On water repellency
Mathilde
Callies
and
David
Quéré
*
Laboratoire de Physique de la Matière Condensée, URA 7125 du CNRS, Collège de France, 75231 Paris Cedex 05, France. E-mail: david.quere@college-de-france.fr
First published on 22nd April 2005
Abstract
Water-repellency is a property of some materials, either natural or synthetic, which makes water hardly stick to them: drops roll very easily off these solids, and bounce back upon impacting them. Here we discuss recent advances in this field, which has been particularly lively in recent years. We first examine the physical causes for this effect. Then we discuss the loss of adherence of the drops in such a state, and stress their remarkable dynamic behaviour. We finally suggest several remaining challenges in the field.
 Mathilde Callies | Mathilde Callies carried out her PhD on superhydrophobic materials. Using microfabrication techniques, she achieved solids decorated with well-controlled micropillars, and studied quantitatively the behaviour of these materials when exposed to water (drops, dew, rain). |
 David Quéré | David Quéré is a Director of Research at CNRS. His main research interests (often inspired by industrial questions) are in the field of fluid interfaces, including coating, wetting and non-wetting, wicking, morphogenesis and singularities. |
1. Introduction
Let us start with a kitchen experiment: take a piece of glass, and pass it through the yellow part of the flame of a match. The glass quickly darkens, owing to the deposition of soot. Wait till the temperature gets uniform, and deposit a water drop on this substrate. Though water partially spreads on glass (wetting may even be complete if the glass is perfectly clean), the globule adopts on soot the shape of a pearl, which rolls off very easily (and in the process becomes coated by the soot particles). If the drop impacts the soot layer, it bounces back, further evidence of water repellency.Water repellency was discovered very early: C. V. Boys for example noticed that water deposited on a layer of lycopodium rolls itself up into perfect little balls.1 However, it mainly attracted attention in the late nineties, following two achievements: 1) a systematic study of the water repellency of plants, by two German botanists, Barthlott and Neinhuis, who emphasized the role of micro-textures on the surface of the leaves to promote such an effect;2 2) the making of fractal hydrophobic surfaces by Kao engineers, in Japan, who reported contact angles as high as 174°.3
We do not intend here to provide a comprehensive report on water repellency, but rather to summarize some recent developments on this topic (with the usual subjectivity inherent to this kind of anthology). Then, we discuss what appears to us as promising questions in the field.
2. A few (more or less) recent advances
2.1. The two states of superhydrophobicity
Water repellency on solid materials has not only a chemical origin: these materials are of course hydrophobic, but they are also microtextured. Hence, there are two possible origins for this effect: either the liquid follows the solid surface, or it leaves air inside the texture (Fig. 1).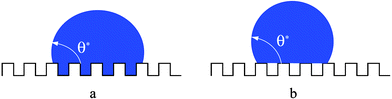 | ||
| Fig. 1 The two superhydrophobic states: in the Wenzel state (a), the liquid follows the solid surface. In the Cassie state (b), it only contacts the top of the asperities, leaving air below. | ||
In the first case (Wenzel scenario), the increase of the surface area (due to the presence of the texture) amplifies the natural hydrophobicity of the material.4 Thus the key parameter controlling the contact angle θ* on this surface is the solid roughness r, defined as the ratio between the true surface area over the apparent one (r is a number larger than unity). In other words, the solid surface energy can be seen as multiplied by the factor r, which yields:
| cos θ* = r cos θ | (1) |
Eqn. (1) predicts that the contact angle on a hydrophobic material (θ > 90°) will increase with the roughness (θ* > θ). This looks like a simple and attractive solution for inducing superhydrophobicity: the rougher the material, the higher the contact angle. However, this is not that simple, for two reasons: firstly, contact angles generally spread in quite a large interval, contrasting with eqn. (1) which predicts a unique angle. This interval, often referred to as the contact angle hysteresis, is responsible for the sticking of drops, an effect in contradiction with water repellency. In a Wenzel state, the contact angle hysteresis will be very large: trying to remove a liquid makes it contact itself (owing to the fraction left in the textures), which yields a low “receding” contact angle—values as low as 40° were reported, making this state hydrophilic-like in the receding stage.5 The second reason which makes it impossible to reach high values of θ*, as expected from eqn. (1) for r large and θ > 90°, can be guessed quite easily: for very rough hydrophobic materials, the energy stored for following the solid surface is much larger than the energy associated with the air pockets sketched in Fig. 1b.5–8
In this state (first suggested by Cassie and Baxter), the liquid only contacts the solid through the top of the asperities, on a fraction that we denote as ϕs.9 If only air were present between the solid and the liquid (as for a water drop on a very hot plate), the “contact angle” would be 180°: the smaller ϕs, the closer to this extreme situation, and thus the higher the hydrophobicity. More precisely, the contact angle θ* of such a “fakir” drop (Fig. 1b) is an average between the angles on the solid (of cosine cosθ), and on the air (of cosine −1), respectively weighed by the fractions ϕs and 1 − ϕs, which yields:
| cos θ* = − 1 + ϕs (cos θ + 1) | (2) |
For θ = 110° and ϕs = 10%, we find that θ* is about 160°. In this case, 90% of the drop base contacts air! This makes it understandable that the corresponding hysteresis is observed to be very low (typically around 5 to 10°), as first reported by Johnson and Dettre:10 the liquid has very little interactions with its substrate. Hence, this state will be the (only) repellent one, since it achieves both a large contact angle and a small hysteresis (this can be observed further, in Fig. 3).
