Solid-supported chemiluminescence and electrogenerated chemiluminescence based on a tris(2,2′-bipyridyl)ruthenium(II) derivative†
G. M.
Greenway
,
A.
Greenwood
,
P.
Watts
and
C.
Wiles
*
Department of Chemistry, The University of Hull, Cottingham Road, Hull, UK HU6 7RX. E-mail: c.wiles@chem.hull.ac.uk; Fax: +44 (0) 1482 466416; Tel: +44 (0) 1482 466369
First published on 11th November 2005
Abstract
We report a simple and efficient technique for the covalent immobilisation of a tris(2,2′-bipyridyl)ruthenium(II) derivative suitable for both chemiluminescence and electrochemiluminescence detection.
The inherent sensitivity and selectivity demonstrated by the chemiluminescent reaction of tris(2,2′-bipyridyl)ruthenium(III) 11 has led to its widespread application for the determination of reducing species, in both static and flowing systems.2 A major drawback of the technique, however is the need to constantly supply the reagent to the site of reaction, increasing both analysis costs and waste generated.
![Mechanism of [Ru(bipy)3]2+2 regeneration.](/image/article/2006/CC/b511071h/b511071h-s1.gif) | ||
| Scheme 1 Mechanism of [Ru(bipy)3]2+2 regeneration. | ||
As Scheme 1 illustrates tris(2,2′-bipyridyl)ruthenium(II) 2 ([Ru(bipy)3]2+) is oxidised, either chemically3 (CL) or electrochemically4 (ECL), to afford [Ru(bipy)3]3+1 which is subsequently reduced by an analyte, resulting in the emission of a photon of light. As [Ru(bipy)3]2+2 is regenerated, immobilisation of the chemiluminescent reagent would not only enable the reagent to be recycled, leading to reduced analysis costs and waste generation, but would also greatly simplify instrumental set-up.5 With these factors in mind, many groups have attempted to immobilise chemiluminescent reagents, using approaches such as the doping of sol–gels,5,6 fabrication of composite films7 or more recently the preparation of solid-supported derivatives.8 Problems with these techniques, however, include a leaching of the reagent,9 instability to organic solvents10 and the inability to use the immobilisation technique for both CL and ECL systems. To address these problems, we describe herein an efficient technique for the synthesis and covalent immobilisation of a tris(2,2′-bipyridyl)ruthenium(II) derivative 3. Preliminary results are subsequently reported for the detection of codeine12 using complex 3 in both CL and ECL based systems.
Using methodology reported by Donnici et al.,13 2,2′-bipyridine-4,4′-carboxylic acid 4 was synthesised in excellent yield (98%) from commercially available 4,4′-dimethyl-2,2′-bipyridine 5. Treatment of the resulting carboxylic acid 4 with thionyl chloride 6 afforded the respective acid chloride 7 which was subsequently reacted with 3-aminopropyltriethoxysilane 8, in the presence of triethylamine 9, to afford 2,2′-bipyridyl-4,4′-dicarboxylic acid bis[(3-triethoxysilylpropyl)amide] 10, in 96% yield.14 Ligand 10 was subsequently reacted with cis-dichlorobis(bipyridine)ruthenium1511 (synthesised in 63% yield) to afford 4,4′-bis[(3-triethoxysilylpropyl)amide]-2,2′-bipyridine]bis(2,2′-bipyridine) ruthenium(II) dichloride 3 in high yield (73%) (Scheme 2).† Consequently, by synthesising a compound that not only contained a ligand for cis-dichlorobis(bipyridine)ruthenium 11 but also a linker, we proposed that the [Ru(bipy)3]2+ derivative 3 could be covalently immobilised onto a silica surface, by simple hydrolysis of the triethoxysilane moieties (Fig. 1).
