Linkage of N3 dye to N3 dye on nanocrystalline TiO2 through trans-1,2-bis(4-pyridyl)ethylene for enhancement of photocurrent of dye-sensitized solar cells
Song-Rim
Jang
a,
R.
Vittal
a,
Jiwon
Lee
b,
Nakcheol
Jeong
a and
Kang-Jin
Kim
*a
aDivision of Chemistry and Molecular Engineering, Korea University, Seoul 136-701, Korea. E-mail: kjkim@korea.ac.kr; Fax: 82-2-3290-3121; Tel: 82-2-3290-3127
bSamsung SDI, Co. Ltd., Gyeonggi-Do, 449-577, Korea
First published on 17th November 2005
Abstract
Linking of N3 dye to another TiO2-attached N3 dye rendered an enhanced short-circuit photocurrent and thereby higher efficiency for the dye-sensitized solar cell with the pertinent TiO2 film electrode.
Linking of N3 dye to N3 dye immobilized on TiO2 particles in TiO2 film through trans-1,2-bis(4-pyridyl)ethylene (tbpe) has rendered higher short-circuit photocurrent density (Jsc) for the corresponding dye-sensitized solar cell (DSSC), relative to that of a usual cell; this enhanced Jsc is attributed to the increased concentration of electrons in the conduction band of TiO2 arising from the efficient transfer of photogenerated electrons from the linked N3 dye through the tbpe linker.
Modification of the dye is one of the strategies to improve the performance of DSSCs. Best energy conversion to date for the DSSC has been with N3 dye (cis-bis(4,4′-dicarboxy-2,2′-bipyridine)dithiocyanato ruthenium(II)).1 Efforts have been made to further improve the cell performance achieved with N3 dye by attaching hydrophobic chains to the pyridine rings, thus increasing the open-circuit voltage (Voc),2 by substituting the NCS ancillary ligands with numerous ligands,3 or finding analogues to Ru(II)-based sensitizers with metal centers such as Re,4 Rh,5 and Os.6 So far, the new dyes have not been able to further improve the cell efficiency. The search for new dyes is thus challenging.
Recently, a Ru(II)-bpt-Ru(II) polypyridyl complex, where two N3 dyes are bridged by 3,5-bis(pyridin-2-yl)-1,2,4-triazole (bpt, a very rigid linker) replacing all NCS ligands of N3 dye, was used as a sensitizer in a DSSC. However, the conversion efficiency was found to be half that of the usual cell.7 Similar complexes with the metal centers Os(II), Ru(II)-bpt-Os(II) produced no photocurrent.6,7
In this paper we report a new in-situ route for the attachment of an N3 dye molecule, using tbpe, to another N3 dye molecule which is already immobilized on nanocrystalline TiO2, and the consequent enhancement of Jsc for the thus modified cell. The basis of selection of this linker lies in the fact that it connects the two Ru(II) metal centers of the N3 dyes by a chain of conjugated double bonds, which is expected to facilitate electron transfer.
N3-immobilized TiO2 film (15 µm thick, N3–TiO2) was prepared on F-doped SnO2, as described elsewhere.8 In a typical synthesis, 81.6 mg (0.448 mol) of tbpe was dissolved in 100 mL ethanol and the solution was sonicated under Ar atmosphere. After 1 min, the N3-immobilized TiO2 film was immersed in the tbpe ethanol solution and the mixture was refluxed for 4 h at 70–80 °C under Ar.9 After cooling to room temperature, the tbpe bonded N3–TiO2 film (tbpe-N3–TiO2) was washed with ethanol. For the N3 dye linking, this film on FTO was immersed in 0.5 mM N3 dye–ethanol solution, followed by refluxing for 4 h at 70–80 °C under Ar. It should be mentioned here that the same synthetic procedure as above in ethanol solution to link the two N3 dye molecules using tbpe led to the production of an insoluble polymeric material which could not be used as a sensitizer. After cooling, the resulting N3-tbpe-N3–TiO2 (Fig. 1) film was washed with ethanol and then used to fabricate DSSCs.8 The redox electrolyte solution consisted of 0.05 M I2, 0.1 M LiI, 0.6 M 1,2-dimethyl-3-hexylimidazolium iodide and 0.5 M 4-tert-butylpyridine in 3-methoxypropionitrile.