θ* monotonously increases as ϕs decreases, suggesting that ϕs should be made as small as possible. But reducing ϕs also makes the roughness decrease, so that we reach the critical roughness rc below which the Wenzel state is favoured.6,7 The quantity rc is easily deduced from the intersection of eqn. (1) and (2), and is found to be (ϕs − 1)/cos θ + ϕs, which is generally close to −1/cosθ (since we will often have: ϕs ≪ 1). For θ = 120° (a high value for the Young angle, obtained on fluorinated substrates), the fakir state will thus be favoured for roughness factors larger than 2. Conversely, Öner and McCarthy experimentally observed that below a critical density of defects (i.e. below a critical roughness), there is indeed a serious deterioration of the water-repellent properties.11
2.2. Examples of water-repellent materials
More than 200 plants, and many insects are (at least partially) water-repellent (to protect themselves against water). The morphology of the plant surfaces was studied comprehensively by Barthlott and Neinhuis, and many different designs were reported.2 However, it seems that the most efficient ones (in term of contact angle) consist of two hierarchical structures: typically bumps of about 10 µm, and submicronic microfibers. Such structures decorate for example the surface of the lotus leaf, the archetype of a natural water-repellent surface.These surfaces are thus very rough, which favours robust fakir states.12 Extending the idea of hierarchical structures naturally leads to fractal surfaces, which were achieved by the Kao group, and were indeed found to be superhydrophobic.3 Contact angles as high as 174° were measured on these surfaces, with a corresponding hysteresis smaller than 5°—yielding amazing non-stick properties for water drops. Many techniques for achieving disordered materials were proposed since, as reported by Nakajima et al.13
On the other hand, microfabrication techniques recently promoted much more regular structures such as pillars (as sketched in Fig. 1), and it was shown that such textures can also induce superhydrophobicity.11,14Fig. 2 shows a millimetric water drop sitting on such a substrate, whose colours originate from the regularity of the structures. This was also observed for spherical regularly spaced microbeads,15 and it suggests more generally the possibility of taking advantage of the microtextures for inducing other properties than the water-repellency alone.
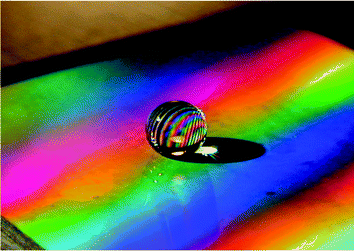 | ||
| Fig. 2 Millimetric water drop on a hydrophobic surface textured with regularly-spaced micropillars. The texture also induces structural colours. | ||
Since pillar surfaces can be of low roughness, the Wenzel state might be preferred. However, it turns out that very often, fakir drops are observed, in spite of a higher surface energy: the Cassie state can be metastable.5,8,16,17 This is observed in Fig. 3, where the two states are coexisting on the same substrate, for which the density of pillars (of diameter 2 µm and height 12 µm) is about 1%. Two drops of same volume were deposited, but the one on the right was (gently) pressed, inducing a Wenzel state of smaller contact angle, and for which we no longer observe the light passing below the drop. Moreover, waiting a few minutes makes the contact angle change (because of evaporation, the angle becomes the receding one), confirming a fakir state of comparable receding angle for the left drop and a sticky Wenzel state of very large hysteresis for the right drop.
