A water-soluble hexa-peri-hexabenzocoronene: synthesis, self-assembly and role as template for porous silica with aligned nanochannels†
Jishan
Wu
a,
Jixue
Li
b,
Ute
Kolb
b and
Klaus
Müllen
*a
aMax-Planck Institut für Polymerforschung, Ackermannweg 10, 55128 Mainz, Germany. E-mail: muellen@mpip-mainz.mpg.de
bInstitute of Physical Chemistry, Johannes-Gutenberg Universität, D-55128 Mainz, Germany
First published on 15th November 2005
Abstract
A water-soluble hexa-peri-hexabenzocoronene was prepared and shown to undergo ordered columnar self-assembly either in water solution or bulk and therefore served as template for the fabrication of porous silica with aligned nanochannels.
Amphiphilic conjugated oligomers or polymers1 based on thienylene,2 phenylene,3 phenylenevinylene,4 and phenyleneethynylene5 units self-assemble into nano- or micro- scale ribbons, fibers and micelles due to strong π-stacking and hydrophobic interactions. Studies on their self-assembly open the possibility to fabricate devices from individual assembled nanostructures in “supramolecular electronics”.6 Recently, disc-like molecules such as triphenylene,7 metallophthalocyanines8 and hexa-peri-hexabenzocoronenes (HBC)9 have attracted interest because of their high tendency to aggregate into columnar superstructures which serve as one-dimensional charge transport channels.10 Their amphiphilic derivatives formed unique nanostructures in mesophase, solution and thin film.11,12 HBC derivatives substituted by branched oligo(ethylene glycol) chains 11d showed extremely ordered columnar superstructures in the bulk state.
The self-assembled amphiphilic conjugated π-molecules were also used as templates to fabricate inorganic nanostructures such as mesoporous silica which have potential applications as catalyst supports, low-κ materials and membranes.13 For example, the synthesis of ordered porous silica using columnar charge-transfer assemblies based on triphenylenes substituted by oligo(ethylene glycol) chains has been reported. However, without the existence of suitable electron acceptors no aligned silicate nanochannels were formed due to the lack of long range order in the columnar structure.11a The HBC has a larger rigid core and promises stronger π-interactions as well as higher ordered columnar structures. Therefore, we now report how the assembly of an amphiphilic HBC 1 (without additional acceptors) in water can be used as template to fabricate porous silica with aligned nanochannels.
Recently, we prepared an insoluble hexaiodo-peri-hexabenzocoronene (2, Scheme 1), and found that it showed high reactivity in Pd-catalyzed coupling reactions.14 Therefore using such a building block, compound 1 bearing oligo(ethylene glycol) chains was easily prepared as shown in Scheme 1. 1-Bromo-3,4,5-trihydroxylbenzene (3)13b was first subjected to etherification with tosylate-protected oligoethylene glycol 415 by K2CO3 in DMF and afforded compound 5 in 75% yield. Sonogashira coupling of 5 with trimethylsilylacetylene in refluxing triethylamine gave 6 and subsequent desilylation with K2CO3 in methanol afforded compound 7 in 82% yield. Coupling reaction between the insoluble HBC building block 213 and 7 was performed in piperidine at 50 °C for 48 hours whereupon the insoluble suspension became soluble during the reaction. After column chromatography with acetyl acetate/methanol as eluents, pure waxy compound 1 was obtained in 72% yield. The use of Pd-catalyzed coupling reaction as the last step avoided the tedious removal of FeCl3 (soluble in methanol) in the previous synthetic method.11d
