Measurement strategy and instrumental performance of a computer screen photo-assisted technique for the evaluation of a multi-parameter colorimetric test strip
D.
Filippini
* and
I.
Lundström
Division of Applied Physics, Institute of Physics and Measurement Technology, Linköping University, S-581 83 Linköping, Sweden. E-mail: danfi@ifm.liu.se
First published on 23rd November 2005
Abstract
A measuring strategy for the evaluation of a seven parameters colorimetric test using a computer screen photo-assisted technique (CSPT) is demonstrated. CSPT is a versatile approach aimed at point of care or home tests that uses regular computer sets and web cameras as the whole instrument. Issues such as the stability and the equivalency on different platforms of the determinations have been addressed in the present work. The method uses an embedded local reference simultaneously measured with the tests and solves the evaluation as a classification problem. The achieved performance tested along 580 classifications covering all the ranges of the assay, using synthetic samples, yielded 97.2% correct determinations compared with 89.7% for the case of colorimetric determinations. The errors were concentrated in only two parameters that show a significant correlation with a set of quality indices used to assess the performance of the classification.
Introduction
Computer screens used as light sources together with regular web cameras as image detectors have been demonstrated in recent years as a feasible platform for the evaluation of colorimetric assays, a method named computer screen photo-assisted technique (CSPT1). CSPT has been used to evaluate assays based on different principles such as whole cell assays (e.g. for drugs detection2,3), or fluorescent fingerprinting of polythiophene derivatives reporting complementary DNA attachment.4,5 The method can be used for both transparent indicators (in different formats such as microtiterplates,3 or printed spots on glass slides) by evaluating transmitted light and for opaque samples,6 in which case CSPT performs reflectance fingerprinting.CSPT evaluates color substances by illuminating them with different light colors provided by the computer screen and calculating their spectral fingerprints from selected regions of interest (ROI) in the recorded images. Since the ROIs can be numerically defined the method can be easily adapted to any assay layout, which makes it a versatile technique for home tests evaluation, or primary health care diagnostics, especially considering the diversity of possible assay formats demanded by these applications and the extended availability of the suggested setup.
However, neither computer screens nor web cameras are intentionally designed for analytical purposes, and the measuring strategy must compensate this fact, typically by exploiting the inherent computing power.
In this study we demonstrate the use of a CSPT platform for the evaluation of a commercial multi-parameter urine test (Roche, Combur7) commonly used for diagnostics in primary care units or doctor's offices. The repeatability of the determination is examined along 100 measurements of 5 different sets of samples covering the possible outcomes of the different indicators, which enables assessment of the instrumental performance and formulation of representative quality indices.
Experimental
The CSPT platform was composed by a laptop computer (Dell Latitude D800, with a Pentium M processor of 1.6 GHz) with a WXGA screen (operating at a resolution 1280 × 800 pixels with a color resolution of 32 bits, and at a refresh frequency of 60 Hz, keeping standard user settings of brightness, contrast and gamma) and a web camera (Philips PCVC740 K ToU Cam Pro, with a CCD detector operating at a resolution of 320 × 240 pixels). The other camera settings were: brightness = 25%, gain = minimum, shutter speed = 1/33 s, saturation = 70%, gamma = 70%, white balance = outdoor.A sample holder was used to position the test strips and the corresponding evaluation chart in front of the screen and also provided a light shield preventing external illumination (Fig. 1a). The web camera was fixed to one extreme of the holder, with its lens parallel to the surface of the samples and focused on them. The dimensions of the holder and imaged areas are indicated in Fig. 1a. Since the only purpose of this accessory is to provide a fixed geometry for the measurements and to shield ambient light there are not demanding constraints regarding materials, and aluminium, opaque plastic or even cardboard can be used.
