The electroanalytical detection of hydrazine: A comparison of the use of palladium nanoparticles supported on boron-doped diamond and palladium plated BDD microdisc array
Christopher
Batchelor-McAuley
a,
Craig E.
Banks
a,
Andrew O.
Simm
a,
Timothy G. J.
Jones
b and
Richard G.
Compton
*a
aPhysical and Theoretical Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, UK OX1 3QZ. E-mail: Richard.Compton@chem.ox.ac.uk; Fax: +44 (0) 1865 275410; Tel: +44 (0) 1865 275413
bSchlumberger Cambridge Research, High Cross, Madingley Road, Cambridge, UK CB3 0EL
First published on 23rd November 2005
Abstract
We show that both a random distribution of palladium nanoparticles supported on a BDD electrode or a palladium plated BDD microelectrode array can each provide a sensing platform for the electrocatalytic detection of hydrazine. The palladium nanoparticle modified electrode displays a sensitivity and limit of detection of 60 mA mol−1 L and 2.6 µM respectively while the array has a sensitivity of 8 mA mol−1 L with a detection limit of 1.8 µM. The beneficial cost implications of using palladium nano- or micro-particles in sensors compared to a palladium macroelectrode are evident. Interestingly the array of the nanoparticles shows similar sensitivity and limit of detection to the microelectrode array which probably indicates that the random distribution of the former leads to ‘clumps’ of nanoparticles that effectively act as microelectrodes.
Introduction
Hydrazine finds widespread usage in rocket fuels, missile systems, weapons of mass destruction, fuel cells and corrosive inhibitors1,2 Hydrazine however is also a neurotoxin and produces carcinogenic and mutagenic effects. Accidental hydrazine poisoning induces vomiting, severe irritation of the respiratory tract and long-term effects on the central nervous system.3 Furthermore it is believed to be oxidized by peroxidases in the body into reactive intermediates capable of causing a variety of adverse side effects including DNA damage.1 In industrial applications, hydrazine is added to boilers which act as an oxygen scavenger and removes dissolved oxygen reducing corrosion time and extending the life of the boilers.It is therefore obvious that reliable and sensitive analytical methods for the determination of hydrazine are needed. Electrochemical techniques offer the opportunity for portable, cheap and rapid methodologies. The main drawback with the electrochemical oxidation of hydrazine is that large overpotentials are required at carbon electrodes.4 An alternative is to modify carbon surfaces with electrocatalysts such as pyrogallol red,4 copper-cobalt hexacyanoferrate5 chlorogenic acid6 or metal phthalocyanines.7,8 Despite the sensitivity and selectivity observed with chemically modified electrodes, the electrodes require regeneration to obtain a reproducible response.9 Alternatively electrodes such as platinum,10 gold, rhodium11 and palladium have been reported as being electrocatalytic for the electrochemical oxidation of hydrazine.
Note that novel electrodes such as microelectrode arrays12–14 and nanoparticle modified electrodes15–17 have attracted attention in electroanalysis due to increased sensitivity and low detection limits in comparison to macroelectrodes.
Recently we reported on a boron-doped diamond (BDD) microelectrode array with the BDD microdiscs arranged in a hexagonal pattern which has the advantage of the array being co-planar to the insulating (undoped diamond) surroundings. We demonstrated that the BDD-microelectrode array could act as an excellent substrate for the deposition of a range of metals, allowing a single electrode array to act as a template for a microelectrode array of many different electrode materials for a variety of analytical tasks.13
In this report we explore and compare the sensing possibilities of a palladium nanoparticle assemble supported on a BDD electrode with that of a palladium plated BDD microelectrode array for the electrocatalytic oxidation of hydrazine. While palladium has been deposited, for example, on highly ordered pyrolytic graphite18 and glassy carbon electrodes;19 the electroanalytical use of a palladium modified electrode for hydrazine detection has been limited to use in capillary electrophoresis.20,21
We show that a random distribution of palladium nanoparticles (assemble) supported on a BDD electrode provides an electrocatalytic route to the sensitive detection of hydrazine. This nanoparticle modified electrode is compared with a palladium plated BDD microelectrode array, viz. palladium microelectrode array which also permits micro-molar detection limits for the detection of hydrazine. We note that the assemble of palladium nanoparticles show similar sensitivity and limit of detection to the microelectrode array and this observation probably indicates that the random distribution of the former leads to ‘clumps’ of nanoparticles acts effectively acts as microelectrodes.
