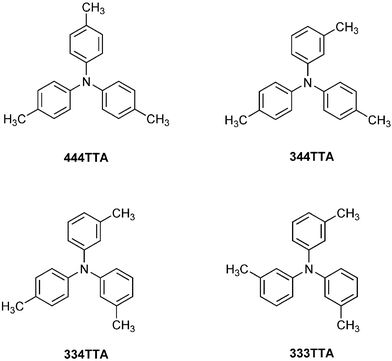
Crystals of isomeric tritolylamines: embrace motifs in crystals, and their thermochemical properties†
Touraj
Manifar
a,
Sohrab
Rohani
a,
Michael
Jennings
b,
Douglas
Hairsine
b and
Ian
Dance
*c
aCrystallization and Control Laboratory, Department of Chemical and Biochemical Engineering, University of Western Ontario, London, Ontario, Canada
bDepartment of Chemistry, University of Western Ontario, London, Ontario, Canada
cSchool of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: I.Dance@unsw.edu.au
First published on 13th January 2006
Abstract
Molecules NAr3 with planar stereochemistry at N and canted Ar rings can form the intermolecular sixfold aryl embrace (6AE). The presence of 3-methyl groups on the aryl groups enhances the stabilisation of the 6AE, by contributing to the EF motifs which comprise it. Analysis of the crystal packing of four tritolylamines, N,N,N-tris(4-methylphenyl)amine [444TTA], N-(3-methylphenyl)-N,N-bis(4-methylphenyl)amine [344TTA], N,N-bis-(3-methylphenyl)-N-(4-methylphenyl)amine [334TTA] and N,N,N-tris(3-methylphenyl)amine [333TTA], reveals the presence of (333TTA)2 dimers maintained by an excellent 6AE, an unprecedented almost linear chain of repeated 6AE in 334TTA (cryst), and poorly formed embrace motifs in 344TTA (cryst), and 444TTA (cryst). Calculated intermolecular potentials for the actual or possible 6AE embraces formed by 333TTA, 334TTA and 444TTA confirm the stabilisation of the 6AE by 3-methyl groups, and it is estimated that each such methyl group augments the stabilisation of the underlying 6AE embrace by ca 5 kJ mol−1. The dominant dimerisation that occurs in crystalline 333TTA is reflected in the phase change enthalpies for the four isomers. The enthalpy of sublimation of 333TTA (51 kJ mol−1) is ca 45 kJ mol−1 less than the enthalpies of sublimation of 334TTA (97 kJ mol−1), 344TTA (98 kJ mol−1) and 444TTA (93 kJ mol−1), which leads to the hypothesis that (333TTA)2 dimers occur in the gas phase at ambient pressure. Mass spectrometric attempts to detect (333TTA)2(g), as [(333TTA)2]+, were unsuccessful, possibly due to dissociation under high vacuum. The unknown crystal packing of tris(3,5-dimethylphenyl)amine is predicted to be dominated by linear chains of methyl-stabilised 6AE.
Introduction
One of the weaknesses of many current investigations of crystal engineering is the absence of thermodynamic data on which to base both analysis and prediction of crystal structure: most efforts use experimental structural information and theoretical energetic information, because the experimental energy data have not been measured. This is unfortunate, since equilibrium thermodynamics and transformation thermodynamics determine the outcomes of crystallisation experiments.1,2In this context, the availability of crystal structures and phase-change enthalpies for a set of four isomeric tritolylamines (444TTA, 344TTA, 334TTA, 333TTA)3 prompted us to analyse relationships between crystal packing and thermochemical data for these related compounds. These compounds constitute an informative set, in which the intermolecular interactions are not complicated by hydrogen bonding or ion association.
The enthalpy of sublimation of one compound, 333TTA, is distinctly different from those of the other three, and therefore we sought explanation of this through examination of the crystal packing. This analysis, supplemented by calculations of relevant intermolecular potentials, led to a prediction that a stable embracing dimer of 333TTA might exist in the gas phase, and we attempted to detect this dimer by mass spectrometric measurements.