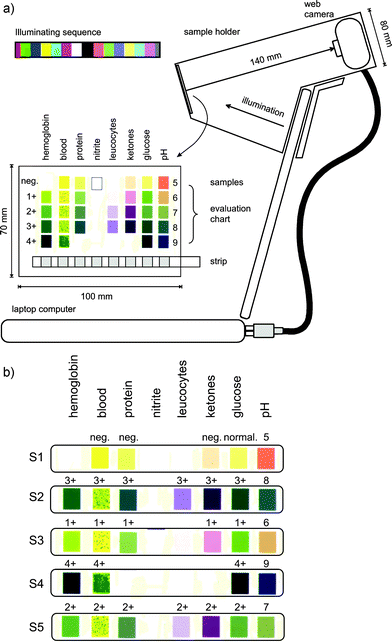 | ||
| Fig. 1 (a) Scheme of a computer screen photo-assisted platform, showing the sample holder and the considered test and corresponding evaluation chart. The 15 color illuminating sequence used is indicated in the upper part of the figure. (b) Five tested samples covering most ranges of the different indicators. | ||
In a typical experiment with a urine test strip (in this case a Roche, Combur7) the strip is dipped into a urine sample and after a certain reaction time the colors of the indicators are evaluated. The strips are disposable and contain different reagents immobilized in paper patches. In Combur7 the strips detect hemoglobin (∼10 to ∼250 erythrocytes µL−1), blood (∼10 to ∼250 erythrocytes µL−1), proteins (30 to 500 mg dL−1), nitrites (negative or positive), leucocytes (∼10 to 500 leucocytes µL−1), ketones, glucose (∼50 to 1000 mg dL−1) and pH (5 to 9).
The alternatives for the evaluation of such tests are either comparison by visual inspection with an evaluation chart (Fig. 1) or using dedicated readers that measure the reflectance of the indicators at certain specific wavelengths (e.g. Urisys 1100 systems). Modern devices of this kind are able to evaluate different tests from the same manufacturer, but are typically dedicated to specific brands and require specific assay layouts, which make them inadequate as general instruments, such as for instance for evaluating different tests at home.
The present contribution is not the result of a clinical trial but a previous stage aimed at checking the instrumental quality of the CSPT for evaluating this kind of colorimetric tests. Accordingly, the use of clinical samples at this point was still premature and instead synthetic samples generated as color printouts (Xerox Phaser 1235 PS) of possible assay outcomes (digitalized with a 24 bit color resolution from the original evaluation chart with a CanoScan 8000F scanner) were used. In the present context hemoglobin and blood were treated as separate indicators.
Standard scanning and printing procedures imply an inherent color degradation that makes the task of distinguishing different colors more demanding for CSPT, making the present results a conservative estimation of the actual performance.
The different color samples are illustrated in Fig. 1b. With these 5 different sets of indicators most of the assay's outcomes were represented (nitrites were not possible due to poor color reproduction in the printouts), enabling a full range estimation of the instrumental capabilities of CSPT for this application.
In the CSPT measurement the image of the test and evaluation chart were simultaneously acquired in synchronism with each screen color of the illuminating sequence. This can be done in different ways as described elsewhere,1,5,6 but in this case was carried out using specially designed software (ScreenLab v1.0, developed in Delphi 7) conceived as a simple interface for users in primary health care units. ScreenLab controlled camera settings, image acquisition, illuminating colors and the numeric masking and data extraction of the ROIs, whose coordinates were read from a configuration file. The software produced a data array containing intensity values of the selected ROIs that were transferred for analysis to a standalone mathematic kernel written in MatLab® 7 R14. This program performed calibration and control routines, extracting CSPT spectral fingerprints from the ROIs that were then used for classification and subsequent evaluation of the assay. The details of the processing and classification routines are discussed in the next section.
All these processing steps are transparent to the user in ScreenLab, which only interacts with a graphic user interface that requests the patient identification (introduced either by typing or using a barcode reader) and displays the results of the evaluation. The present illuminating sequence is composed of 15 different colors displayed at a rate of 1 color s−1. The total measuring time is accordingly these 15 seconds used for acquisition plus 1 or 2 seconds for processing depending on the particular computer. It must be noted that the imaging capabilities of CSPT allows acquisition of all the ROIs at the same time independently of the number of ROIs by contrast with conventional sequential reading that takes about 70 s for the same test.