Experimental
All chemicals used were of analytical grade and were used as received without any further purification. These were: palladium (II) chloride (Aldrich, 99.9%), hydrazine sulfate (Aldrich, 99%) and sulfuric acid (Aldrich, 98%, AR grade). All solutions were prepared with deionised water of resistivity not less than 18.2 MΩ cm (Millipore water systems, UK).Voltammetric measurements were carried out using a μ-Autolab III (ECO-Chemie, Utrect, The Netherlands) potentiostat. All measurements were conducted using a three electrode cell. The BDD microelectrode arrays were constructed in house via sealing the BDD disk into Teflon housing. The fabrication process for the BDD microelectrode array is detailed elsewhere.22 A boron-doped diamond macroelectrode (3 mm diameter, Windsor Scientific, UK) and a palladium macroelectrode (1 mm diameter) where also used as working electrodes. The counter electrode was a bright platinum wire, with a saturated calomel electrode completing the circuit. All experiments were typically conducted at 20 ± 2 °C
All optical microscopy was performed with a Digital Instruments OMV-PAR microscope based on a Sony XC-999P CCD camera, having a maximum resolution of 752 × 582 pixels over an area of 540 × 400 µm. The atomic force microscope (AFM) used was a Digital Instruments (now a division of Veeco) Multimode SPM, operating in ex-situ tapping mode. A model “J” scanner was used, having a lateral range of 125 × 125 µm and a vertical range of 5 µm. Standard silicon nitride probes (Veeco part number NP), having a force constant of approximately 0.58 N m−1, were used. Scans were collected at a scan rate of 1 Hz or below.
Results and discussion
First the cyclic voltammetric response of a boron-doped diamond (BDD) macroelectrode was explored in a solution of 2.4 mM PdCl2 in 1 M H2SO4. Fig. 1 shows the response from sweeping the potential from +1 V to 0 V (vs. SCE), where a reduction wave at ca. + 0.11 V (vs. SCE) is apparent corresponding to the electrochemical reduction of Pd2+ to Pd0 on the electrode surface. On reversing the voltage scan the deposited Pd0 is then voltametrically stripped at ca. + 0.66 V reforming Pd2+. Note that a current crossover is observed indicative of a nucleation and growth mechanism, where the initial formation of palladium nuclei on the BDD surface serves to increase the rate of further deposition; thus on the return scan the cathodic current flows at less negative potential than those required to initial deposition.23 It is clear from Fig. 1 that the charge under the peak for the oxidation wave is less than that for the reduction wave indicating that not all the palladium is re-dissolved into the sulfuric acid solution indicating incomplete stripping.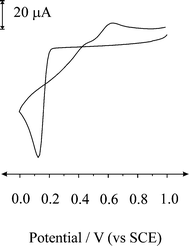 | ||
| Fig. 1 Cyclic voltammetric response obtained at a BDD macroelectrode in a 2.4 mM PdCl2 (in 1 M H2SO4) solution recorded at a scan rate of 100 mV s−1. | ||
The decoration of a BDD electrode with palladium particles was optimised by varying the deposition conditions and concentration of the plating solution. The procedure for producing palladium nanoparticles was found to occur when the potential was held at 0 V (vs. SCE) for 40 s in a 0.177 mM PdCl2 (in 1 M H2SO4) solution. AFM images, depicted in Fig. 2 reveal a random distribution of palladium nanoparticles over the electrode surface (Fig. 2A). Closer inspection, note the change of scale, as shown in Fig. 2B reveals palladium nanoparticles in the range of 10–200 nm in diameter are evident. The density of palladium nanoparticles on the electrode was found to be ca. 5 µm−2.