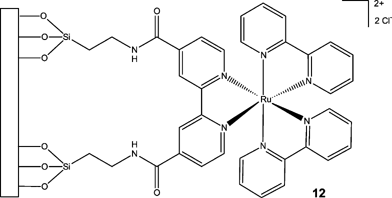 | ||
| Fig. 1 Schematic illustrating the covalent immobilisation of a tris(2,2′-bipyridyl)ruthenium(II) complex 3 onto a silica surface. | ||
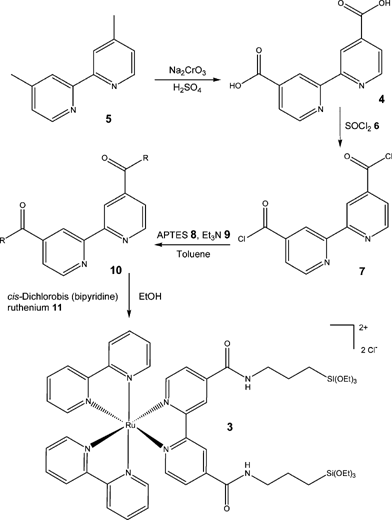 | ||
| Scheme 2 Reaction scheme illustrating the synthesis of a tris(2,2′-bipyridyl)ruthenium(II) derivative 3 (R = CONH(CH2)3Si(OEt)3). | ||
In order to evaluate the chemiluminescent properties of the resulting ruthenium derivative 3, we initially investigated its covalent immobilisation onto controlled pore glass (CPG), whereby a free flowing orange material was obtained (2.61 × 10−3 mmol Ru g−1) (Fig. 1). The functionalised CPG 12 (0.050 g, 1.305 × 10−3 mmol Ru) was subsequently packed into a flow cell (Fig. 2) and its response to codeine (1 × 10−3 M) evaluated using sequential injection analysis (SIA) (Table 1). Using cerium(VI) sulfate (0.1 M in 1.0 M sulfuric acid) as the oxidising agent,16 we were able to confirm the successful immobilisation of a CL reagent to CPG and its application to the detection of codeine, whereby an average signal of 35.5 mV was obtained (RSD = 2.98%, n = 30) with an analysis time of 60 s.
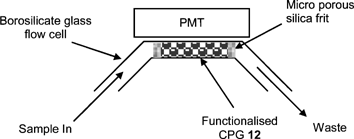 | ||
| Fig. 2 Flow cell used for the evaluation of functionalised CPG 12. | ||
In comparison to the immobilised ruthenium derivative described by Barnett and co-workers,8 no conditioning of the immobilised reagent was found to be necessary in order to obtain a steady response. Having successfully demonstrated the covalent immobilisation of the tris(2,2′-bipyridyl) ruthenium(II) derivative 3 to CPG, the investigation was extended to evaluate the incorporation of complex 3 into a silicate sol–gel, enabling the fabrication of a regenerable ECL sensor.
Silicate sol–gels are glass-like materials synthesised via the acid or base-catalysed hydrolysis, and subsequent polycondensation, of alkoxides such as tetramethylorthosilicate (TMOS). Due to the low temperature processing used for their fabrication, silicate sol–gels can be readily doped with modifiers such as dyes,17 enzymes18 and as demonstrated herein, chemiluminescent reagents; the resulting gels are then cast into monoliths, films or coatings depending on the final application. As the dopant molecules are often smaller than the pores within silicate sol–gels, leaching frequently occurs; leading to irreproducible responses and a limited sensor lifetime.17 With this in mind, many techniques have been reported within the literature to overcome this problem, these include the preparation of composite films, using additives such as Nafion19 or titania,20 or attaching the dopant to large anchor molecules such as dextran;21 few have however, resulted in the fabrication of stable and reproducible sensors.
In order to address this widespread problem, MacCraith et al.11 reported the synthesis of 4,4′-bis[(3-triethoxysilylpropyl)amide]-2,2′-bipyridine]bis(2,2′-bipyridine)ruthenium(II) dichloride 3 and demonstrated its incorporation into a fluorescence based sensor for oxygen determination. However, as we were interested in the evaluation of tertiary amines by chemiluminescence, the approach taken by MacCraith and co-workers11 could not be used as it was essential that residual triethylamine 9 was removed from the ruthenium complex 3. With this in mind, amide 10 was synthesised and purified prior to complexation with cis-dichlorobis(bipyridine) ruthenium 11, affording complex 3 in high yield (73%) and purity.