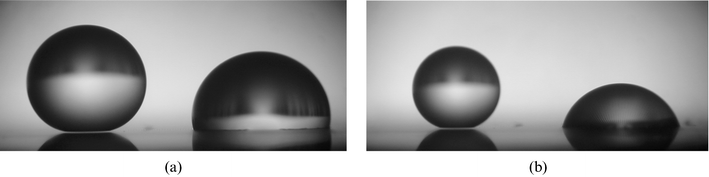 | ||
| Fig. 3 Millimetric water drops (of the same volume) deposited on a superhydrophobic substrate consisting of dilute pillars (ϕs = 0.01). (a) The right drop has been pressed, which induced a Wenzel state, characterized by a smaller angle (the roughness is very low, and equal to 1.1). The light passes below the left drop, indicating a Cassie state. (b) Ten minutes later, the drop volumes have decreased, owing to evaporation, and angles became receding ones. The difference of hysteresis between both states is clearly visible: the Wenzel drop even became hydrophilic! | ||
Increasing the height of the pillars will favour the Cassie state, and a particular case of superhydrophobic material worth mentioning is fibrous solids. Different solutions were proposed recently, with either polymer or carbon nanofibers;18 in the latter case, it was shown that regular arrays of vertical nanotubes coated with fluorinated molecules can be grown on solid substrates, giving rise to water-repellent nanograss.19
2.3. Switchable wettability
A wetting liquid might behave very differently, when contacting a textured solid. It can impregnate the texture, so that it finally contacts a solid filled with liquid, yielding superhydrophilic behaviour. Research on materials of tunable wettability (acting on drops through an external field, such as temperature, light, pH, electric field) started long ago, but it found here an interesting field of application because of the enhancement of wettability due to the presence of textures. Several very nice achievements can be quoted, classified by the field controlling the transition.Light can modify the solid surface energy: photocatalytic oxides can be made hydrophilic by UV exposure, and materials decorated with ZnO rods were indeed observed by the group of Jiang to be either superhydrophilic or superhydrophobic, after exposure to light or dark, respectively.20 Similarly, using changes of conformation of polymers owing to temperature allowed the same group to realize a textured material on which the contact angle could be tuned between (about) 0° and 160° for an increase of temperature of a few degrees (around 35 °C).21 Finally, Krupenkin et al. proposed to tune quite instantaneously the wettability of textured materials using an electric field.22 The Lippman law relates the change of contact angle to the voltage applied on a drop. It is generally a modest effect, which was observed to be amplified dramatically in the presence of textures: for liquids such as water, a transition between a fakir and a Wenzel (stuck) drop could be induced by applying about 20 V; for oils, a transition between partial wetting and nearly complete wetting (revealing an impregnation of the texture) was obtained with the application of about 50 V.
In all these cases, the authors stressed the “reversibility” of the transitions, meaning that the material could recover its initial properties after a given treatment (for example, leaving the ZnO sample for a few days in dark allowed it to be superhydrophobic again). However, this does not (yet) correspond to what we generally mean by a switcher: the liquid impregnation in the texture is irreversible, in the sense that we do not know how to make it escape from these traps—the achievement of a genuine switcher, permitting a reversible and rapid transition between wetting and drying states, remains a challenge.
2.4. Dynamics
The practical interest of superhydrophobic materials becomes quite obvious when looking at the dynamic properties they generate for drops. The first remarkable property is a very low degree of sticking: unlike on usual solids, millimetric water drops move whatever the solid slope.23 To give orders of magnitude, a drop of radius R = 1.5 mm will move on a common flat hydrophobic solid (such as Teflon) if the solid is tilted by about 10 to 30°, while this angle can be reduced to a value smaller than 1° for a water-repellent material. This effect is much more sensitive for a drop squeezed in a channel, since the tilting angle drops from about 90° to about 1°, when texturing a Teflon surface.24 This very strong decrease of the adhesion results from the conjunction of a very high contact angle (which reduces the solid/liquid surface area) and a very small hysteresis (in the fakir regime).Once the drops move, they are observed to reach amazingly large velocities, which raises the stimulating question of a possible slip. A liquid flowing on a solid is postulated not to slip at the interface between both phases, but a microscopic slip might exist if the solid is hydrophobic. This effect can be dramatically amplified if the hydrophobic solid is textured, provided that air is trapped in the textures (Cassie regime). This was first proposed numerically,25 and confirmed in a beautiful series of experiments by Ou et al. who measured the pressure drop Δp necessary to drive water at a prescribed rate in a channel of thickness of about 100 µm.26 Comparing Δp for smooth and textured hydrophobic surfaces showed a decrease of this quantity for textured surfaces (by about 10%), this reduction increasing up to 40% when diluting pillars. Of course, as discussed above, we expect that too high dilutions of pillars provoke a Wenzel transition, in which case the slippage should be mainly lost.25
Another way to quantify the slippage consists of introducing a so-called slip length. This quantity is the distance below the solid/liquid interface for which the velocity vanishes, as extrapolating the velocity profile. This length is microscopic on most solids and may be of the order of 10 nm on hydrophobic flat solids. But in the experiments of Ou et al., slip lengths were found to be in the range of 10 to 20 µm,26 showing the relevance of these materials in the context of microfluidics, where the size of the channels can be of the same order.