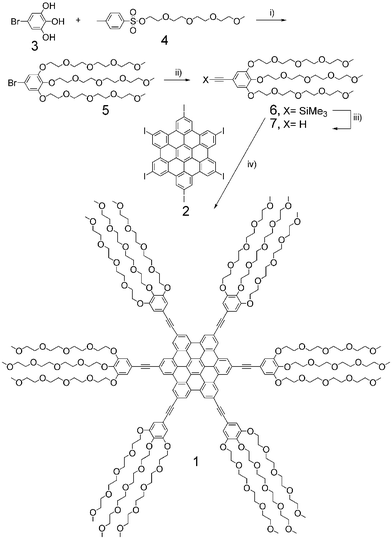 | ||
| Scheme 1 i) K2CO3, DMF, 60 °C, 75%; ii) trimethylsilylacetylene, Pd(PPh3)2Cl2–CuI, triethylamine, 80 °C; iii) K2CO3, MeOH, 82% for ii) and iii) steps; iv) Pd(PPh3)4–CuI, piperidine, 50 °C, 72%. | ||
In chlorinated solvents such as 1,1,2,2-tetrachloroethane, the existence of non-aggregated individual molecules of 1 was confirmed by the independency of 1H NMR signals on temperature (not shown). However, in polar protic solvents such as methanol and water compound 1 showed a strong tendency to aggregate by π-stacking and hydrophobic interactions. The fluorescence spectra of compound 1 in a mixture of dichloromethane and methanol with different volume ratios are shown in Fig. 1. In pure dichloromethane, a well-resolved fluorescence spectrum was observed between 470 nm and 600 nm. Upon addition of methanol, the fluorescence intensity decreased and the emission maximum shifted to longer wavelength. When the methanol/dichloromethane ratio reached 7 ∶ 3, an unresolved band centered at 540 nm was observed. The longer wavelength band typically arises from the excimer emission, thus gradual addition of methanol enhances the hydrophobic interactions and leads to an increase of the size of aggregates.
![Fluorescence spectra of compound 1 in mixed solvents of dichloromethane (DCM) and methanol ([1] = 5.0 x 10−6 M), λexc = 380 nm.](/image/article/2006/CC/b511868a/b511868a-f1.gif) | ||
| Fig. 1 Fluorescence spectra of compound 1 in mixed solvents of dichloromethane (DCM) and methanol ([1] = 5.0 x 10−6 M), λexc = 380 nm. | ||
Compound 1 at room temperature displayed a thermotropic columnar liquid crystalline phase as disclosed by differential scanning calorimetry (DSC), polarized optical microscopy (POM) and X-ray diffraction. It should be noted that it can be processed in the melt because the isotropization temperature (Tiso) of 1 has been dramatically decreased to 150 °C compared with the normal alkyl substituted HBCs which usually have high Tiso (> 400 °C). After slow cooling from the isotropic melt, obvious birefringence was observed under cross POM (see Fig. 2a) at 90 °C. A typical 2D X-ray diffraction pattern recorded at 120 °C on a mechanically extruded fiber is shown in Fig. 2b. The wide-angle reflexes at meridional direction can be assigned to the π-stacking between the HBC discs in the columns. The distance of 3.8 Å is larger than for the normal alkyl substituted HBC, probably because of the higher temperature and the bulky substitution of the HBC core. Two strong spots were found at low scattering angle along the equatorial direction resembling the distances 3.54 nm and 2.68 nm which are correlated to the intercolumnar distances. However, because of the lack of clearer reflexes, an exact 2D unit cell can not be figured out. Interestingly, at the diagonal directions (±20° from the meridian), four strong reflexes with the same distance of 2.68 nm were observed. This distance is about seven times the π–π distance between the HBC discs, and the deviation from the equator and meridion suggests a probably helical stacking of the discs in the column due to the steric hindrance and space-filling limit during columnar structure formation.13b In addition, some weak reflexes at the middle-angle area were found which can be correlated to higher order scattering. After cooling to room temperature (below the phase transition temperature of 56 °C observed in the second cooling scan), no obvious changes were observed in the X-ray diffraction pattern, even after long-time annealing at 120 °C and at slow cooling rate.