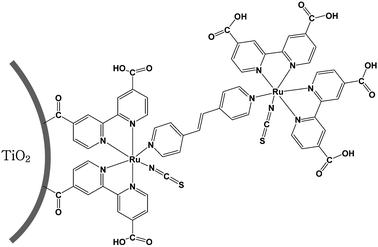 | ||
| Fig. 1 Schematic representation of N3 dye linked to another N3 dye which is immobilized on a TiO2 particle. | ||
A similar synthesis was performed starting with Ru(diCl), denoting cis-bis(4,4′-dicarboxy-2,2′-bipyridine)dichloro ruthenium(II) to produce Ru(Cl)-tbpe-Ru(Cl)–TiO2 film on FTO, to find the influence of replacing NCS ligands with Cl ligands on the performance of DSSCs.10
J–V curves were obtained using a Keithley M236 source measure unit. A 300 W Xe arc lamp (Oriel) through an AM 1.5 solar simulating filter was used to illuminate the working electrode of 0.4 × 0.4 cm. The light intensity was adjusted to 100 mW/cm2, using a Si solar cell.
Vibration spectra by FT-IR (using a Bowman, Hartman & Braun MB-series spectrometer), Raman spectra (with a Jobin-Yvon T64000 spectrometer) and elemental analysis by ICP (using a Thermo Jarrell Ash Polyscan 61E spectrometer) confirmed the linkage of N3 dye viatbpe to the N3 dye immobilized on the TiO2 film. A tbpe links two Ru(II) centers as shown schematically in Fig. 1. Fig. 2 shows the FT-IR spectra of powders of N3-tbpe-N3–TiO2, Ru(Cl)-tbpe-Ru(Cl)–TiO2, Ru(Cl)–TiO2, and bare TiO2. A peak of the NCS stretching mode appears at 2100 cm−1 for N3-tbpe-N3–TiO2, while a characteristic peak of Ru–Cl appears at 2000 cm−1 for both Ru(Cl)-tbpe-Ru(Cl)–TiO2 and Ru(Cl)–TiO2. Fig. 3 compares the Raman spectra of N3-tbpe-N3–TiO2 film and N3–TiO2 film. Their C–C stretching vibrations11,12 at 1461, 1528 and 1596 cm−1 are enlarged in the right inset and the Eg mode of anatase TiO2 at 144 cm−1 in the left inset. The ratio of each of the intensities of C–C stretching vibrations to the intensity of the Eg mode of TiO2 is greatly enhanced with the N3 linking, relative to the similar ratio without N3 linking, indicating the increased amount of N3 dye. In addition, ICP analysis with a Thermo Jarrell Ash Polyscan 61E ICP spectrometer revealed that the atomic percent of ruthenium in the N3-tbpe-N3–TiO2 film has increased to 0.49% from 0.40% obtained in the case of the N3–TiO2 film, supporting the observations made with the Raman data. From the ICP result, it is estimated that about one fifth of the immobilized N3 dye molecules on TiO2 are linked to N3 dye through the tbpe linkage.
