A steep one-step [HS–HS] to [LS–LS] spin transition in a 4,4′-bipyridine linked one-dimensional coordination polymer constructed from a pyrazolato bridged Fe(II) dimer†
Ko
Yoneda
a,
Keiichi
Adachi
a,
Shinya
Hayami
b,
Yonezo
Maeda
b,
Motomi
Katada
c,
Akira
Fuyuhiro
a,
Satoshi
Kawata
*a and
Sumio
Kaizaki
*a
aDepartment of Chemistry, Graduate School of Science, Osaka University, Toyonaka, Osaka, 560-0043, Japan. E-mail: kawata@chem.sci.osaka-u.ac.jp; kaizaki@chem.sci.osaka-u.ac.jp; Fax: +81-6-6850-5473
bDepartment of Chemistry, Graduate School of Science, Kyushu University, Fukuoka, 812-8581, Japan
cDepartment of Chemistry, Graduate School of Science, Tokyo Metropolitan University, Hachiouji, 192-0397, Japan
First published on 20th October 2005
Abstract
The variable temperature magnetic susceptibility, X-ray crystallography, and Mössbauer and Raman spectra of a new dinuclear complex-based one-dimensional coordination polymer [{FeII2(NCS)2(μ-bpypz)2}(μ-4,4′-bpy)]·MeOH demonstrated a steep one-step [HS–HS] to [LS–LS] spin transition.
The spin-crossover phenomenon is one of the most spectacular examples of molecular bistability driven by external constraints leading to molecular switch or memory.1,2 The fundamental origin of the spin-crossover phenomenon is molecular, but the magnetic behavior strongly depends on intermolecular interaction. Up to now, the major source of information on spin transition systems has been mononuclear iron(II) compounds. However, controlling both intra- and intermolecular interactions if polynuclear compounds are employed would enhance the cooperativity in the systems. Thus, the cooperativity can be achieved by using suitable bridging ligands. A series of dimeric and polymeric iron(II) spin-crossover compounds were synthesized and magnetically characterized along these lines while only a few crystal structures were determined.3,4 In previous papers, we have structurally and magnetically characterized dinuclear complexes, [{FeII(NCE)(X-py)}2(μ-bpypz)] (E = S or BH3, Hbpypz = 3,5-bis(2-pyridyl)-pyrazole):5,6 we have been showing the possibility of controlling the magnetic behavior, that is, the spin transition temperature and cooperativity, by changing the axial ligands. The dinuclear unit is robust and can be used as a building block of assemblies. These results prompt us to construct polymer compounds, which consist of the dinuclear cluster unit and an additional linking ligand, for a fundamental understanding of cooperativity between clusters and the synergy between the SCO phenomenon and intra- or intercluster magnetic exchange interaction. We report here the synthesis and magnetic characterization of one-dimensional coordination polymers, [{Fe2(NCS)2(μ-bpypz)2}(μ-4,4′-bpy)]·MeOH (1).‡
ORTEP drawings of the structures at 200 K and 150 K around the iron ions in 1 are shown in Fig. 1a (at 200 K) and Figure S1 (at 150 K).§ Both structures are isomorphous with each other. The structure at 200 K shows that the desired coordination has been achieved with the iron atoms being coordinated to two bpypz− to form a dinuclear complex. The dinuclear unit is centrosymmetric, thus, only the one kind of iron and ligands exist in the molecule. Each iron atom is bound to the four nitrogen atoms of two pyridyl and two pyrazolate moieties in bpypz− in the basal plane and two nitrogen atoms of 4,4′-bpy and NCS− anion at the axial positions forming a distorted octahedral environment. Neighbor dimers are connected by the 4,4′-bpy bridges through axial coordination of the Fe(II) ions, defining a 1D chain. The chain forms a three-dimensional structure by two types of π–π stacking interactions between the bpypz− ligands of the adjacent dimers on the neighbor chains (Fig. 1b, 1c); (1) between pyridyl rings and (2) between pyridyl and pyrazole rings of the adjacent complexes. There are voids between the chains. Interstitial MeOH molecules are introduced in the void and disordered in two positions. Further, they have OH–π interaction with the bridged 4,4′-bpy (nearest neighbor O–C distance: 3.60 Å (150 K) and 3.66 Å (200 K)). The average Fe–N bond length at 200 K is 2.17 Å, the value of which is typical of high spin (HS) iron(II) centers while the corresponding value at 150 K is 2.00 Å. The changes