Intermolecular interactions between molecules containing aryl groups are based on two fundamental geometrical motifs, the offset-face-to-face (OFF) and edge-to-face (EF) arrangements.2,4 In molecules containing multiple aryl groups these pairwise primary motifs may operate in concert to form intermolecular embraces. Molecules containing triaryl entities Ar3E are well-known to form intermolecular embrace motifs in crystals: the two most common embraces are the sixfold phenyl/aryl embrace (6PE/6AE) which is an (EF)6 concert involving all six aryl groups (Fig. 1(b)), and the (OFF)(EF)2 concert involving four aryl groups, named the parallel fourfold phenyl/aryl embrace (P4PE/P4AE, Fig. 1(c))
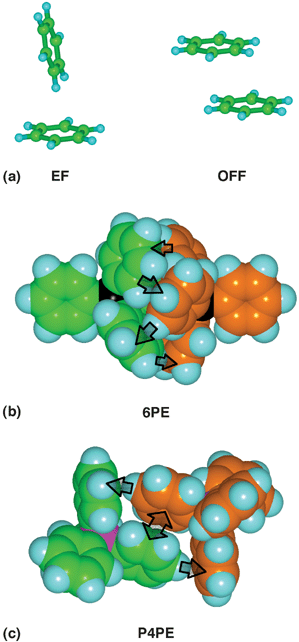 | ||
| Fig. 1 (a) The common edge-to-face (EF) and offset-face-to-face (OFF) interactions between a pair of aromatic groups. (b) The centrosymmetric sixfold phenyl embrace (6PE), or (EF)6 embrace, between a pair of Ph4P+ molecules (colour-differentiated C atoms). The six vectorial EF interactions (those visible are marked with arrows) alternate between the molecules around the embrace. (c) The centrosymmetric parallel fourfold phenyl embrace (P4PE) or (OFF)(EF)2 embrace between a pair of Ph4P+ molecules: the double-ended arrow is the OFF interaction. | ||
In almost all of the molecules known to form these embraces the Ar3E moieties are pyramidal at E (usually P or As), and for the sixfold embraces the Ar3E moieties are threefold rotors.5 The triarylamines (E = N) are essentially planar at N, but the inclinations of the aryl groups to the C3N plane permit embrace motifs, although these had not been investigated prior to this work.
Experimental
The syntheses and solubilities of the triarylamines have been described.6The crystallographic data for these compounds is available in the literature.7
Thermochemical measurements
Thermal analysis was conducted by differential scanning calorimetry (DSC, Mettler). For heat of vaporisation measurements, aluminum hermetic containers with a capacity of less than 50 µL, a specimen size of 2–4 mg and lids with a pinhole of diameter 25–127 µm were used. The heating rate was 5–10 °C min−1, from ambient to 400 °C. Sensors and crucibles were maintained under a flow of nitrogen during the experiment. For the measurement of heat of fusion, standard aluminum pans were used, with a heating rate of 2 °C min−1 from 0–140 °C.Mass spectrometry
Electrospray positive ion spectra were obtained with a Micromass LCT spectrometer, with samples dissolved in toluene or methanol. Spectra were obtained with the sample cone voltage varing from 200 to 0 V, and with a 30 V cone voltage the capillary voltage was varied from 5 to 3.5 KV. No dimers were detected.Theoretical calculations
Calculations of intermolecular potentials have been made by two independent methods, density functional and empirical van der Waals/coulombic methods. Details of the methodology and the results are presented in the supplementary material.†Results
Crystal packing
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) symmetry for the embrace.
symmetry for the embrace.