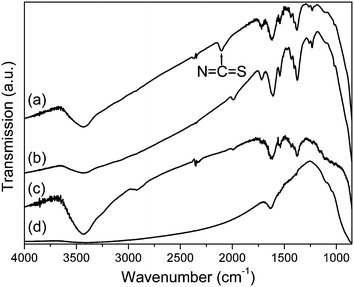 | ||
| Fig. 2 FT-IR spectra of powders of (a) N3-tbpe-N3–TiO2, (b) Ru(Cl)-tbpe-Ru(Cl)–TiO2, (c) Ru(Cl)–TiO2, and (d) bare TiO2. | ||
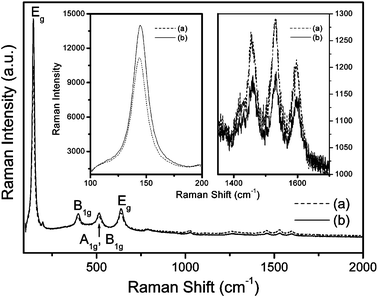 | ||
| Fig. 3 Raman spectra of TiO2 films anchored by (a) N3-tbpe-N3, and (b) N3 only. Insets represent larger versions of peaks at 144, 1461, 1528 and 1596 cm−1. | ||
Fig. 4 shows that the absorbance of the desorbed dye from TiO2 films (0.8 × 0.8 cm) into aqueous 1 mM KOH, measured with a Hewlett-Packard HP 8453A spectrophotometer, has increased by about 37% in the case of N3-tbpe-N3–TiO2 film, compared with that from N3–TiO2 film, supporting the ICP and Raman results. Though to a discernible extent, the absorption maxima of N3-tbpe-N3 shift to the blue compared with those of N3. A similar blue shift was observed by Garcia et al., when the NCS ligands of N3 were replaced by one or two 4-phenylpyridine ligands.9
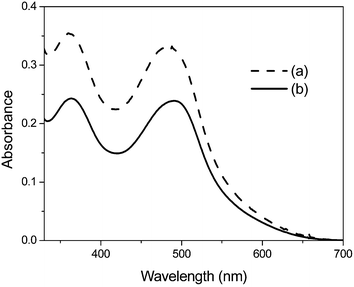 | ||
| Fig. 4 Absorption spectra of dye desorbed from (a) N3-tbpe-N3–TiO2 film and (b) N3–TiO2 film into 1 mM KOH. | ||
Fig. 5 shows the desirable influence of the linked N3 dye on J–V characteristics. The Jsc of the cell fabricated with N3-tbpe-N3–TiO2 film increases to 12.1 mA/cm2 from 10.4 mA/cm2 of the cell prepared with N3–TiO2 film. The N3 dye linking resulted essentially in identical open-circuit voltage (Voc) and fill factor of 680 mV and 0.63, respectively. As a result of mainly the Jsc enhancement, the overall energy conversion efficiency of the N3 dye-linked cell increases from 4.6 to 5.3%. The Jsc increase in the case of the N3-linked cell is consistent with the IPCE increase (measured using an Amino-Bowman fA-256 luminescence spectrometer), as shown in the inset, where the cell formed with the N3-tbpe-N3–TiO2 electrode shows unfailingly higher IPCE values over the visible region, compared with those obtained with a cell containing an N3–TiO2 electrode. The Jsc enhancement can be correlated with the increased amount of dye molecules (Fig. 4) owing to the linking of N3 dye to the immobilized N3 dye, albeit about one half of the linked dye contributes to the enhancement. This implies that the linked N3 dye molecules act as electron donors upon excitation and transfer electrons to the conduction band of TiO2 through the chain consisting of conjugated double bonds between two Ru(II)s. This also implies that the linked dye upon excitation can regenerate the oxidized, immobilized N3 dye in the pores of the TiO2 film possibly with a higher rate constant than that of I− ions. The combined regeneration process of oxidized TiO2-attached dye by I− ions and by linked dye in effect reduces the hole density relative to the situation in the absence of linked dye. The reduction in hole population in turn suppresses the recombination chances of the injected electrons with the holes formed on the TiO2-attached dye. Moreover, the electron injection to the conduction band of TiO2 from its attached dye involves a metal-to-ligand transfer process, which cannot be visualized in the case of the linked dye.