Alternatively, Lee et al.22 recently reported the synthesis of a ruthenium derivative containing six trimethoxysilane moieties suitable for ECL sensing at an ITO electrode. Although this approach is advantageous as no tertiary amines are employed during the synthesis, the resulting ruthenium complex is extremely air sensitive and therefore undesirable. In comparison, the ruthenium complex 3 described herein has been shown to be air stable for in excess of eight months.
In brief,† the organosilicate sol–gels were prepared using a modified method described by Collinson et al.5 whereby a solution of TMOS and the ruthenium derivative 3 (∼6.5% wrt TMOS) were hydrolysed in the presence of an acid catalyst (0.1 M aq. HCl), followed by base catalysed gelation. In order to investigate the leaching properties of the organosilicate sol–gels, monoliths were prepared in addition to the dip coated electrodes. With respect to monolith fabrication, gelation was induced by the addition of aq. NaOH (1 × 10−2 M) and the resulting sol–gel cured in a dessicator for 24 h (in the absence of a vacuum), to afford an orange translucent glassy material.‡ Coated electrodes were prepared by dipping a glassy carbon electrode into the hydrolysed solution containing complex 3, withdrawing slowly to leave a thin film; gelation was subsequently induced by placing the electrode in borate buffer (pH 8.5, 0.1 M) for a period of 24 h in order to age the film.
Using an ECL detection system built in-house,† the coated glassy carbon electrodes were placed in an electrochemical cell in front of a photomultiplier tube. A three electrode potentiostat was used to apply the required voltages and cyclic voltammetry employed to assess the performance of the electrode. In order to evaluate the ECL activity of the organosilicate sol–gel films, the following operating conditions were used; sample volume 5 ml, 1 × 10−3 M codeine, voltage range 0.7 to 1.3 V, step potential 0.0024 V and a scan rate of 0.01 V s−1.
Other groups report that when employing [Ru(bipy)3]2+2 doped silicate sol–gel films, irreproducible ECL signals are obtained; demonstrating an overall trend of decreasing signal with time; an observation that can be attributed to leaching of the CL reagent.17 However, by covalently immobilising the CL reagent within a silicate sol–gel, reproducible ECL signals of 7.7 mV (% RSD = 1.07, n = 24) were obtained. Although the ECL signals obtained are not as great as those for the functionalised CPG 12, we are currently investigating the incorporation of a greater proportion of 4,4′-bis[(3-triethoxysilylpropyl)amide]-2,2′-bipyridine]bis(2,2′-bipyridine)ruthenium(II) dichloride 3 into the silicate sol–gels in order to attain higher sensitivities.
In summary, compared to the physical entrapment of CL reagents in silicate sol–gels, covalent immobilisation is advantageous as it ensures homogeneous distribution of the reagent within the matrix while preventing reagent leaching; resulting in reduced analysis costs, reproducible analyte responses and extended sensor lifetimes.
In conclusion, by synthesising a compound that acts as both a ruthenium ligand and a hydrolysable linker, we have demonstrated a simple and efficient technique for the covalent immobilisation of a tris(bipyridyl)ruthenium derivative 3, suitable for employment in both CL and ECL systems. In comparison to adsorption or entrapment of the CL reagent, covalent immobilisation is advantageous as no leaching is observed, leading to increased reproducibility and sensor lifetime. This approach to immobilisation allows the CL reagent to be readily recycled, which is vital for in situ sensor measurements. In addition to the applications described herein, 4,4′-bis[(3-triethoxysilylpropyl)amide]-2,2′-bipyridine]bis(2,2′-bipyridine)ruthenium(II) dichloride 3 could also be employed for the functionalisation of other silica substrates such as glass coils, micro channels, optical fibres and nanosensors.
With these factors in mind, work is currently underway within our laboratories to ascertain the effect of 4,4′-bis[(3-triethoxysilylpropyl)amide]-2,2′-bipyridine]bis(2,2′-bipyridine)ruthenium(II) dichloride 3 concentration on the working range and detection limits of the ECL technique.
