. G. MacKenzie, L. T. Meltzer, T. A. Pugsley, S. J. Smith, L. D. Wise and D. J. Wustrow, J. Med. Chem., 1994, 37, 3523 CrossRef CAS.
- K. Chandramohan and K. Ravikumar, J. Chem. Crystallogr., 1999, 29, 121 CrossRef CAS.
- A. A. Pinkerton, P.-A. Carrupt, F. Claret and P. Vogel, Acta Crystallogr., Sect. C, 1993, 49, 1632 CrossRef.
- S. Allenmark and U. Skogsberg, Enantiomer, 2000, 5, 451 Search PubMed.
- F. Toda, K. Tanaka, H. Ueda and T. Oshima, Isr. J. Chem., 1985, 25, 338 CAS.
- S. S. B. Glover, R. O. Gould and M. D. Walkinshaw, Acta Crystallogr., Sect. C, 1985, 41, 990 CrossRef.
- F. J. J. Dijksma, R. O. Gould, S. Parsons, P. Taylor and M. D. Walkinshaw, Chem. Commun., 1998, 745 RSC.
- F. J. J. Dijksma, M. D. Walkinshaw, S. Person and R. O. Gould, Acta Crystallogr., Sect. C, 1998, 54, 1948 CrossRef.
- G. Quinkert, H.-G. Schalz, E. M. Dzierzynski, G. Durner and J. W. Bats, Angew. Chem., Int. Ed. Engl., 1986, 25, 992 CrossRef.
- S. E. Boiadjiev, R. V. Person, G. Puzicha, C. Knobler, E. Maverick, K. N. Trueblood and D. A. Lightner, J. Am. Chem. Soc., 1992, 114, 10123 CrossRef CAS.
- J. B. Laursen, C. G. Jorgensen and J. Nielsen, Bioorg. Med. Chem., 2003, 11, 723 CrossRef CAS.
- G. Smith, U. D. Wermuth, P. C. Healy, D. J. Young and J. M. White, Acta Crystallogr., Sect. E, 2005, 61, 2646 CrossRef.
- A. Białońska, Z. Ciunik, T. Popek and T. Lis, Acta Crystallogr., Sect. C, 2005, 61, o88 CrossRef.
- E. Cheung, M. R. Netherton, J. R. Scheffer and J. Trotter, Tetrahedron Lett., 1999, 40, 8737 CrossRef CAS.
- M. Yamagishi, Y. Yamada, K. Ozaki, T. Da-te, K. Okumara, M. Suzuki and K. Matsumoto, J. Org. Chem., 1992, 57, 1568 CrossRef CAS.
- S. Kuwata, J. Tanaka, N. Onda, T. Yamada, T. Miyazawa, M. Sugiura, Y. In, M. Doi, M. Inoue and T. Ishida, Bull. Chem. Soc. Jpn., 1993, 66, 1501 CAS.
- J. Bao, W. D. Wulff, J. B. Dominy, M. J. Fumo, E. B. Grant, A. C. Rob, M. C. Whitcomb, S.-M. Yeung, R. L. Ostrander and A. L. Rheingold, J. Am. Chem. Soc., 1996, 118, 3392 CrossRef CAS.
- L. Thumberg and S. Allenmark, Tetrahedron: Asymmetry, 2003, 14, 1317 CrossRef.
- Z. Ciunik and G. R. Desiraju, Chem. Commun., 2001, 703 RSC.
- A. I. Kitaigorodskii, Molecular Crystals and Molecules, Academic Press, Inc., New York, 1973 Search PubMed.
- J. T. Wróbel, Preparatyka i elementy syntezy organicznej, PWN, Warszawa, 1983 Search PubMed.
- Oxford Diffraction. CrysAlis ‘RED’. 2001. Wrocław, Oxford Diffraction (Poland) Sp. z o. o.
- Bruker. SHELXTL-NT. [5.1]. 1999. Bruker AXS.
- F. Toda, K. Tanaka, H. Ueda and T. Oshima, Isr. J. Chem., 1985, 25, 338 CAS.
- W. Humphrey, A. Dalke and K. Schelten, J. Mol. Graphics, 1996, 14(1), 33 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Geometry of selected C–H⋯O and C–H⋯π hydrogen bonds. See DOI: 10.1039/b515365d |
| ‡ CCDC reference numbers 288082 (1), 288083 (2), 288084 (3) and 203141 (4). For crystallographic data in CIF or other electronic format see DOI: 10.1039/b515365d |
| This journal is © The Royal Society of Chemistry 2006 |