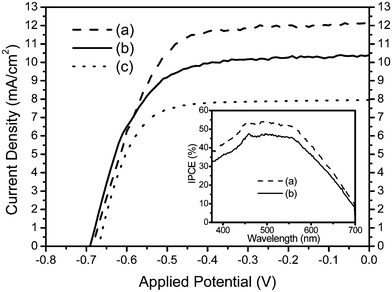 | ||
| Fig. 5 J–V curves of DSSCs prepared with films of (a) N3-tbpe-N3–TiO2, (b) N3–TiO2, and (c) Ru(Cl)-tbpe-Ru(Cl)–TiO2. The inset shows IPCE spectra of DSSCs containing (a) and (b). Light intensity was 100 mW/cm2. | ||
When all NCS ligands of the N3-tbpe-N3 sensitizer were replaced by Cl groups, the resulting cell showed a decrease in the Jsc and Voc by about 35% and 10 mV (Fig. 5), respectively, compared with those of the cell made with the N3-tbpe-N3 composite dye. This result is consistent with previous reports that substitution of NCS ligands of N3 dye with Cl or other ligands did not improve the overall conversion efficiency for the pertinent DSSCs.3,13
In contrast to the Jsc increase, the near invariance of Voc and fill factor indicates that the linking of N3 dye has no effect on the surface properties of TiO2 film. We observed almost identical dark currents irrespective of the N3 linking which is consistent with the invariance of the Voc. The absence of an effect on the surface properties of TiO2 film and the invariance of dark currents suggest that the linked N3 molecules do not directly lie on the TiO2 film and most likely protrude toward the pores in the TiO2 film. We also observed that Jsc increased linearly with the light intensity up to 100 mW/cm2, regardless of the linking of N3 dye, but with a higher slope in the case of the N3-linked DSSC than that of a usual DSSC. This linearity indicates that the photocurrent is not limited by diffusion of I3−/I− in the N3-linked TiO2 film. Further investigations on the effects of multiple linking of N3 dyes and of substitution of the linking dye on the spectral and photovoltaic properties are in progress.
In conclusion we have successfully employed a synthetic route for linking N3 dye to immobilized N3 dye on nanocrystalline TiO2, which enabled a 17% enhancement in Jsc for the pertinent DSSC, compared with the case without N3 dye-linking.
We acknowledge financial support for this work by the CRM-KOSEF of Korea University and by the Sol-Gel Innovation Project.
Notes and references
- M. Grätzel, J. Photochem. Photobiol. A: Chem., 2004, 164, 3 CrossRef CAS.
- J.-J. Lagref, M. K. Nazeeruddin and M. Grätzel, Synth. Met., 2003, 138, 333 CrossRef CAS.
- A. S. Polo, M. K. Itokazu and N. Y. Murakami Iha, Coord. Chem. Rev., 2004, 248, 1343 CrossRef CAS.
- R. Argazzi, C. A. Bignozzi, T. A. Heimer and G. J. Meyer, Inorg. Chem., 1997, 36, 2 CrossRef CAS.
- C. J. Kleverlaan, M. T. Indelli, C. A. Bignozzi, L. Pavanin, F. Scandola, G. M. Hasselman and G. J. Meyer, J. Am. Chem. Soc., 2000, 122, 2840 CrossRef CAS.
- C. Kleverlaan, M. Alebbi, R. Argazzi, C. A. Bignozzi, G. M. Hasselman and G. J. Meyer, Inorg. Chem., 2000, 39, 1342 CrossRef CAS.
- A. C. Lees, C. J. Kleverlaan, C. A. Bionozzi and J. G. Vos, Inorg. Chem., 2001, 40, 5343 CrossRef.
- M. G. Kang, K. M. Kim, K. S. Ryu, S. H. Chang, N.-G. Park, J. S. Hong and K.-J. Kim, J. Electrochem. Soc., 2004, 151, E257 CrossRef CAS.
- C. G. Garcia, N. Y. Murakami Iha, R. Argazzi and C. A. Bignozzi, J. Photochem. Photobiol. A: Chem., 1998, 115, 239 CrossRef CAS.
- P. Liska, N. Vlachopoulos, M. K. Nazeeruddin, P. Comte and M. Grätzel, J. Am. Chem. Soc., 1988, 110, 3686 CrossRef CAS.
- A. Hugot-Le Goff, S. Joiret and P. Falaras, J. Phys. Chem. B, 1999, 103, 9569.
- P. Falaras, A. Hugot-Le Goff, M. C. Bernard and A. Xagas, Sol. Energy Mater. Sol. Cells, 2000, 64, 167 CrossRef CAS.
- O. Schwarz, D. van Loyen, S. Jockusch, N. J. Turro and H. Dürr, J. Photochem. Photobiol. A: Chem., 2000, 132, 91 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2006 |