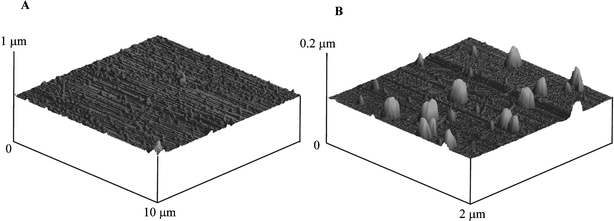 | ||
| Fig. 2 AFM images of palladium particles deposited onto a BDD electrode. Deposition: 0 V for 40 s in 0.17 mM PdCl2 in 1 M H2SO4. | ||
We next turn to exploring the palladium nanoparticle decorated BDD electrode for the electroanalytical detection of hydrazine. A BDD electrode was prepared by depositing palladium for 40 s at 0 V from a 0.177 mM PdCl2 (in 1 M H2SO4) solution. The modified electrode was removed and washed with distilled water and then placed into a pH 7 phosphate buffer solution containing 1.36 mM hydrazine. The electrochemical oxidation was explored with an oxidation wave observed to occur with peak maxima at ca. + 0.11 V (vs. SCE, 100 mV s−1). Note that this catalytic oxidation peak is well resolved from solvent decomposition (ca. > 1 V) which proceeds exclusively at the palladium surface; the electrochemical oxidation of hydrazine at the bare BDD was not observed to occur in the available potential window.
The effect of scan rate was explored where a gentle increase in peak current was observed which is likely due to diffusional overlap; note that not only does this depend on the spacing of the particles but also on the diffusion coefficient and scan rate employed.13
The effect on the oxidation potential from changing the solution pH was explored. It was found that from altering the pH from 7 to 3 the hydrazine oxidation peak shifted from ca. + 0.11 V to ca. + 0.4 V; proceeding further to lower pH values resulted in shifting the oxidation potential of hydrazine into the same region as that corresponding to the stripping of palladium metal from the electrode surface to palladium ions. Thus on potential cycling at pH values lower than 3, the electrocatalytic signal was observed to decrease resulting from the palladium particles on the surface being stripped and consequently the analytical signal is lost. This also explains the reason why Li and co-workers24 observed the decreasing of the electroanalytical signal with potential cycling at a palladium modified glassy carbon electrode in 0.1 M K2SO4 for the electro-catalytic oxidation of hydrazine, which they suggested was due to some impurities in the hydrazine solution but is likely due to the analytical signal corresponding to hydrazine oxidation being in the same region as stripping potential.
Returning to our observed shift of hydrazine oxidation potential with pH, analysis of a peak potentials versus pH produced a linear response over the studied pH range of 3–7 with a gradient of 72.6 mV per pH unit which is close to the expected 73.7 mV per pH unit for a 4 electron, 5 proton process. Thus it is likely that the electrochemical oxidation of hydrazine is as follows:25
| N2H5+ − 4e− → N2 + 5H+ | (1) |
We now turn to exploring the electroanalytical response of the palladium nanoparticle decorated BDD electrode. The BDD electrode was modified via depositing palladium for 40 s at a potential of 0 V (vs. SCE) from a 0.177 mM PdCl2 (in 1 M H2SO4) solution. The modified electrode was removed and washed with distilled water and placed into a pH 7 solution where additions of hydrazine were made. Typical voltammograms are shown in Fig. 3 with analysis of these, as peak current versus added hydrazine reveals a linear plot from 27.2 µM up to 85 µM (IL = 59.6 mA mol−1 L ([hydrazine]/M) + 1.2 × 10−6 A, R2 = 0.99) with a limit of detection (based on 3σ) found to correspond to 2.6 µM. Note the low overpotential that is required to oxidise hydrazine (ca. + 0.1 V vs. SCE) indicative of the electrocatalytic nature of the palladium metal.