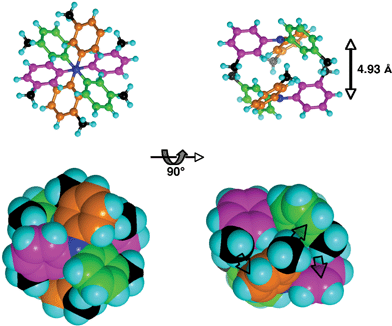 | ||
Fig. 2 Skeletal and space-filling representations of the centrosymmetric sixfold aryl embrace (EF)6 between a pair of 333TTA molecules, viewed along and normal to the N⋯N vector. The three rings on each molecule are coloured green, orange and magenta, and all methyl C atoms are black: N blue. The symmetry of the embrace is close to ![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . Each of the six EF local interactions that comprise the embrace involves 2-C–H and 3-methyl C–H of one tolyl group directed towards the face of a tolyl ring on the other molecule: the three EF interactions evident on the lower right picture are marked with grey arrows. . Each of the six EF local interactions that comprise the embrace involves 2-C–H and 3-methyl C–H of one tolyl group directed towards the face of a tolyl ring on the other molecule: the three EF interactions evident on the lower right picture are marked with grey arrows. | ||
The embracing pairs of molecules are loosely stacked along the c-axis of the crystal, as illustrated in Fig. 3. Apart from one CH3 to tolyl face motif, the lateral interactions between embrace pairs in adjacent stacks are non-specific van der Waals stabilisation (Fig. 4).
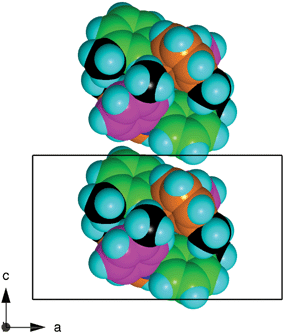 | ||
| Fig. 3 The relationship between adjacent pairs of embracing 333TTA molecules stacked along the c-axis. | ||
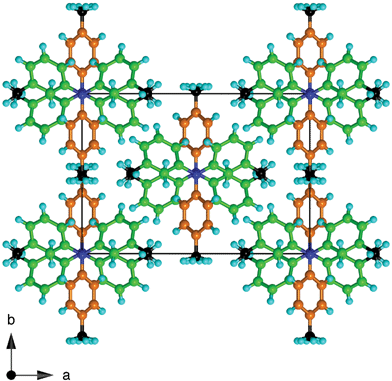 | ||
| Fig. 5 Projection along the chains of molecules in crystalline 334TTA. Twofold axes pass through the 4-tolyl substituents, coloured orange, with both disorder components of the 4-methyl groups included. Note that the methyl groups (C black) are segregated. | ||
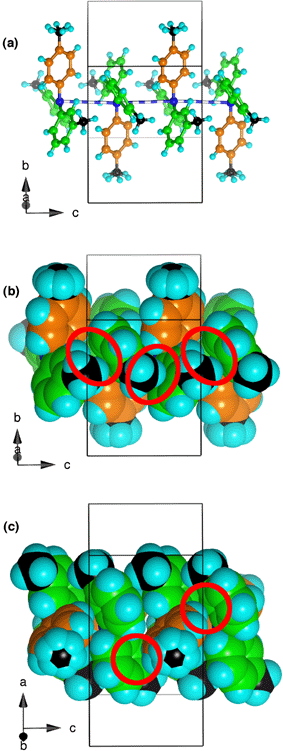 | ||
| Fig. 6 Aspects of the interactions between four contiguous 334TTA molecules along the dominant chains in the crystal. (a) Skeletal view. The blue/white stripes are the N–N separations across centres of inversion: N–N 4.48 Å, N–N–N 176°; twofold axes pass though the orange tolyl groups. (b) Space-filling representation, emphasising the approach (red enclosures) of the 2-C–H and 3-methyl C–H atoms of the green ligands to the faces of green ligands in the next molecule. Edge (green) to face (orange) and edge (orange) to face (green) motifs are also visible. (c) Space-filling view showing how two edges of the orange ligand interact with the faces of green ligands of the two contiguous molecules (red enclosures). | ||
As far as we know this sequence of continuous sixfold aryl embraces has not been observed previously.