As a last dynamical characteristics of water-repellent surfaces, let us quote the one which justifies this denomination: a drop hitting such a material just bounces off (Fig. 4), as first reported in the context of pesticide treatments (in which case bouncing is detrimental, since it scatters the pesticide far from its target).27 However, in many other applications such as waterproof clothes, hydro-protected concrete, or windshields, this effect is of obvious interest, since it preserves the dryness of a solid despite rain.
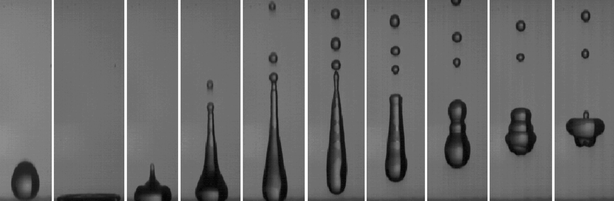 | ||
| Fig. 4 Millimetric water drop bouncing off a superhydrophobic material. The impact velocity is around 1 m s−1, which makes the kinetic energy about 20 times larger than the surface energy: hence, the strong deformations at impact. (Courtesy of Denis Richard and Christophe Clanet.) | ||
The rebound is made possible by the small dissipation as the drop impacts the solid: because of the high contact angle, viscous dissipation close to the moving contact line (which usually is the primary cause of viscous loss) becomes nearly negligible. As very clearly observed in Fig. 4, a drop impacting a superhydrophobic material deforms, and the deformation is all the larger since the impact velocity is high. However, it keeps its contact angle very high, allowing it to store its kinetic energy in surface deformation, and thus to bounce back.28 A drop thus behaves as a spring, whose stiffness is the surface tension of the liquid.
3. Perspectives and questions
3.1. Quantifying the superhydrophobicity
In most cases, the characterization of a water-repellent surface is limited to the measurement of a contact angle. This is obviously not sufficient to distinguish different materials, and to decide which one is the “best” for a given application. Here we first stress a difficulty related to the measurement itself, and discuss other possible tests, in order to discriminate different substrates.As emphasized above, a unique contact angle does not characterize a given solid—the observed static contact angles lie in an interval of amplitude Δθ called the contact angle hysteresis, whose lower and higher bounds are the receding and advancing angles θr and θa, respectively (θa = θr + Δθ). It is important to measure both these angles for two reasons: firstly, because it generally allows one to conclude if a drop is in a Wenzel or Cassie state (of very different adhesion properties);5 secondly, because even in a Cassie state (eqn. 2), we expect the hysteresis to differ according to the materials: the smaller ϕs, the smaller the hysteresis and thus the less sticky the surface. Systematic experimental studies of the hysteresis as a function of the texture would be welcome: a Cassie surface is an ideal substrate for studying hysteresis, because it realizes a perfect substrate (air), with only a few pinning “defects” (the top of the pillars), of controllable density. The discussion on hysteresis is also important, because it clarifies the status of eqn. (1) and (2): the angles predicted by these laws are the ones which minimize the free energy of the drop, but they generally cannot be measured: an observation will produce anything between θr and θa, so that these equations cannot be directly checked. The only favorable limit is a Cassie regime of low Δθ, for which all the angles are comparable (θ* ≈ θr ≈ θa).
It should also be emphasized that the measurement of a contact angle is not an easy task in the limit of very large angles. The drop is slightly flattened by gravity, so that even in a purely non-wetting situation (θ = 180°) as for drops on very hot plates (Leidenfrost effect), usual techniques of observation may suggest a contact angle smaller than 180°. Many authors claim that they could reach angles as high as 160 to 175° with a precision smaller than 2 or 3°, but this view seems to be optimistic, and there is not today a commonly accepted method for measuring such large angles with high precision. Such methods should be developed (as was done in the opposite limit of small contact angles, using interference techniques, for example), in order to allow a real and serious comparison between materials, for both the advancing and receding angles.