in Fe–N distances by ca. 0.17 Å suggest that all Fe(II) ions are in the low spin (LS) state at 150 K. Fe–Fe distances in the dimer unit in the HS state (200 K) and LS state (150 K) are 4.217(1) and 4.047(1) Å, respectively. Furthermore, the inter-dimer Fe–Fe distance across the 4,4′-bpy bridge in the chain also is shortened by 0.54 Å upon spin transition (11.558(1) Å (HS) to 11.020(1) Å (LS)) while the nearest neighbor inter-chain Fe–Fe distance extends from 6.336(4) Å (HS) to 6.473 Å (LS) reflecting the rigidity of 4,4′-bipyridine. It is noted that the unit cell volume decreases sharply from 949.6(6) Å3 at 200 K to 877.9(3) Å3 at 150 K: a 7.6% decrease upon SCO is bigger than that for [{FeII(NCBH3)(py)}2(μ-bpypz)],6 which has a discrete dimer structure, and the change of the unit cell is anisotropic. The contracting direction is along the chain direction. These results suggest that the one-dimensional coordination polymer structure is sensitive to SCO due to the direct bridge between Fe(II) ions by the coordination of 4,4′-bipyridine. Mössbauer spectra also confirm the spin transition of the compound: a pure low-spin state doublet is present at 79.5 K, with an isomer shift δ = 0.505 mm s−1 and a quadrupole splitting ΔEQ = 0.573 mm s−1. The spectrum at 297 K shows a symmetrical doublet with δ = 1.00 mm s−1 and ΔEQ = 2.45 mm s−1, the values of which are typical of high spin iron(II) centers. Interestingly, two distinct quadrupole doublets, the parameters of which coincide with those of two temperature phases mentioned above, are observed at 168 K (Fig. 2 and S2). These features indicate the spin conversion occurs around 168 K and there is only one phase in each temperature range, that is, the dimer unit exhibits fully [HS–HS] and [LS–LS] states in the high and low temperature ranges, respectively. Therefore, there is no intermediate magnetic phase, [HS–LS] unlike the cases of [Fe2(PMAT)2](BF4)4·DMF4 and [{Fe(bpym)(NCSe)2}2(μ-bpym)].2 Temperature dependence of the magnetic susceptibility shows the steep spin crossover from the quintet (χMT = 3.50 emu mol−1 K at 300 K) high spin [HS–HS] state to the singlet low spin (0.15 emu mol−1 K at 100 K) [LS–LS] state around 162 K (Fig. 3). The behavior below the crossover transition arises from the dinuclear units being in the [LS–LS] form, with the non zero χMT ‘plateau’ originating from a combination of 2nd order Zeeman contributions to the LS Fe(II) susceptibilities plus a small Curie-like contribution from a small fraction of trapped [HS–HS] form.6 The magnetic behavior in the heating and cooling modes indicates the occurrence of a small thermal hysteresis (ΔT ∼ 2 K). The differential scanning calorimetry gives one exo- (160.4 K) and endothermic (164 K) peaks, in the cooling and heating modes, respectively (Fig. 3) and thus, the spin-crossover temperature (Tc) is 162.2 K, the value of which is higher than those of the related dinuclear complexes, [{Fe(NCS)(X-py)}2(μ-bpypz)2].6 Detailed comparison of spin-crossover behavior between these complexes could be made by the least-squares fitting of the magnetic susceptibility using the regular solution model through Eqn. (1).7
| ln [(1 − γHS)/γHS] = ΔHHL + Γ(1 − 2γHS)/RT − ΔSHL/R = ΔHHL + Γ(1 − 2γHS)/RT − ΔHHL/Tc/R | (1) |
 | ||
| Fig. 1 ORTEP drawing for 1 at 200 K (a) and perspective views representing the π–π stacking interactions between the bpypz− ligands of the adjacent dimers on the neighbor chains between pyridyl rings (b) and between pyridyl and pyrazole rings (c). | ||
 | ||
| Fig. 2 Temperature dependence of isomer shift (circle) and quadrupole splitting (triangle) values. | ||
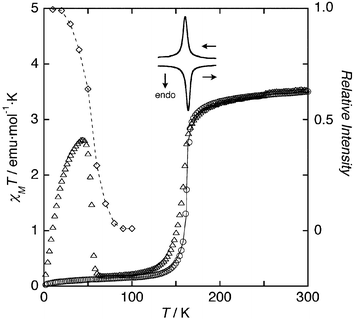 | ||
| Fig. 3 Temperature dependence of χMT (circle), Raman spectral intensity (lozenge), DSC traces (solid lines), and LIESST (triangle). A thin solid line indicates the least-squares fitting of the magnetic susceptibility using the regular solution model. | ||