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . One of these molecules forms an approximate single sixfold aryl embrace with itself across a centre of inversion (Fig. 7), in which the 3-tolyl methyl group is not part of the edge-to-face interaction as it has been in the previous two crystals. There are no other concerted embrace motifs in this crystal, and only a small proportion of the many different intermolecular contacts in this crystal approximate local edge-to-face or offset-face-to-face motifs.
. One of these molecules forms an approximate single sixfold aryl embrace with itself across a centre of inversion (Fig. 7), in which the 3-tolyl methyl group is not part of the edge-to-face interaction as it has been in the previous two crystals. There are no other concerted embrace motifs in this crystal, and only a small proportion of the many different intermolecular contacts in this crystal approximate local edge-to-face or offset-face-to-face motifs.
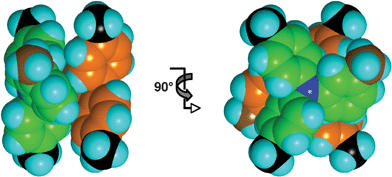 | ||
| Fig. 7 The approximate sixfold aryl centrosymmetric embrace between two 344TTA molecules: the N–N separation (obscured) is 5.0 Å. The methyl group of the 3-tolyl molecule (C brown) is not part of the embrace. Inclinations of the aryl planes to the C3N core plane are 31, 35 and 63°. | ||
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . None of the possible sixfold aryl embraces occur. The packing of molecules in the crystal is most readily understood in terms of alternating corrugated layers of each of the independent molecules, stacked along the b-direction, as illustrated in Fig. 8. The layers of the two molecules are quite similar, and within each there are (EF)2 embraces and OFF motifs, as illustrated in Fig. 9. The (EF)2 embrace is similar to the P4AE shown in Fig. 1(c), but with excess offset of the central OFF component.
. None of the possible sixfold aryl embraces occur. The packing of molecules in the crystal is most readily understood in terms of alternating corrugated layers of each of the independent molecules, stacked along the b-direction, as illustrated in Fig. 8. The layers of the two molecules are quite similar, and within each there are (EF)2 embraces and OFF motifs, as illustrated in Fig. 9. The (EF)2 embrace is similar to the P4AE shown in Fig. 1(c), but with excess offset of the central OFF component.
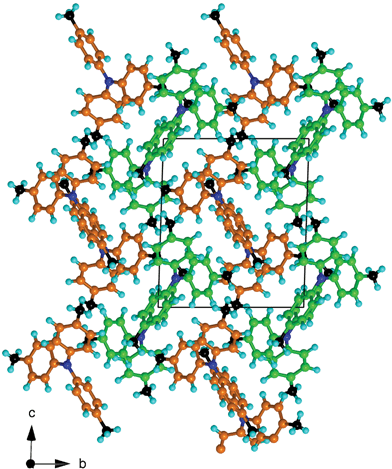 | ||
Fig. 8 The crystal packing of 444TTA (space-group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ), conceptualised as alternating corrugated layers of the two independent molecules (coloured green and orange). ), conceptualised as alternating corrugated layers of the two independent molecules (coloured green and orange). | ||
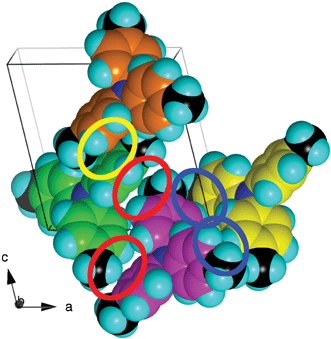 | ||
| Fig. 9 The local motifs in the crystal structure of 444TTA, illustrated for four contiguous molecules, differentiated by C atom colour. A centrosymmetric offset-face-to-face interaction is marked with the yellow enclosure; the red enclosures and the blue enclosures mark centrosymmetric pairs of edge-to-face interactions involving the 3-C–H and 4-CH2 donors. | ||
To summarise the crystal packing and intermolecular interactions in these four crystals: (a) 333TTA molecules occur as pairs maintained by a well-formed 6AE in which all of the 3-methyl groups are involved, and van der Waals interactions of these (333TTA)2 pairs with neighbours are apparently less significant; (b) 334TTA molecules form a continuous chain of 6AE embraces, in which the 4-methyl group is not involved, and the EF local motifs are not as well developed as those in 333TTA; (c) 344TTA molecules pack with poor geometrical efficiency, with only one in three molecules occurring in a concerted motif which is a less well developed 6AE involving none of the tolyl methyl groups; (d) 444TTA molecules do not form sixfold aryl embraces in the crystal, but do engage in some (EF)2 and OFF intermolecular interactions.