The illuminating sequence used in this study was a non-optimal 15 color set (Fig. 1a) empirically chosen for providing a good sample classification in a short measurement time.
Results and discussion
Fig. 2a shows the ROIs considered for the evaluation of the assay. White areas correspond to the masked regions of the evaluation chart and the strip, and will be referred to as sample ROIs. Black areas (called here background ROIs) are used to estimate the background intensity of the image and for the composition of the substance's fingerprints.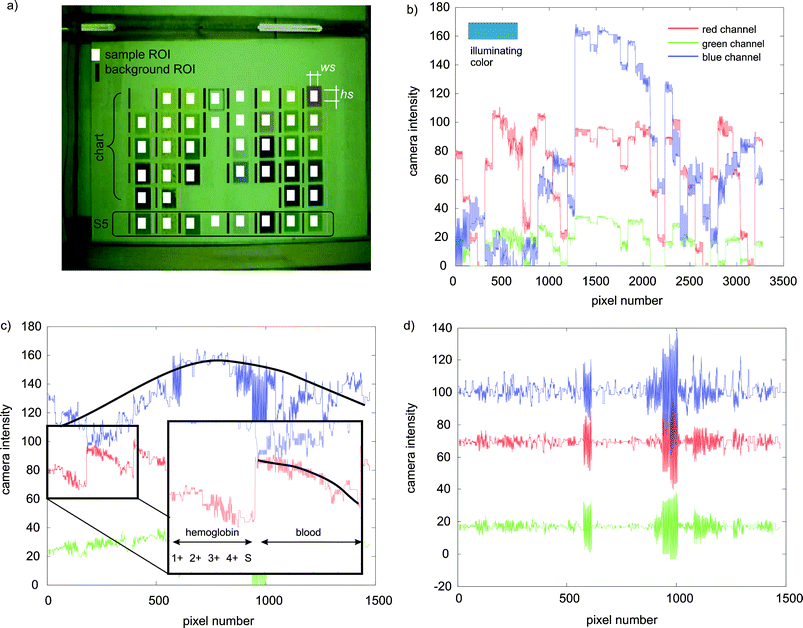 | ||
| Fig. 2 (a) Image of the sample and evaluation chart within the holder as acquired by the web camera for white illumination. The white squares indicate the masked regions of interest (ROIs) corresponding to the samples and the other areas were used for the background compensation. (b) Raw signals of all the pixels from the different sample ROIs for one illuminating color and for the red, green and blue channels of the camera. (c) Raw signals from the background ROIs. The insert shows the composition of the signals ordered by parameters, and how the intensity reduces vertically (the black line is an aid to the eye). (d) Effect of background compensation on the intensity modulation. | ||
Fig. 2b shows the raw data from the red, green and blue channels of the web camera extracted from the sample ROIs and Fig. 2c from the background ROIs for a particular illuminating color (indicated in Fig. 2b). The ideal background characterization requires repeating the measurement on a homogeneous white area, however, this would demand an additional measurement that is here eliminated by estimating the local background from neighbor areas. Thus, the background ROIs corresponding to any particular sample ROI is calculated as the average intensity of the 2 areas beside it. The height of the background ROIs is larger than the sample ROIs in order to account for vertical variations of the intensity.
Fig. 2c illustrates the background intensity modulation. The x axis corresponds to each image pixel of which the CSPT fingerprint is composed. Considering background ROIs of wb = 2 × hb = 18 pixels × 41 sample ROIs in the whole image this corresponds to the 1476 pixels in the figure. The different color lines correspond to the intensity values of these pixels measured through the red, green and blue channels of the web camera for the 15 different illuminating colors, becoming a set of 45 curves (for clarity only the three corresponding to one particular illumination are displayed in Fig. 2).
Since CSPT extracts spectral fingerprints of color substances7–9 by analyzing the light intensity of the ROIs under different illuminating colors, any modulation of the intensity not related to the color of the sample, should be minimized. In ideal conditions (homogeneous background and free of noise) each of the 45 curves would correspond to a constant intensity value. Actual instrumental constraints create the observed intensity modulation.