This work was financially supported by the RSC (A. G.). We are grateful to, Mrs. Brenda Worthington (NMR), Mr Bob Knight (NMR and ICP-MS), Dr Kevin Welham (MS) and Mr Mike Bailey (flow cell fabrication), for their assistance with this investigation.
Notes and references
- D. M. Hercules and F. E. Lytle, J. Am. Chem. Soc., 1966, 88, 4745 CrossRef CAS.
- R. D. Gerardi, N. W. Barnett and S. W. Lewis, Anal. Chem. Acta, 1999, 378, 1 Search PubMed.
- R. D. Geradi, N. W. Barnett and P. Jones, Anal. Chim. Acta, 1999, 388, 1 CrossRef CAS.
- W. L. Wallace and A. J. Bard, J. Phys. Chem., 1979, 83, 1350 CrossRef CAS.
- M. M. Collinson and B. Novak, J. Sol-Gel Sci. Technol., 2002, 23, 215 CrossRef CAS.
- L. L. Schultz, J. S. Stoyanoff and T. A. Neiman, Anal. Chem., 1996, 68, 349 CrossRef CAS.
- H. Wang, G. Xu and S. Dong, Anal. Chim. Acta, 1992, 261, 64.
- N. W. Barnett, R. Bos, H. Brand, P. Jones, K. F. Lim, S. D. Purcell and R. A. Russell, Analyst, 2002, 127, 455 RSC.
- T. M. Downey and T. A. Nieman, Anal. Chem., 1992, 64, 261 CrossRef CAS.
- A. J. Tudos, J. J. Ozinga, H. Poppe and W. Th. Kok, Anal. Chem., 1990, 62, 367 CrossRef CAS.
- C. Malins, S. Fanni, H. G. Glever, J. G. Vos and B. D. MacCraith, Anal. Commun., 1999, 36, 3 RSC.
- (a) N. W. Barnett, T. A. Bowser, R. D. Gerardi and B. Smith, Anal. Chim. Acta, 1996, 318, 309 CrossRef CAS; (b) G. M. Greenway, A. W. Knight and P. J. Knight, Analyst, 1995, 120, 2459 Search PubMed.
- C. L. Donnici, D. H. M. Filho, L. L. C. Moreira, G. teixeira dos Reis, E. S. Cordeiro, I. M. Ferreira de Oliveira, S. Carvalho and E. B. Paniago, J. Braz. Chem. Soc., 1998, 9, 455 CAS.
- A. Franville, D. Zambon and R. Mahiou, Chem. Mater., 2000, 12, 428 CrossRef CAS.
- G. Sprintschnik, H. W. Sprintschnik, P. P. Kirsch and D. G. Whitten, J. Am. Chem. Soc., 1977, 4947 CrossRef CAS.
- B. A. Gorman, N. W. Barnett and R. Bos, Anal. Chim. Acta, 2005, 541, 117 CrossRef.
- T. M. Butler, B. D. MacCraith and C. McDonagh, J. Non-Cryst. Solids, 1998, 224, 249 CrossRef CAS.
- I. Gill and A. Ballesteros, J. Am. Chem. Soc., 1998, 120, 8587 CrossRef CAS.
- I. Rubinstein and A. J. Bard, J. Am. Chem. Soc., 1980, 102, 6642 CrossRef CAS.
- Y. Zhang and H. Ju, Electroanalysis, 2004, 16, 1401 CrossRef CAS.
- M. Plaschke, R. Czolk, J. Reichert and H. J. Ache, Thin Solid Films, 1996, 279, 233 CrossRef CAS.
- J.-K. Lee, S.-H. Lee, M. Kim, H. Kim, D.-H. Kim and W.-Y. Lee, Chem. Commun., 2003, 1602 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/b511071h |
| ‡ To confirm covalent immobilisation of complex 3 into the silicate sol–gel matrix, monoliths were prepared and stored in a range of commonly employed solvent systems, including borate buffer (pH 8.5), phosphate buffer (pH 7.0), aq. sulfuric acid (0.01 M) and acetonitrile; no leaching has been observed over a period of 8 months. |
| This journal is © The Royal Society of Chemistry 2006 |