The preceding analysis of intermolecular motifs has been based solely on geometric juxtaposition, but of course it is the energies of these interactions that are determinative. We next describe our measurements of the phase-change thermochemical properties of these crystals, and their relationships with the crystal packing.
Phase-change thermochemical properties
Table 1 contains our measurements of the enthalpies of fusion and vapourisation, and the enthalpy of sublimation, for the four compounds. The notable property is that these molar enthalpies are appreciably smaller for 333TTA than for the other compounds: for example, the enthalpies of sublimation of 334TTA, 344TTA and 444TTA are all in the range 93–98 kJ mol−1, whereas ΔHsub for 333TTA (51 kJ mol−1) is just over half these values.| Compound | 333TTA | 334TTA | 344TTA | 444TTA |
|---|---|---|---|---|
| Mp/°C | 39.8 | 89.5 | 56.7 | 115.6 |
| ΔHfus/kJ mol−1 | 13.07 | 26.39 | 21.71 | 19.95 |
| Bp/°C | 294.8 | 274.2 | 315.9 | 300.9 |
| ΔHvap/kJ mol−1 | 37.59 | 69.29 | 75.93 | 72.57 |
| ΔHsub/kJ mol−1 | 50.66 | 95.69 | 97.64 | 92.53 |
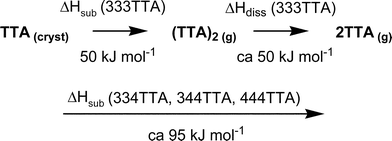
These data prompt the hypothesis that the favourable 6AE evident in crystalline 333TTA might be retained in a dimeric molecule in the gas phase: the hypothesis is that eqn (1) is the sublimation process for 333TTA, while the other three compounds dissociate according to eqn (2). The reduced value of ΔHsub for 333TTA is proposed to occur because the [(tolyl)3N]2 dimer is not dissociated.
| (tolyl)3N (cryst) ⇒ [(tolyl)3N]2 (g) | (1) |
| (tolyl)3N (cryst) ⇒ (tolyl)3N (g) | (2) |
This interpretation of the ΔHsub data is summarised in Scheme 1.
 | ||
| Scheme 1 | ||
The differentiation of 333TTA from the other compounds occurs also for the enthalpies of fusion and vapourisation, and so the hypothesis is also that the [(tolyl)3N]2 dimer occurs in the liquid phase more so for 333TTA than for the other isomers.
