AFM study of cationically charged polymer brushes: switching between soft and hard matter
Tamer Farhan, Omar Azzaroni and Wilhelm T. S. Huck*
Melville Laboratory for Polymer Synthesis, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge, UK CB2 1EW. E-mail: wtsh2@cam.ac.uk; Fax: (+44) 1223 334866
First published on 20th April 2005
Abstract
AFM studies on cationic polymer brushes in water (a good solvent) show that brushes in an extended conformation can be easily deformed or indented by an AFM tip. Experiments on polymer brushes in a more collapsed conformation, using methanol–water as a ‘poor solvent’ environment, show similar properties. Conversely, this ‘soft’ behaviour is dramatically different in the presence of electrolytes containing anions that are strongly coordinated to cationic groups of the polymer brush. Our initial studies in electrolyte solutions at different concentrations show that these brushes become so rigid that they cannot be indented or deformed by the AFM tip, even at high loads.
Surface-tethered polymer chains, usually described as polymer brushes, have generated considerable interest for their use in controlling the interfacial properties of surfaces.1,2 Recent advances in the ‘grafting from’ approach have paved the way for preparing brush films with controllable thickness ranging from a few nm to several 100s of nm, as well as the preparation of block copolymer brushes of almost any composition. Of particular interest in this study are surface-tethered polyelectrolyte chains:3,4 positively or negatively charged polymer chains that undergo a physical transition when exposed to a variety of different environmental stimuli, such as a change in pH, solvent or salt conditions, depending on the chemical functionality of the polymer.5 These properties could be exploited in the development of ‘smart’ surfaces and nanoactuators.6,7 One key feature related to the nanoscale properties of these charged systems that have scarcely been explored concerns the stiffness of polymer brushes in different environments. This study looks at the properties of the cationically charged polyelectrolyte system of poly[2-(methacryloyloxy)ethyl trimethylammonium chloride] (PMETAC) brushes grown via surface-initiated aqueous Atom Transfer Radical Polymerization (ATRP).8 The work presented here is based on tapping-mode AFM characterisation of these brushes to probe the responsive nature of the polyelectrolyte chains and the effect of counterion exchange on the physical properties of the brushes.
The synthesis of surface-tethered PMETAC was carried out using a combination of micro-contact printing (µCP)9 and ATRP to create µm-patterned brushes from gold surfaces. Initially, a patterned self-assembled monolayer (SAM) of a thiol-ATRP initiator was deposited onto a gold surface using an elastomeric PDMS stamp, and subsequently backfilled with an inert methyl-terminated thiol SAM (undecanethiol). Under a N2 atmosphere, these µm-patterned initiator surfaces were immersed in an ATRP mixture comprising of [METAC] : [CuICl] : [CuIICl2] : [Bipy] = 100 : 2 : 5 : 0.1 and 4 : 1 methanol : water and left to polymerize at room temperature. After removal from the reaction mixture the surfaces were washed with Milli-Q water and dried under N2.
Fig. 1 represents a TM-AFM image of a 4 × 2 µm patterned PMETAC brush from gold that was left for 3 h in the polymerization bath giving rise to approximately 20 nm high brush features. In this case (Fig. 1a) the image is that of a PMETAC brush in a ‘dry’ state directly after polymerization. It should be noted that these polymers are very hygroscopic and could hence be slightly swollen. By immersing the brushes in water, the chloride counterions are allowed to dissociate. This dissociation, within a “good solvent”, causes electrostatic repulsions to occur between the cationic charges on neighbouring polymer chains, and in addition to solvation effects, forces them to swell into a more extended conformation.10 Care was taken not to indent the AFM tip into the brush film and it should be noted that the actual height can deviate slightly from the one measured by AFM due to long range interactions between the tip11 and the charged surface.12 It must be noted that no significant differences were observed when the AFM experiments were repeated using tips modified with octadecyltrichlorosilane monolayers. This fact indicates that the measurements are not seriously affected by electrostatic interactions between the tip and the sample.13 The TM-AFM image in Fig. 1b clearly show the responsive effect of PMETAC when exposed to a ‘good’ solvent, in this case Milli-Q water. In the dry state, AFM analysis shows brush height features of approximately 20 nm and in its swollen state features of up to 60 nm are observed, hence a three-fold increase in brush thickness.