Among these factors are the limited angular vision of the web camera and the distance to the samples, which is trade off to maximize the image size of the ROIs while minimizing the length of the holder. In the present case the size of the evaluation chart is imposed by the manufacturer, and determines the minimum length of the holder in order to accommodate its whole area within the image. An additional 15 mm length is used to improve the background homogeneity. Another contributing factor to the background modulation is the angular distribution of the illumination from the LCD screen.
In Fig. 2c all the background pixels corresponding to the first sample ROI (hemoglobin 1+) are followed by all the pixels of the second sample ROI (hemoglobin 2+) until completing the first column with the test strip patch of hemoglobin. As can be seen the intensity decreases down this row of the assay (inset in Fig. 2c, the black line is an aid to the eye). The next group of values corresponds to the column of blood and again the background intensity decreases in the downward vertical direction, but additionally a global increase of intensity towards the center of the image can be observed.
A perfectly illuminated assay, perhaps with customized sample dimensions and a more sophisticated holder, would allow minimization of the background modulation already in the raw signal, however, it must be considered that a central aspect of CSPT is its simplicity and low cost, which could be adversely affected by favoring more sophisticated holders. Thus, in the present study we focus on investigating the possibility of correcting these deficiencies just using the inherent computer power of CSPT.
The background compensation consists of averaging the pixels corresponding to the two background ROIs beside each sample ROI. This process provides an intensity profile for each curve in Fig. 2c. Since the goal is to eliminate the intensity modulation but to retain the relative intensities of the different curves, the minimum value of each profile is subtracted and only the background variation, Δ, is extracted:
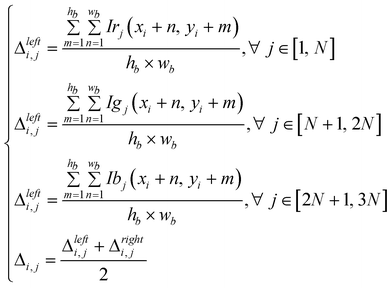 | (1) |
These intensities contain the spectral characteristics of the particular setup and sample reflectance, which for instance, for the red channel can be written as:7
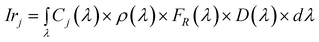 | (2) |
Cj(λ) is the spectral radiance of the screen for illuminating color j and in principle can be written as the linear combination of the spectral radiances of the screen primary sources10R(λ), G(λ) and B(λ):
| Cj(λ) = rj × R(λ) + gj × G(λ) + bj × B(λ) | (3) |
The result of the background characterization is a set of 3N curves, each with 41 points (the total number of sample ROIs in the present case). Actually, this way of representing the information aims at showing the spatial variation of the background, but for substance classification the color directions (41 vectors each of them with 3N elements) is considered.
In order to compensate for the background variability in the samples, Δ is re-sampled along 3280 points (ws = 8 × hs = 10 × 41) and subtracted from the sample ROIs data.
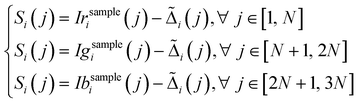 | (4) |
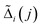 is the re-sampled background compensation for the pixel i and for the color index j.
is the re-sampled background compensation for the pixel i and for the color index j.
In order to illustrate the effect of the compensation, Fig. 2d shows the effect of the background subtraction when is applied to the background signal itself. As can be observed the intensity modulations have disappeared, just leaving the acquired noise of the individual pixels.
The S(j) vectors given by eqn (4) are the CSPT fingerprints of the chosen sample ROIs, which are divided into 8 groups corresponding to hemoglobin, blood, proteins, nitrites, leucocytes, ketones, glucose and pH.
The first subset to be analyzed corresponds to the 4 labels of hemoglobin (1+, 2+, 3+ and 4+ in Fig. 1b) that contain the possible outcomes of that indicator. These 4 ROIs are represented for a set of 4 × ws × hs = 320 S vectors of 3N = 45 elements, which are standardized and used as input for principal components analysis (PCA11).