 | ||
| Fig. 2 a) POM images for 1 recorded at 90 °C during slow cooling from the isotropic liquid; b) 2D WAXD pattern recorded at 120 °C on an extruded fiber of compound 1. | ||
The ordered columnar structure in the solid-state and in water solution suggested the fabrication of nanoscale organic–inorganic composites. Sol–gel polymerization was thus conducted according to the reported method:16 compound 1 was dissolved in aqueous HCl and then tetraethoxylsilane (TEOS) was added; the mixture was stirred at room temperature overnight. The precipitate was collected and further calcinated at 500 °C for 6 hours in air to remove the organic part. Powder X-ray diffraction on the solid sample before calcination revealed two weak peaks at 3.33 nm and 1.67 nm in the small-angle area, suggesting that the sol–gel polymerization of oligo(ethylene glycol) chains and TEOS maintained the columnar structure of HBCs during the reactions (See supporting information†). It is worthy of note that in our previous report, sixfold acryloyl-terminated HBC derivatives underwent thermal polymerization and afforded an insoluble network in which the columnar superstructure of the liquid crystalline phase was also preserved.17 Transmission electron microscopy (TEM) measurements on the calcinated powder disclosed ordered nanoporous silica with one-dimensionally aligned nanochannels (Fig. 3). The diameters of the channels are about 1.0 nm, which is smaller than the size of the rigid part of molecule 1. This may be explained by the shrinkage during the calcination, a phenomenon previously observed.13c To our knowledge, this is the smallest pore size that has ever been reached by template synthesis. Gas storage based on the obtained materials will be next issue.
 | ||
| Fig. 3 TEM image of porous silica fabricated by sol–gel polymerization. | ||
In summary, a water soluble HBC 1 substituted by oligo(ethylene glycol) chains was prepared by a simple method. The obtained molecule showed an ordered columnar liquid crystalline phase in the solid-state and strong aggregation in polar solvents. The aligned nanochannels in the obtained porous silica are replicas of the self-assembled columnar structures of the disc-like compound 1. This success also suggests using amphiphilic π-conjugated systems as templates to fabricate other functional organic–inorganic composites under controllable sol–gel procedures.18 Thus, both the π-systems (e.g. electronic donor and charge transporting HBCs) and the inorganic part (e.g. electronic acceptor such as TiO2) can be used as electronic functional units in photovoltaic devices in the future.
Financial supports from EU projects NAIMO (NMP4-CT-2004-500355) and RADSAS (NMP3-CT-2004-001561) are acknowledged. We also thank Sirma Koynova for her assistance with the syntheses.
Notes and references
- (a) F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491–1546 CrossRef CAS; (b) T. Shimizu, M. Masuda and H. Minamikawa, Chem. Rev., 2005, 105, 1401–1443 CrossRef CAS.
- (a) A. P. H. J. Schenning, A. F. M. Kilbinger, F. Biscarini, M. Cavallini, H. J. Cooper, P. J. Derrick, W. J. Feast, R. Lazzaroni, Ph. Leclere, L. A. McDonell, E. W. Meijer and S. C. J. Meskers, J. Am. Chem. Soc., 2002, 124, 1269–1275 CrossRef CAS; (b) T. Bjϕrnholm, D. R. Greve, N. Reitzel, T. Hassenkam, K. Kjaer, P. B. Howes, N. B. Larsen, J. Bϕgelund, M. Jayaraman, P. C. Ewbank and R. D. McCullough, J. Am. Chem. Soc., 1998, 120, 7643–7644 CrossRef CAS.
- Y.-S. Yoo, J.-H. Choi, J.-H. Song, N.-K. Oh, W.-C. Zin, S. Park, T. Chang and M. Lee, J. Am. Chem. Soc., 2004, 126, 6294–6300 CrossRef CAS.
- (a) B. S. Gaylord, S. Wang, A. J. Heeger and G. C. Bazan, J. Am. Chem. Soc., 2001, 123, 6417–6418 CrossRef CAS; (b) H. Xiong, L. Qin, J. Sun, X. Zhang and J. Shen, Chem. Lett., 2000, 35, 586–587 CrossRef.
- I.-B. Kim, J. N. Wilson and U. H. F. Bunz, Chem. Commun., 2005, 1273–1275 RSC.
- E. W. Meijer and A. P. H. J. Schenning, Nature, 2002, 419, 353–354 CrossRef CAS.
- (a) D. Adam, P. Schuhmacher, J. Simmerer, L. Häussling, K. Siemensmeyer, K. H. Etzbach, H. Ringsdorf and D. Haarer, Nature, 1994, 371, 141–143 CrossRef CAS; (b) S. Chandrasekhar and S. K. Prasad, Contemp. Phys., 1999, 40, 237–245 CrossRef CAS.