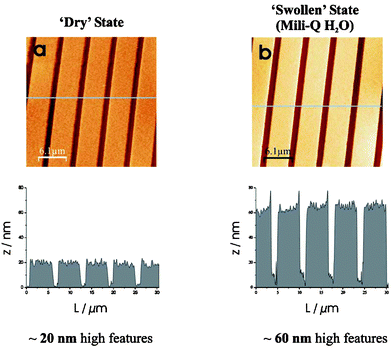 | ||
| Fig. 1 TM-AFM images of the same 4 × 2 µm patterned PMETAC brushes from Au substrates (a) in air, with average brush height features of 20 nm, and (b) in Milli-Q water, described as the swollen state with features of up to 60 nm. | ||
Subsequently, the same PMETAC brushes were scanned in a ‘poor’ solvent (a 1 : 1 mixture of methanol : water; Fig. 2) in which the brushes are expected to be in a partial, not fully, collapsed state. With the AFM tip scanning at the lightest load, at an amplitude setpoint of 7 V (where the tip is just in contact with the sample) features heights around 45 nm are observed as opposed to 60 nm in water. By changing the load (amplitude setpoint) of the AFM tip during the scan, a dramatic difference in the height features was seen. During the same scan, the tip load was increased by halving the amplitude setpoint to 3.5 V, resulting in far thinner features of approximately 3–5 nm. Although it could be possible to ‘squeeze’ all remaining solvent out of the brush layer, we assign this large change in height features to indentation of the AFM tip through the polymer brush during scanning, rather than compression of the polymer film. Based on this observation (i.e. facile indentation under moderate loads of the AFM tip) we classify these brushes as ‘soft’.14
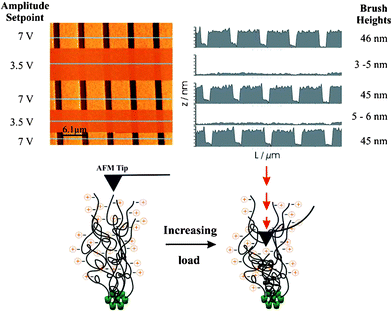 | ||
| Fig. 2 (top) 31 × 31 µm2 TM-AFM image of swollen 4 × 2 µm patterned PMETAC brushes (shown in Fig. 1) in 1 : 1 methanol : water with a switching amplitude setpoint of 7 V and 3.5 V. The corresponding line profiles show that the increasing load has a dramatic effect on the topographic heights of the brushes, from ∼45 nm at 7 V to ∼5 nm at 3.5 V. | ||
By placing the charged polymer brushes in electrolyte solutions, the cationic charges along the polymer chains become effectively screened and electrically neutral, thereby reducing any electrostatic repulsion between neighbouring chains, leading to a ‘collapsed’ state relative to that in pure water.15,16Fig. 3 represents AFM data of the same PMETAC brushes in a solution of 3 mM LiClO4. The line scans show feature heights around 25 nm, indicating significant collapse of the polymer brushes. As before the AFM tip load was increased in order to observe any difference in topographic features. However, no indentation or deformation of the features was observed when decreasing the amplitude setpoint. Instead, the topographic features remained unchanged at heights of approximately 25 nm when halving the amplitude setpoint from 7 V to 3.5 V. Similar AFM experiments performed under 1 M NaCl solutions showed that even in the fully collapsed state (brush height around 20 nm) the Cl−-coordinated METAC brushes can be indented: when the setpoint was reduced from 7 V to 2 V, a 10 nm tip indentation was observed (brush height compressed to around 10 nm), while the ClO4−-METAC brushes remain completely unchanged, even when subjected to high loads at 2 V. These results are dramatically different from the properties seen for Cl−-PMETAC brushes and suggests a change in the physical properties of the brushes from soft to hard matter. We believe that the Cl− anions are exchanged for ClO4− ions in a LiClO4 solution and these anions permeate into the brush structure and bind electrostatically to the cationically charged quaternary ammonium groups along the polymer chains, leading to a rigid structure. This dramatic change in the physicochemical properties of the polyelectrolyte brush can be explained by the interactions of the two different anions with the polymer chain pendant groups. The ClO4− ions are larger and more highly polarizable, in addition to being much less hydrated, than Cl− anions allowing them to interact much more strongly with the quaternary ammonium groups through ion pairing interactions, hence the change from soft to hard behaviour.