The result of the PCA enables representation of such fingerprints in a 2D space accounting for ∼95% of the variance in the case of hemoglobin (and between ∼87% and ∼97% for the rest of the indicators). In this representation the 320 vectors become 320 points clearly separated in four different clusters.
Direct calculation of the average values of the original vectors is less computer demanding, but especially at this stage of the development it was preferred to keep the information of the variability in the classification space in order to better determine the quality of the evaluation.
The remaining 80 vectors correspond to the hemoglobin indicator on the strip, and are projected into the principal components space forming a new cluster of 80 points representing the test response. This result is then classified by measuring the Euclidean distances between the centroids of the different clusters (Fig. 3).
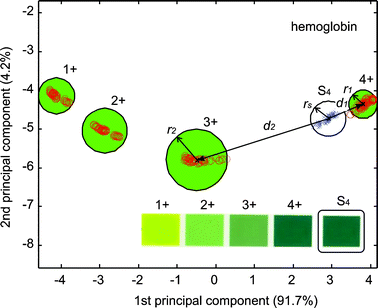 | ||
| Fig. 3 First two principal components of the classification corresponding to the parameter hemoglobin. The circles correspond to 3 times the maximum standard deviation of the ROIs coordinates in the PCs space. ○ correspond to the ROIs on the evaluation chart and × to the projections of the strip ROI of hemoglobin on the PCs space. d1 is the closest distance between the centroid of the strip projection and a label cluster and d2 the distance to the second closest label. | ||
In order to assess the quality of the evaluation four different quality indices are tested:
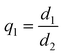 | (5) |
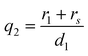 | (6) |
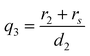 | (7) |
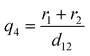 | (8) |
These quality indices are tested for their correlation with misclassifications. For this purpose, five different samples combining different response ranges were fabricated using outcomes from the evaluation chart. Since all of them should be perfect matches, the obtained results allow assessment of the performance of the platform to detect problematic indicators, and to determine representative ranges for the quality indices.
The overall result indicates that in 100 experiments involving 580 classifications, only 13 determinations are incorrect, which corresponds to a global error of 2.24%. However, these errors are concentrated only in two particular cases: glucose = 2+ (11 misclassifications in 20 measurements, 55% errors) and blood = 3+ (2 misclassifications in 20 measurements, 10% errors).
The quality indices vary along the different determinations within certain limits (Fig. 4), since the CSPT platforms are not necessarily designed for fulfilling analytical requirements such as stability. It is actually this kind of aspect that the present measuring strategy aims to correct.
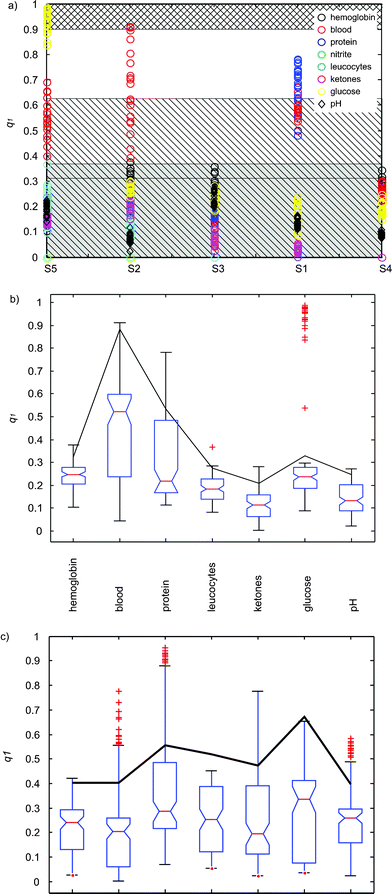 | ||
| Fig. 4 (a) Quality index q1versus the different samples (Fig. 1b). The different parameters are indicated with color in the legend. The gray hatched area corresponds to q1 values associated with no misclassifications, and the hatched area to a less safe region involving large values of q1 occurring for proteins. The cross hatched area corresponds to values of detected mismatches. (b) Quality index q1versus the detected parameters for all the classifications, calculated from CSPT fingerprints. The box shows the inter-quartile interval with the median indicated by a line. The 95% interval is depicted with the whiskers and + indicate outliers. The black line corresponds to the median plus the inter quartile interval. (c) Quality index q1versus the detected parameters for all the classifications, calculated for white illumination. | ||
The number of possible combinations of screens and cameras is immense and makes the spectral characterization of each particular CSPT platform impossible. However, it is necessary to deal with these conditions since it is the possibility of building up a measuring platform from the casual assembly of ubiquitous devices that confers to CSPT its versatility and low cost. In the concept of CSPT it is implicit that potential users of this method already own computer sets or web cameras (the cost of the holder can be estimated as fraction of the cost of a standard web camera). In this context no other measuring approach with similar capabilities is available at such a global scale. These advantages of CSPT motivate the development of measuring strategies able to manage the assumed diversity of setups.