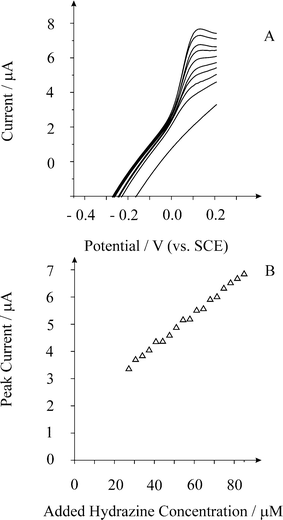 | ||
| Fig. 3 Linear sweep voltammograms from increasing additions of hydrazine recorded at a palladium decorated BDD macroelectrode in a pH 7 buffer solution at a scan rate of 100 mV s−1. Additions from bottom to top are: 0, 27.2, 34, 40.8, 47.6, 54.4, 61.2, 68, 74.8, and 81.6 µM. | ||
Next the response of a palladium macroelectrode was explored. Fig. 4 shows the voltammetric responses from increasing additions of hydrazine in a pH 7 phosphate buffer solution. Analysis of the current versus added hydrazine concentrations are shown in Fig. 4 where two linear ranges can be identified; the first is from 31 µM to 71 µM (IL = 19.2 mA mol−1 L ([hydrazine]/M) + 1.2 × 10−6 A, R2 = 0.98) and the second is from 71 µM to 204 µM (IL = 7.6 mA mol−1 L ([hydrazine]/M) + 1.96 × 10−6 A, R2 = 0.97). Using the first linear section of this curve, a limit of detection (based on 3σ) was found to correspond to 12.1 µM. However the analytical curve is not ideal due to its curvature, the origins of which are unknown.
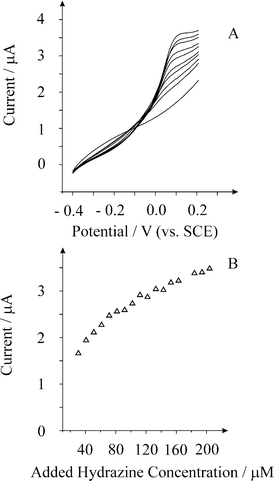 | ||
| Fig. 4 Linear sweep voltammograms from increasing additions of hydrazine recorded at a palladium macroelectrode in a pH 7 buffer solution at a scan rate of 100 mV s−1. Additions from bottom to top are: 0, 30.6, 40.8, 61.2, 91.8, 122, 143, 153, 173, and 204 µM. | ||
We next turn to exploring a palladium plated BDD microelectrode array for the possible electroanalytical detection of hydrazine. This array consists of 362 microdiscs which are 25 microns in diameter separated from their nearest neighbour by 250 microns. The array was plated with palladium by depositing palladium metal from a 1.77 mM PdCl2 (in 1 M H2SO4) via holding the potential at 0.0 V for 40 s. An optical image of the palladium coated array is shown in Fig. 5A. Closer inspection with AFM (Fig. 5B) reveals that the array consists of microdiscs which are 25 microns in diameter (Fig. 5B) which appear to be fully covered with palladium metal.