Photogeneration of the metastable HS state at low temperatures, the so-called Light-Induced Excited Spin-State Trapping (LIESST) experiment, was carried out on a microcrystalline sample. The results are displayed in Fig. 3 (triangles). At 4.2 K, the sample was irradiated with green light (532 nm) for 5 h to reach a value of χMT = 0.44 emu mol−1 K, which is much lower than the high spin value. However, after light irradiation, χMT increases dramatically up to a value of 2.64 emu mol−1 K then drops rapidly in the temperature region 2–44 K as the temperature is increased. The temperature dependence of χMT after light irradiation is totally different from what is observed in LIESST experiments performed on mononuclear species, and it resembles the magnetic behavior of [{FeII(NCS)(DMSO)}2(μ-bpypz)2] (Fig. S3).6 The increase of χMT represents the change in thermal populations of the different microstates or excited pair spin states, arising from a weak iron(II)–iron(II) antiferromagnetic interaction,8 which is expected to occur in the bpypz-bridged [Fe2(bpypz)2] dimer unit due to the inefficient exchange ability of the bridging 4,4′-bipyridine.9 This magnetic behavior, therefore, is attributed to the synergy between magnetic interaction and spin transition under light irradiation. At temperatures higher than 45 K, χMT drops rapidly to reach a value close to 0.15 emu mol−1 K at 65 K (the critical LIESST temperature, determined as the extreme of the derivative ∂χMT/∂T was found as 54 K), representative of the occurrence of a complete HS to LS relaxation in a relatively lower temperature range. To confirm the LIESST phenomenon and the spin state of 1 after light irradiation, we have also measured Raman spectra as an accessible probe of vibrations that are most affected by the geometric and electronic changes around the metal ion. The LIESST can qualitatively be followed by monitoring a change in intensity of the CN stretching for the SCN− ligand (observed at 2071 and 2104 cm−1 for HS and LS, respectively) by irradiation with the excitation light source (632.8 nm) for Raman spectroscopy.5 At 10 K, the intensity of the HS signal for the CN vibration is twenty times bigger than that of the LS signal (Figure S4) and the intensity of the signal decreases on heating and reaches zero at 100 K (Fig. 3). This observation of the spin transition from HS to LS demonstrates the LIESST effect. Moreover, the steep decrease in intensity is a mirror image of the SCO suggestive of the high cooperativity in 1.
In summary, 1 provides the first dinuclear iron(II) complex-based one-dimensional polymer compound in which the spin transition from high- to low-spin states has been followed by crystal structural analyses and in which no step in χMT due to the presence of [HS–LS] states is observed. The magnetic transition is sharp, with a hysteresis, indicating that cooperativity is occurring between the Fe(II) dinuclear units through the bridging 4,4′-bipyridine due to its rigidity and inefficient magnetic exchange ability. Further dinuclear iron(II)-based polynuclear examples of the present and related types are required.
This research was supported by a Grant-in-Aid for Scientific Research (No. 17350026) and by a Grant-in-Aid for Scientific Research on Priority Areas (No. 434) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Notes and references
- P. Gütlich, Y. Garcia and H. A. Goodwin, Chem. Soc. Rev., 2000, 29, 419 RSC; G. J. Halder, C. J. Kepert, B. Moubaraki, K. S. Murray and J. D. Cashion, Science, 2002, 298, 1762 CrossRef CAS; O. Kahn and C. J. Martinez, Science, 1998, 279, 44 CrossRef CAS; S. Hayami, K. Danjobara, K. Inoue, Y. Ogawa, N. Matsumoto and Y. Maeda, Adv. Mater., 2004, 16, 869 CrossRef CAS; S. Bonhommeau, G. Molnár, A. Galet, A. Zwick, J.-A. Real, J. J. McGarvey and A. Bousseksou, Angew. Chem., Int. Ed., 2005, 44, 4069 CrossRef CAS; A. Bousseksou, G. Molnár and G. Matouzenko, Eur. J. Inorg. Chem., 2004, 4353 CrossRef CAS.
- J.-A. Real, A. B. Gaspar, V. Niel and M. C. Munoz, Coord. Chem. Rev., 2003, 236, 121 CrossRef CAS.
- B. A. Leita, B. Moubaraki, K. S. Murray, J. P. Smith and J. D. Cashion, Chem. Commun., 2004, 156 RSC; G. S. Matouzenko, G. Molnar, N. Bréfuel, M. Perrin, A. Bousseksou and S. A. Borshch, Chem. Mater., 2003, 15, 550 CrossRef CAS; V. Niel, J. M. Martínez-Agudo, M. C. Muñoz, A. B. Gaspar and J.-A. Real, Inorg. Chem., 2001, 40, 3838 CrossRef CAS.
- M. H. Klingele, B. Moubaraki, J. D. Cashion, K. S. Murray and S. Brooker, Chem. Commun., 2005, 987 RSC.
- N. Suemura, M. Ohama and S. Kaizaki, Chem. Commun., 2001, 1538 RSC.