Although the spectral characterization of the platforms is impracticable, nonetheless, the spectral characteristics of the instrument become embedded in the CSPT fingerprints together with the spectral signatures of the target substances. It is feasible to completely disentangle the spectral reflectance of the samples from the spectral characteristics of the platform when the latter are known, such as in the case of spectral reconstruction techniques (e.g. used in art work digitalization12,13), but this approach is not compatible with the purpose of CSPT. In our solution the references are embedded with the assay, which becomes a combination of chemically sensitive areas (test strip) and possible strip outcomes (evaluation chart). Therefore, even if the CSPT fingerprints differ from platform to platform, it is still possible to compare (and classify) sample and reference signatures simultaneously measured in the same platform.
This approach minimizes the influence of the platform in the measurements, but also eliminates additional calibration measurements, which makes the system more robust to instrumental instabilities and simpler for the end user.
Misclassifications occurred for samples S2 and S5 (Fig. 1b), and are correlated with q1 (for assessing the correlation the probability of getting a correlation as large as the observed value by random chance, is tested. If this probability is less than 5%, then the correlation is significant). The uncertainty in the correlation test is 0.73% for S5 and 0.48% for S2.
In the case of S2, the indicators q2, q3 and q4 are also significantly correlated with the misclassifications (with uncertainties lower than 0.64%).
These misclassifications are due to different causes. In the case of S5 misclassification is due to the colors of the labels becoming degraded upon scanning and printing, making glucose 1+ and 2+ areas practically identical. Thus, q1 that measures proximity to the second closest reference cluster, shows a group of values close to 1 (Fig. 4a). Values of q1 close to 1 reflect this loose classification.
By analyzing q1 along all the experiments it is possible establish a band of values where errors have not occurred, in this case for q1 ≤ 0.3238 + 0.0552 (gray hatched area in Fig. 4a). This interval is calculated as the mean value (+ standard deviation) of all the maximum q1 values exhibited by all the samples excluding the indicators of blood, glucose (the faulty ones) and proteins. Since this interval follows the values of the q1 index, intervals closer to 1 indicated a poorer overall performance.
Proteins were excluded from this analysis since they appear to be in an intermediate situation between the safely classified and the mismatches (cross hatched area for q1 > 0.9 in Fig. 4a). If the protein indicator is introduced in the analysis, then q1 ≤ 0.4155 + 0.2106 (hatched area in Fig. 4a), which establishes a less secure classification limit.
It is also important to examine the performance of each parameter and to establish their valid ranges. Thus, outliers indicate potential misclassifications, and large inter-quartile ranges describe variability of the quality indicators. Fig. 4b collects the statistics of the different parameters. q1 shows a large variation for blood due to the dithering in the patches, and glucose shows several outliers close to 1. It is also possible to identify proteins as a risky parameter with q1 values larger than 0.7.