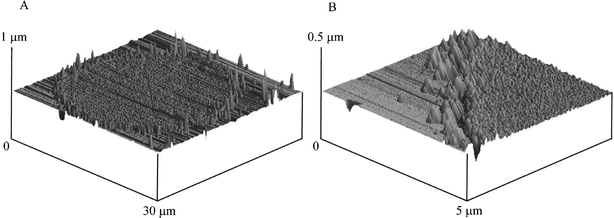 | ||
| Fig. 5 A: Optical image of the BDD microelectrode array after plating with palladium. The image size is 540 × 400 µm. B: AFM images of a single BDD electrode making up the array (picture A) while picture B shows the edge of the BDD microelectrode. The array was plated by depositing palladium metal at 0 V for 40 s in 0.17 mM PdCl2 in 1 M H2SO4. | ||
The BDD microelectrode array was plated with palladium, washed and placed into a pH 7 phosphate buffer solution with additions of hydrazine made. Fig. 6A shows linear sweep voltammetric responses from additions of hydrazine from 10 µM to 102 µM. A steady-state response is observed consistent with the high current density conditions associated with the microelectrode array. Analysis of the limiting current (IL) taken at + 0.1 V (vs. SCE) vs. added hydrazine concentration is shown in Fig. 6B where a linear response is observed (IL = 7.7 mA mol−1 L ([hydrazine]/M) + 3.9 × 10−7 A, R2 = 0.99) with a limit of detection (based on 3σ) found to correspond to 1.8 µM; the sensitivity in terms of electrode area based on the geometric size of the BDD discs on the array equates to 4.7 A M−1 cm−2. Note that given the magnitude of the peak current from the lowest addition, the predicted detection limit based on three sigma which is commonly used to evaluate analytical systems is unlikely to be seen in reality.
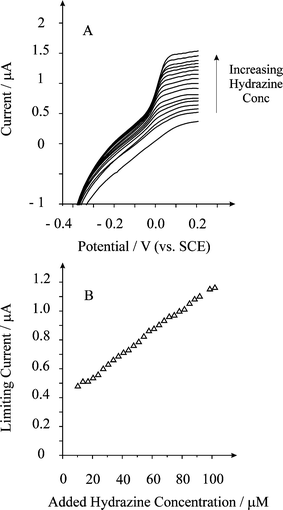 | ||
| Fig. 6 Linear sweep voltammograms from the oxidation of hydrazine using a palladium plated BDD microelectrode array. Deposition: 0 V for 40 s in 0.17 mM PdCl2 in 1 M H2SO4. Scan rate used: 100 mV s−1. The curves are resulting from additions of (bottom to top): 0, 6.8, 10.2, 13.6, 17, 20.4, 23.8, 27.2, 34, 41, 47.6, 54.9, 61.2, 68, 74.8, 81.6, 88.4, 88.4, 95.2, and 102 µM. | ||
The detection limits observed at the palladium nanoparticle BDD electrode and the palladium plated microelectrode array is comparable to pyrogallol red modified glassy carbon electrodes4 and is improved over copper-cobalt hexacyanoferrate films5 and metal phthalocyanine films.8
Comparison of the palladium nanoparticle decorated BDD electrode with the palladium plated BDD microdisc array reveals that comparable low micro-molar detection and highly linear response toward hydrazine is possible. The former occurs because there are 362 palladium 25 µm diameter microdiscs which individually are working in parallel. Thus given the similar response at the nanoparticle palladium decorated electrode it is likely that the palladium nanoparticles are also acting as an array of microelectrodes. In fact inspection of Fig. 2 reveals nanoparticles which are close together which effectively makes them act as single, larger particles.
Note that both the array and the nanoparticle assemble provide lower detection limits and highly linear responses compared to the palladium macroelectrode, making these electrodes ideal for routine sensing and inclusion in Clark-type sensors given the reduced cost due to the low amount of palladium metal that is required but also provides improved sensing capabilities.
Conclusions
The electroanalytical detection of hydrazine is shown to be possible at palladium nanoparticles supported on a BDD electrode and a palladium microelectrode array using a BDD microelectrode array as a template. The advantages of these electrodes for hydrazine sensing lie in the low detection limit and high sensitivity compared to existing electrochemical techniques and a palladium macroelectrode. Also the cost implications of using palladium nano- or micro-particles in sensors compared to a palladium macroelectrode are clearly evident.References
- J.-W. Mo, B. Ogorevc, X. Zhang and B. Pihlar, Electroanalysis, 2000, 12, 48 CrossRef CAS
.