In order to explore this idea that the 6AE association of 333TTA is special, and to assess the merits of the observed and unobserved 6AE pairings of TTA isomers, intermolecular potentials have been calculated, by two independent methods. Details of the methods and the resulting potentials are provided in the supplementary material.† The energy of the 6AE in (333TTA)2 is estimated to be 65 ± 10 kJ mol−1, and in (334TTA)2 about 50 ± 10 kJ mol−1. The unobserved 6AE for (444TTA)2 is estimated to have an energy of 35 ± 10 kJ mol−1. From the participation of the tolyl methyl groups in the EF motifs of these embraces—6 methyl groups per 6AE in (333TTA)2, 2 methyl groups per 6AE in (334TTA)2, none in (444TTA)2—it could be concluded that each methyl group augments the stabilisation of the underlying embrace by ca 5 kJ mol−1. The calculated dissociation potential energy of the (333TTA)2 dimer, ca 65 kJ mol−1, is of similar magnitude to the deficit in ΔHsub for 333TTA, ca 45 kJ mol−1 (Scheme 1).
Since paired 333TTA molecules are postulated to exist in the gas phase, we made mass spectrometric measurements in attempt to detect them. Since it is known that the intermolecular stabilisation of positively charged benzene pairs [(C6H6)2]+ is much larger than that of uncharged pairs—experimental measurements for [(C6H6)2]+ range 35 to 90 kJ mol−1,8,9 compared with 7 to 10 kJ mol−1 for (C6H6)2—it was expected that ionized [(333TTA)2]+ would be strongly stabilised in the gas phase. Solutions of the four isomeric TTA molecules in either toluene or methanol were investigated by electrospray ionisation techniques using ranges of sample cone voltages and capillary voltages, but no evidence of dimer cations was found in the resulting spectra. Experiments with the solids on a solid probe and electron ionisation also yielded no evidence of dimers. We suspect that the failure to observe the predicted dimer cations is due to the high vacuum conditions which favour dissociation, and believe that detection of the dimer in the gas phase should be attempted at ambient pressure.
Discussion
We have shown that EAr3 molecules with planar stereochemistry at E can engage in the sixfold aryl embrace, provided that the aryl rings are canted to the molecular plane: this canting is obligatory in NAr3 molecules to avoid steric clashes between 2- and 6-hydrogen atoms. Further, the presence of 3-methyl groups on the aryl groups enhances the stabilisation of the 6AE, by contributing to the EF motifs which comprise the 6AE: it is estimated that each such methyl group augments the energy of the 6AE by ca 5 kJ mol−1. The consequences of this for the organisation of TTA isomers in their crystals are: (1) 333TTA with the strongest 6AE uses this embrace alone to create (333TTA)2 dimers in the crystal, utilising all of the substituent methyl groups which are therefore unavailable to promote any other concerted motifs between the (333TTA)2 dimers; (2) in crystalline 334TTA the 6AE are less stable, the 4-methyl group is uninvolved and therefore disordered, and each molecule forms poorer 6AE on both sides using only two 3-methyl groups per 6AE rather than the ideal six; (3) in 344TTA the 6AE is more poorly formed, and is hardly competitive with general unoriented van der Waals interactions; (4) in crystalline 444TTA the 6AE motif is uncompetitive with other concerts of EF and OFF motifs.As far as we know, the almost linear chain of repeated 6AE in 334TTA is unprecedented. It cannot occur with pyramidal EAr3 molecules. Tetrahedral EAr4 molecules can form zig-zag chains of repeated 6AE,10 but these do not use both edges of all aryl rings as occurs in the 334TTA crystals. The principles developed above lead to the expectation that the molecule (tris(3,5-dimethylphenyl)amine) which has correctly placed methyl groups on both edges of the phenyl substituents will be able to form two of the favourable 6AE motifs demonstrated by 333TTA. Therefore we predict that tris(3,5-dimethylphenyl)amine will crystallise with the formation of linear chains repeating the most stable 6AE, illustrated in Fig. 10: this is an infinite extension of the 6AE that occurs in 333TTA (Fig. 2).