The misclassification of blood 3+ (occurring in only 10% of the measurements) is due to a different cause. The blood indicator does not produce a homogeneous color area, but increasing erythrocyte concentration produces a denser dithering of green points on a yellow background instead. Since CSPT classifies the fingerprints of each recorded pixel, the effect of the dithering is to increase the cluster radius in the principal component space. This is reflected by the overlapping of clouds and is correlated to the values of the quality indices q1, q2, q3 and q4.
From these indices, q1 is the most relevant, but does not preclude that correct matches can still occur by chance for values close to 1. Representative values of the indices (median + inter-quartile range of the indices) are collected in Table 1. The larger the values of typical q1s the larger risk of misclassification for that parameter. The other indices can thus be used to corroborate the presumption of unstable detection. In all cases wide distributions and outliers are elements of warning. q2 measures the overlapping with the closer reference and good determinations are associated with large q2s (a perfect coincidence with the reference corresponds to q2 = ∞). On the other hand, q3 is the overlapping to the closest mismatch. q3 values larger than 1 do not necessarily indicate a misclassification but a large dispersion of the sample or reference clusters. A stronger condition to be satisfied is q3 < q2.
| Representative quality indices | ||||||||
|---|---|---|---|---|---|---|---|---|
| Index | Hemoglobin | Blood | Protein | Leucocytes | Ketones | Glucose | pH | |
| Fingerprint | q 1 | 0.3319 | 0.8863 | 0.4775 | 0.2423 | 0.2335 | 0.3317 | 0.2504 |
| q 2 | 2.1212 | 10.1282 | 3.8974 | 4.4345 | 6.3282 | 4.1077 | 3.2556 | |
| q 3 | 0.4769 | 4.6054 | 1.2571 | 0.7992 | 0.3576 | 0.9514 | 0.5246 | |
| q 4 | 0.7353 | 3.2631 | 1.1885 | 0.6261 | 0.3030 | 1.3764 | 0.4843 | |
| 1 color | q 1 | 0.4033 | 0.4021 | 0.5549 | 0.5193 | 0.4732 | 0.6705 | 0.3964 |
The index q4 gives a qualification of the references, and q4 > 1 indicates overlapping clusters and potentially problematic parameters. From the indicative values collected in Table 1, q4 provides a good guide for assessing the three most compromised parameters in these experiments. Eventually, this analysis can be automatically implemented using statistical learning methods.14
In practice the blood and hemoglobin indicators compete for the determination of the erythrocyte concentration. The observation of individually separated green dots on the test paper points to the presence of intact erythrocytes whereas a homogeneous green color indicates the presence of free hemoglobin or lysed erythrocytes. Thus, the dispersion of the principal component clusters could eventually be used to determine the source of the response.
As indicated in the experimental section, in order to estimate the variability of the determinations, the complete set of pixels composing each ROI has been classified, by contrast with the average reflectance of each sensing patch that is typically evaluated.
It must be noted that only two ranges of two indicators have shown wrong evaluations, mostly due to processing conditions that can be refined or color degradation in the print outs. Even if these errors were not solved the rest of the categories and ranges of the tests remain valid not limiting its overall use, especially considering that CSPT is not a dedicated device.
The characteristics of the present assay (size of the patches, contrast of the indicators, and design suitable for visual inspection), makes it a favorable candidate for direct color evaluation by contrast with the use of CSPT fingerprints. Accordingly, the same samples were evaluated by their rgb-colors when acquired under white screen illumination in the same platform and using the same settings.
In this evaluation the average intensity of each sample ROI produces a point in the rgb-space. The rest of the procedure consists of measuring Euclidean distances in this color space. The result is the detection of 60 misclassifications (corresponding to different ranges of the indicators) in 580 classifications, which gives 10.34% of wrong determinations, ∼4.6 times worse than using CSPT fingerprints with a non-optimum sequence. Also the q1s are in general larger, with wider distributions and more frequent presence of outliers (Fig. 4c) that suggest a less reliable result. The values of the indices are also collected in Table 1.
Considering the CSPT fingerprints, other alternatives are feasible to enhance the present classification, such as adapting the illuminating colors to maximize a particular target,15 nevertheless, for practical applications it is also important to minimize the illuminating sequence in order to keep the measurement time short.