- (a) C. Piechoki, J. Simon, A. Skoulios, D. Guillon and P. Weber, J. Am. Chem. Soc., 1982, 104, 5245–5247 CrossRef; (b) S. Chandrasekhar, Mol. Cryst. Liq. Cryst., 1981, 63, 171–180 CrossRef CAS.
- (a) M. D. Watson, A. Fechtenkötter and K. Müllen, Chem. Rev., 2001, 101, 1267–1300 CrossRef CAS; (b) C. D. Simpson, J. Wu, M. D. Watson and K. Müllen, J. Mater. Chem., 2004, 14, 494–504 RSC; (c) J. Wu, A. C. Grimsdale and K. Müllen, J. Mater. Chem., 2005, 15, 41–52 RSC.
- R. J. Bushby and O. R. Lozman, Curr. Opin. Solid State Mater. Sci., 2002, 6, 569–578 CrossRef CAS.
- (a) A. Okabe, T. Fukushima, K. Ariga and T. Aida, Angew. Chem., Int. Ed., 2002, 41, 3414–3417 CrossRef CAS; (b) A. Arnaud, J. Belleney, F. Boué, L. Bouteiller, G. Carrot and V. Wintgens, Angew. Chem., Int. Ed., 2004, 43, 1718–1721 CrossRef CAS; (c) M. Kimura, K. Wada, K. Ohta, K. Hanabusa, H. Shirai and N. Kobayashi, J. Am. Chem. Soc., 2001, 123, 2438–2439 CrossRef CAS; (d) M. Lee, J.-W. Kim, S. Peleshanko, K. Larson, Y.-S. Yoo, D. Vaknin, S. Markutsya and V. V. Tsukruk, J. Am. Chem. Soc., 2002, 124, 9121–9128 CrossRef CAS.
- J. P. Hill, W. Jin, A. Kosaka, T. Fukushima, H. Ichihara, T. Shimomura, K. Ito, T. Hashiyume, N. Ishii and T. Aida, Science, 2004, 304, 1481–1483 CrossRef CAS.
- (a) F. Schüth and W. Schmidt, Adv. Mater., 2002, 14, 629 CrossRef CAS; (b) G. Wirnsberger, P. Yang, B. J. Scott, B. F. Chmelka and G. D. Stucky, Spectrochim. Acta, Part A, 2001, 57, 2049 CrossRef CAS; (c) A. Yamaguchi, F. Uejo, T. Yoda, T. Uchida, Y. Tanamura, T. Yamashita and N. Teramae, Nat. Mater., 2004, 3, 337–341 CrossRef CAS.
- (a) J. Wu, M. D. Watson and K. Müllen, Angew. Chem., Int. Ed., 2003, 42, 5329–5333 CrossRef CAS; (b) J. Wu, M. D. Watson, L. Zhang, Z. Wang and K. Müllen, J. Am. Chem. Soc., 2004, 126, 177–186 CrossRef CAS; (c) J. Wu, M. Baumgarten, M. G. Debije, J. M. Warman and K. Müllen, Angew. Chem., Int. Ed., 2004, 43, 5331–5335 CrossRef CAS; (d) J. Wu, A. Fechtenkötter, J. Gauss, M. D. Watson, M. Kastler, C. Fechtenkötter, M. Wagner and K. Müllen, J. Am. Chem. Soc., 2004, 126, 11311–11321 CrossRef CAS.
- K. A. Arnold, J. Mallen, J.E. Trafton, B. D. White, F. R. Fronczek, L. M. Gehrig, R. D. Gandour and G. W. Gokel, J. Org. Chem., 1988, 53, 5652–5657 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- J. D. Brand, C. Kübel, S. Ito and K. Müllen, Chem. Mater., 2000, 12, 1638–1647 CrossRef CAS.
- P. D. Cozzoli, A. Kornowski and H. Weller, J. Am. Chem. Soc., 2003, 125, 14539–14548 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: synthetic details and structural characterizations for all new compounds; X-ray diffraction data for the HBC-SiOx. See DOI: 10.1039/b511868a |
| This journal is © The Royal Society of Chemistry 2006 |