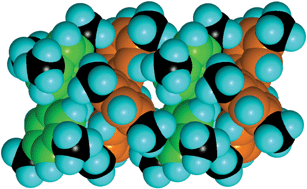 | ||
| Fig. 10 A four molecule segment of an infinite chain, predicted to be the principal intermolecular feature in crystalline tris(3,5-dimethylphenyl)amine. Ring C atoms are coloured green and orange in alternating molecules, and all methyl C atoms are black. Each molecule is involved in a complete 6AE with its neighbours: the 2-CH, 3-CH3, 5-CH3 and 6-CH of each ring are EF donors, and all ring faces are EF acceptors. | ||
For these four isomers of TTA there is good general agreement between the observed crystal packing and the experimental phase change enthalpies.11 The isomer with optimally located 3-methyl groups and able to form the most energetically stabilised 6AE, occurs as such in the crystal. With diminishing energy of the 6AE as the methyl groups are relocated to 4-positions, the integrity of this 6AE motif in the crystals also diminishes.
Acknowledgements
IGD acknowledges computing facilities provided by the Australian Centre for Advanced Computing and Communications.References
- T. Beyer, T. Lewis and S. L. Price, CrystEngComm, 2001, 44 RSC
; R. J. Davey, K. Allen, N. Blagden, W. I. Cross, H. F. Lieberman, M. J. Quayle, S. Righini, L. Seton and G. J. T. Tiddy, CrystEngComm, 2002, 4, 257–264 RSC
.
- I. Dance, CrystEngComm, 2003, 5, 208–221 RSC
.
-
N,N,N-tris(4-methylphenyl)amine, N-(3-methylphenyl)-N,N-bis(4-methylphenyl)amine, N,N-bis-(3-methylphenyl)-N-(4-methylphenyl)amine and N,N,N-tris(3-methylphenyl)amine, respectively
.
-
I. Dance, in Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. M. Steed, Dekker, New York, 2004, p. 1076–1092 Search PubMed
.
- I. G. Dance and M. L. Scudder, J. Chem. Soc., Dalton Trans., 2000, 1579–1586 RSC
; I. G. Dance and M. L. Scudder, J. Chem. Soc., Dalton Trans., 2000, 1587–1594 RSC
.
- T. Manifar and S. Rohani, Can. J. Chem. Eng., 2004, 82, 323–334 CAS
; T. Manifar, S. Rohani, T. Bender, B. Goodbrand, M. Saban and R. Gaynor, Ind. Eng. Chem. Res., 2005, 44, 789–798 CrossRef CAS
; T. Manifar, S. Rohani and M. Saban, Ind. Eng. Chem. Res., 2005, 44, 970–976 CrossRef CAS
.
- S. L. Reynolds and R. P. Scaringe, Cryst. Struct. Commun., 1982, 11, 1129–1134 CAS
; T. Manifar, S. Rohani and M. Jennings, Acta Crystallogr., Sect. E, 2004, 60, O2301–O2303 CrossRef
; T. Manifar, S. Rohani and M. Jennings, Acta Crystallogr., Sect. E, 2005, 61, O1297–O1299
; T. Manifar, S. Rohani and M. Jennings, Acta Crystallogr., Sect. E, 2005, 61, O1032–O1034
.
- M. Moet-Ner, P. Hamlet, E. P. Hunter and F. H. Field, J. Am. Chem. Soc., 1978, 100, 5466–5471 CrossRef
; K. Hiraoka, S. Fujimaki, K. Aruga and S. Yamabe, J. Chem. Phys., 1991, 95, 8413–8418 CrossRef CAS
.
- E. Miyoshi, T. Ichikawa, T. Sumicheme, Y. Sakai and N. Shida, Chem. Phys. Lett., 1997, 275, 404–408 CrossRef CAS
.
- I. Dance and M. Scudder, J. Chem. Soc., Dalton Trans., 1996, 3755–3769 RSC
.
- Note that, as expected, the high melting points (Table 1) do not correlate with crystal packing features.
Footnote |
| † Electronic supplementary information (ESI) available: Calculations of intermolecular potentials. See DOI: 10.1039/b516210f |
| This journal is © The Royal Society of Chemistry 2006 |