In principle, the illuminating colors are just linear combinations of three screen spectral radiances, and only three colors should be enough to reconstruct any spectra in about 9 points (considering the unfolding in the three channels of the web camera), however, in practice the classification of sets of color samples depends on the choice of the illuminating colors, which are not necessarily the same for a different set of samples. The selection of an optimum illuminating sequence for several sets of color indicators is not trivial, and has been solved ad hoc, by means of longer sequences that despite the redundancy have better chances to compensate for the ignored optimum conditions.
Even with a non-optimum illuminating sequence, the present study shows an appropriate performance of the CSPT evaluation for the intended use. In particular, considering measuring strategies with assays containing embedded references, it is possible to simplify the procedure and most importantly to make the classification more independent of the measuring platform. Under these conditions, an imaging technique able to easily adapt to any assay layout, operating on familiar and highly available hardware, demonstrates captivating possibilities for primary care or home testing applications.
Conclusions
An effective strategy for using CSPT platforms for the evaluation of colorimetric assays has been demonstrated. This approach compensates several instrumental weaknesses by exploiting the inherent computational power of the setup and by measuring samples and references simultaneously. In this way, the influence of the CSPT platform in the classification is minimized, making it more robust to random fluctuations in the instrument and allowing a simpler use, free of additional calibrating measurements.The tested performance shows a global 97.2% accuracy, but the observed errors can be traced back to their causes and eventually prevented. Quality indices correlated with possible misclassifications have been identified as well as indicative ranges suitable for safe determinations.
References
- D. Filippini, S. P. S. Svensson and I. Lundström, Chem. Commun., 2003, 2, 240–241 RSC.
- D. Filippini, T. P. M. Andersson, S. P. S. Svensson and I. Lundström, Biosens. Bioelectron., 2003, 19, 35–41 CrossRef CAS.
- A. Suska, D. Filippini, T. Andersson and I. Lundström, Biosens. Bioelectron., 2005, 21, 727–734 CrossRef CAS.
- K. P. Nilsson and O. Inganas, Nat. Mater., 2003, 2, 419–424 CrossRef CAS.
- D. Filippini, P. Åsberg, P. Nilsson, O. Inganäs and I. Lundström, Sens. Actuators, B, 2005 Search PubMed in press.
- D. Filippini, G. Comina and I. Lundström, Sens. Actuators, B, 2005, 107, 580–586 CrossRef.
- D. Filippini and I. Lundström, Anal. Chim. Acta, 2004, 512, 239–246.
- F. H. Imai and R. S. Berns, Spectral estimation using trichromatic digital cameras, in Proceedings of the International Symposium on Multispectral Imaging and Color Reproduction for Digital Archives, Chiba University, Chiba, Japan, 1999, pp. 42–49 Search PubMed.
- S. Westland, J. Shaw and H. Owens, Sens. Rev., 2000, 20, 50–55 CrossRef.
- G. Wyszecki and W. Stiles, Color Science: Concepts and Methods, Quantitative Data and Formulae, Wiley, New York, 1982 Search PubMed.
- R. Johnson and D. Wichern, Applied multivariate statistical analysis, Pearson Education Ltd, Upper Saddle River, NY, 2002 Search PubMed.
- R. S. Berns, J. Imaging Sci. Technol., 2001, 45, 373–383 Search PubMed.
- J. Y. Hardeberg, F. Schmitt, H. Brettelm, J. Crettez and H. Maitre, Multispectral image acquisition and simulation of illuminant changes in Colour Imaging: Vision and Technology, ed L. W. MacDonald and R. Luo, Wiley, Chichester, UK, 1999 Search PubMed.
- T. Hastie, R. Tibshirani and J. H. Friedman, The elements of statistical learning: Data mining, inference, and prediction, Springer Verlag, New York, 2001 Search PubMed.
- D. Filippini and I. Lundström, Appl. Phys. Lett., 2005, 86, 84101_1-3.
| This journal is © The Royal Society of Chemistry 2006 |