- S. D. Zelnick, D. R. Mattie and P. C. Stepaniak, Aviat., Space Environ. Med., 2003, 74, 1285 Search PubMed
.
- S. Garrod, M. E. Bollard, A. W. Nicholls, S. C. Connor, J. Connelly, J. K. Nicholson and E. Holmes, Chem. Res. Toxicol., 2005, 18, 115 CrossRef CAS
.
- A. A. Ensafi and E. Mirmomtaz, J. Electroanal. Chem., 2005, 583, 176 CrossRef CAS
.
- A. Abbaspour and M. A. Kamyabi, J. Electroanal. Chem., 2005, 576, 73 CrossRef CAS
.
- S. M. Golabi and H. R. Zare, J. Electroanal. Chem., 1999, 465, 168 CrossRef CAS
.
- K. M. Korfhang, R. Ravichandran and R. P. Baldwin, Anal. Chem., 1984, 56, 1514 CrossRef CAS
.
- K. I. Ozoemena and T. Nyokong, Talanta, 2005, 67, 162 CrossRef CAS
.
- P. Anda, J. Weber, L. Dunsch and A. B. P. Lever, Anal. Chem., 1966, 68, 960
.
- M. D. Garcia-Azorero, M. L. Marcos and J. Gonzalez Velasco, Electrochim. Acta, 1994, 39, 1909 CrossRef CAS
.
- B. Alvarez-Ruiz, R. Gomez, J. M. Orts and J. M. Feliu, J. Electrochem. Soc., 2002, 149, D35 CrossRef CAS
.
- D. W. M. Arrigan, Analyst, 2004, 129, 1157 RSC
.
- A. O. Simm, C. E. Banks, S. Ward-Jones, T. J. Davies, N. S. Lawrence, T. G. J. Jones, L. Jiang and R. G. Compton, Analyst, 2005, 130, 1303 RSC
.
- A. Berduque, G. Herzog, Y. E. Watson, D. W. M. Arrigan, J.-C. Moutet, O. Reynes, G. Royal and E. Saint-Aman, Electroanalysis, 2005, 17, 392 CrossRef CAS
.
- M. Tominaga, T. Shimazoe, M. Nagashima and I. Taniguchi, Electrochem. Commun., 2005, 7, 189 CrossRef CAS
.
- J.-J. Xu, X.-L. Luo, Y. Du and H.-Y. Chen, Electrochem. Commun., 2004, 6, 1169 CrossRef CAS
.
- L. Zhang and X. Jiang, J. Electroanal. Chem., 2005, 583, 292 CrossRef CAS
.
- Y. Gimeno, A. Hernandez Creus, S. Gonzalez, R. C. Salvarezza and A. J. Arvia, Chem. Mater., 2001, 13, 1857 CrossRef CAS
.
- F. Li, B. Zhang, S. Dong and E. Wang, Electrochim. Acta, 1997, 42, 2563 CrossRef CAS
.
- J. Liu, W. Zhou, T. You, F. Li, E. Wang and S. Dong, Anal. Chem., 1996, 68, 3350 CrossRef CAS
.
- J. Wang, M. P. Chatrathi, B. Tian and R. Polsky, Electroanalysis, 2000, 12, 691 CrossRef CAS
.
- M. Pagels, C. E. Hall, N. S. Lawrence, A. Meredith, T. G. J. Jones, H. P. Godfried, C. S. J. Pickles, J. Wilman, C. E. Banks and R. G. Compton, Anal. Chem., 2005, 77, 3705 CrossRef CAS
.
- C. Prado, S. J. Wilkins, F. Marken and R. G. Compton, Electroanalysis, 2002, 14, 262 CrossRef CAS
.
- F. Li, B. Zhang, S. Dong and E. Wang, Electrochim. Acta, 1997, 42, 2563 CrossRef CAS
.
- A. J. Bard, Anal. Chem., 1963, 35, 1602 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2006 |