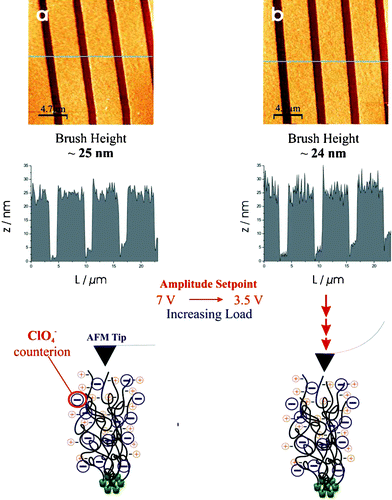 | ||
| Fig. 3 TM-AFM images of rigid PMETAC brushes (again the same samples shown in Fig. 1 and 2) in 3 mM LiClO4. Top left image (a) is a 31 × 31 µm2 scan of 4 × 2 µm patterned PMETAC brushes on Au in 3 mM LiClO4 with an amplitude setpoint of 7 V and ∼25 nm high features. Right image (b) also represents a 31 × 31 µm2 scan of the same PMETAC brush but at a higher load, i.e. an amplitude setpoint of 3.5 V, which seems to have no impact on the topographic features giving rise to similar height brushes of ∼24 nm. | ||
This ‘switch’ between ‘soft’ and ‘hard’ matter is completely reversible. Immersion the ClO4−-METAC brushes in a 1 M NaCl solution and subsequent washing with Milli-Q water, led to full restoration of the soft behaviour shown in Fig. 2. This suggests that the exchange of counterions within the brushes is easily achieved through simply immersing the sample in the relevant solution,17 such that an excess of Cl− ions (from 1 M NaCl) displaces the equilibrium ClO4−/quaternary ammonium resulting in the original chemical/ionic (Cl−-coordinated PMETAC) composition. In addition, washing with Milli-Q water removes any NaCl excess and thus PMETAC brushes convert to their ‘swollen’ state.
TM-AFM studies on PMETAC brushes in water (a “good” solvent) show that charged polymer brushes in an extended conformation can be easily deformed or indented by an AFM tip. Similar properties are observed when the same experiments are performed on the polymer brushes in a slightly more collapsed conformation, using methanol–water as a ‘poor solvent’ environment. Conversely, this ‘soft’ behaviour is dramatically different in the presence of electrolytes containing anions that are strongly coordinated to cationic groups of the polymer brush. Our initial studies in electrolyte solutions at different concentrations show that these brushes become so rigid that they cannot be indented or deformed by the AFM tip, even at high loads. We are currently further investigating the response of polyelectrolyte brushes to different salts and the precise mechanical properties of ‘hard’ polyelectrolyte brush films.
Acknowledgements
Financial support from the EPSRC (GR/T11555/01) and DuPont Teijin Films is gratefully acknowledged. O.A. acknowledges a Marie Curie Research Fellowship.References
- Polymer Brushes, eds. R. C. Advincula, W. J. Brittain, K. C. Caster and J. Rühe, Wiley-VCH, Weinheim, 2004 Search PubMed.
- S. Edmondson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 2004, 33, 14 RSC.
- R. A. L. Jones and R. W. Richards, Polymers at Surfaces and Interfaces, Cambridge University Press, Cambridge, 1999 Search PubMed.
- J. Rühe, M. Ballauff, M. Biesalski, P. Dziezok, F. Grohn, D. Johannsmann, N. Houbenov, N. Hugenberg, R. Konradi, S. Minko, M. Motornov, R. R. Netz, M. Schmidt, C. Seidel, M. Stamm, T. Stephan, D. Usov and H. N. Zhang, Adv. Polym. Sci., 2004, 165, 79.
- A. Halperin, M. Tirrell and T. P. Lodge, Adv. Polym. Sci, 1992, 100, 31 CAS.
- N. Nath and A. Chilkoti, Adv. Mater., 2002, 14, 1243 CrossRef CAS.
- R. A. L. Jones, Soft Machines, Oxford University Press, Oxford, 2004 Search PubMed.
- V. L. Osborne, D. M. Jones and W. T. S. Huck, Chem. Commun., 2002, 1838 RSC.
- Y. Xia and G. M. Whitesides, Langmuir, 1997, 13, 2059 CrossRef CAS.
- E. B. Zhulina, O. V. Borisov and T. M. Birshtein, Macromolecules, 1999, 32, 8189–8196 CrossRef CAS.
- For AFM experiments we have used Type II MACLevers (Molecular Imaging) silicon cantilevers. The tip has the shape of a pyramid with a polygon as the base. Tip radius is typically less than 10 nm and its height is ∼15 µm.
- S. M. Lindsay, in Scanning Probe Microscopy: Theory, Techniques and Applications, ed. D. Bonnell, VCH-Wiley, New York, 2000, ch.9, p. 296 Search PubMed.
- D. J. Muller and A. Engel, Biophys. J., 1997, 73, 1633 CrossRef CAS.
- Y. Jiao and T. E. Scheffer, Langmuir, 2004, 20, 10038 CrossRef CAS.
- E. B. Zhulina, J. Klein Wolterink and O. V. Borinrov, Macromolecules, 2000, 33, 4945 CrossRef CAS.
- M. Bielsaski, D. Johannsmann and J. Ruhe, J.Chem.Phys., 2004, 120, 8807 CrossRef.
- R. Konradi and J. Ruhe, Macromolecules, 2004, 37, 6954 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