- K. Nakano, N. Suemura, S. Kawata, A. Fuyuhiro, T. Yagi, S. Nasu, S. Morimoto and S. Kaizaki, Dalton Trans., 2004, 982 RSC; K. Nakano, S. Kawata, K. Yoneda, A. Fuyuhiro, T. Yagi, S. Nasu, S. Morimoto and S. Kaizaki, Chem. Commun., 2004, 2892 RSC; K. Nakano, N. Suemura, K. Yoneda, S. Kawata and S. Kaizaki, Dalton Trans., 2005, 740 RSC; K. Yoneda, K. Nakano, J. Fujioka, K. Yamada, T. Suzuki, A. Fuyuhiro, S. Kawata and S. Kaizaki, Polyhedron, 2005 Search PubMed , in press (DOI: 10.1016/j.poly.2005.03.047).
- O. Kahn, Molecular Magnetism, VCH, New York, 1993 Search PubMed.
- J.-F. Létard, J. A. Real, N. Moliner, A. B. Gaspar, L. Capes, O. Cador and O. Kahn, J. Am. Chem. Soc., 1999, 121, 10630 CrossRef CAS; G. Chastanet, A. B. Gaspar, J.-A. Real and J.-F. Létard, Chem. Commun., 2001, 819 RSC; A. B. Gaspar, V. Ksenofontov, J.-A. Real and P. Gütlich, Chem. Phys. Lett., 2003, 373, 385 CrossRef CAS.
- N. Moliner, M. C. Muñoz, S. Létard, L. Salmon, J.-P. Tuchagues, A. Bousseksou and J. A. Real, Inorg. Chem., 2002, 41, 6997 CrossRef; P. Jensen, S. R. Batten, B. Moubaraki and K. S. Murray, J. Chem. Soc., Dalton Trans., 2002, 3712 RSC.
- G. M. Sheldrick, SHELXL97, University of Göttingen, Germany, 1997 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1: ORTEP drawing at 150 K, Fig. S2: Mössbauer spectra, Fig. S3: details of magnetic susceptibility, Fig. S4: Raman spectrum. See DOI: 10.1039/b512266j |
| ‡ A methanolic solution (2 mL) of trans-[Fe(NCS)2(py)4] (3.7 mmol L−1) was transferred to a glass tube, and then a methanolic solution of 2 mL of Hbpypz (3.7 mmol L−1) and 4,4′-bpy (1.8 mmol L−1) with a 10% methanol solution of tetra-n-butylammonium hydroxide (n-Bu)4NOH (3.7 mmol L−1) was poured into the glass tube without mixing the solutions under an N2 stream. Brown crystals began to form at ambient temperature in 1 month. Yield 80%. Calcd (%) for C39H30N12OS2Fe2: C, 54.56; H, 3.52; N, 19.58. Found (%): C, 54.43; H, 3.27; N, 19.80. |
| § Crystal data for 1: Data collections were carried out on a Rigaku/MSC Mercury CCD diffractometer with graphite-monochromated Mo-Kα radiation. The structures were solved by direct methods (Rigaku CrystalStructure crystallographic software package of Molecular Structure Corporation) and refined with a full-matrix least-squares technique (SHELXL-9710). For 200 K: orange, triclinic, space group P-1, Z = 1, a = 9.529(3), b =10.386(4), c = 10.680(4) Å, α = 78.50(6), β = 73.16(5), γ = 70.91(5)°, V = 949.6(6) Å3, dcalcd = 1.501 g cm−3, μ(MoKα) = 9.24 cm−1. 10604 Reflections were collected in the range 8 < 2θ < 55° and 4253 independent reflections [R(int) = 0.055] were used in the structural analysis. R1 = 0.0475 [for 2897 F < 4σ(F); wR2 = 0.1069 and goodness of fit = 1.038 for all 4253 F2; 260 parameters; all non-hydrogen atoms anisotropic except for the atoms of the disordered MeOH; all hydrogen atoms except for the atoms on the disordered MeOH were placed in the calculated positions]. For 150 K: black, triclinic, space group P-1, Z = 1, a = 9.2371(9), b =10.273(2), c = 10.2499(10) Å, α = 78.43(4), β = 73.91(4), γ = 71.16(4)°, V = 877.9(3) Å3, dcalcd = 1.624 g cm−3, μ(MoKα) = 9.99 cm−1. 8849 Reflections were collected in the range 8 < 2θ < 55° and 3957 independent reflections [R(int) = 0.053] were used in the structural analysis. R1 = 0.0661 [for 3081 F < 4σ(F); wR2 = 0.1530 and goodness of fit = 1.073 for all 3957 F2; 260 parameters; all non-hydrogen atoms anisotropic except for the atoms of the disordered MeOH; all hydrogen atoms except for the atoms on the disordered MeOH were placed in the calculated positions]. CCDC 283072 & 283073. For crystallographic data in CIF or other electronic format see DOI: 10.1039/b512266j |
| This journal is © The Royal Society of Chemistry 2006 |