A complementary test is the maximum radius of the drop which can remain stuck on a material inclined by an angle α,23 as observed in Fig. 5. For water-repellent surfaces, this radius can be typically 10 times smaller (that is, 103 smaller for the drop volume!) than on a common surface. This test is practically useful (we need to know the ability of these surfaces to remove droplets), and it allows us to compare different surfaces in a straightforward way.
 | ||
| Fig. 5 Collection of polydisperse water drops on a textured surface (the bar indicates 5 mm). When tilting the surface by α = 90°, most of the drops roll off, but the ones of radius smaller than Rc remain stuck. The mobile drops stop upon reaching the non-textured zone (without colors), to which they can stick, indicating a much larger value of Rc in this zone. | ||
At equilibrium, the drop weight balances the sticking force. We consider that the rear and front halves of the drop meet the solid with a contact angle θr and θa, respectively, and we denote θ as the average angle (θ = (θa + θr)/2). At the threshold of entrainment, the sticking force is expressed as πRsinθγ (cosθr − cosθa), where Rsinθ is the radius of the base of the drop (supposed larger than the gravitational base, due to the drop weight). Expanding the formula in the Cassie limit (Δθ ≪ θ, and ε = π − θ ≪ 1), we find that the critical radius Rc above which a drop rolls off is given by:
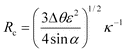 | (3) |
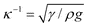 ); κ−1 is 2.7 mm for water. It has been often proposed that the critical tilt angle αc, above which the drop rolls off (for a given drop size R), should rather be considered. However we prefer a test on Rc, since the latter quantity can always be defined, unlike αc which may not exist if R if too small.
); κ−1 is 2.7 mm for water. It has been often proposed that the critical tilt angle αc, above which the drop rolls off (for a given drop size R), should rather be considered. However we prefer a test on Rc, since the latter quantity can always be defined, unlike αc which may not exist if R if too small.
Eqn. (3) suggests that the wetting parameter controlling the non-stick behavior of a fakir drop is the quantity σ = Δθε2, that we might call the sticking factor; σ should in general be an intrinsic property of a superhydrophobic material. It would be interesting to tabulate values of σ for natural and synthetic materials, for comparing them according to this criterion.
Other measurements also remain to be done. It was proved that pressing on a fakir drop possibly induces a Wenzel state, when the surface is not very rough (pillar-decorated materials).5 It seems important to be able to specify the critical pressure Δp* above which the transition occurs, which quantifies the robustness of the fakir state.7 This would also allow, from a more fundamental point of view, the specification of the energy barrier between both states.15,16 A small droplet exerts a Laplace pressure which increases with decreasing drop size, which might also make it fall in the Wenzel state below a critical radius. Fig. 6 shows that the state of a water droplet on a superhydrophobic material of low ϕs (same substrate as in Fig. 3) may indeed depend on its size: unlike the large fakir drop, a small drop (as produced by a spray) is in a Wenzel state.5 This was also observed for water drops evaporating on a superhydrophobic substrate of small defect density: below a critical size, the drop falls in the Wenzel state.29 The measurement of the critical radius R* (found for example in Fig. 6 to be 0.20 mm) would also be an interesting characteristic of a water-repellent surface, and the origin of this transition should be clarified.
 | ||
| Fig. 6 Two water drops on the structured surface described in Fig. 3, and their reflections. The state may depend on the size: the large drop is a fakir one, while the small one is in the Wenzel state. The bar indicates 1 mm. | ||
Another useful test concerns the ability of drops to bounce off. Although full rebounds occur at moderate impact velocities V, the situation is different at small and large V. Unlike very hot plates (for which we have σ = 0), there is a threshold velocity V* below which an impacting drop sticks.28 It would be useful to understand if V* is simply correlated with σ (the contact angle hysteresis being a natural cause for sticking). Conversely, only part of the drop bounces (partial rebound) at large V, because of a possible impalement in the texture which keeps it in. The transition between full and partial bouncing is quite clear-cut, so that the value of the velocity at which it occurs could be a useful parameter to know, in particular for applications related to rain exposure.
3.2. The choice of a texture
A more comprehensive characterization of superhydrophobic samples is necessary to compare them with each other. But it does not tell which design must be chosen for the surface, for a given application. Owing to the different processes of fabrication, a large panel of designs were produced or imagined,3,8,11–15,18–23,30–33 and natural superhydrophobic materials display the same diversity.2 This raises two questions: 1) How does one optimize a given design (e.g. pillars)? 2) Can special designs bring special properties?For the first question, and focusing on micropillars, we stressed that a “good” fakir state requires a high dilution of pillars (i.e. small ϕs) for increasing the contact angle (eqn. (2)) and reducing the hysteresis. But if the dilution is too high, the pillars cannot support the liquid which falls in, leading to a Wenzel state. Thus, there is an optimum density which minimizes ϕs, keeping the fakir regime robust—which remains to be determined, as a function of pillar sizes. Note also that the shape of the pillars can also influence the contact angle and hysteresis, as reported by Öner and McCarthy.11
The second question is more general and difficult. For example, many plants have two levels of texture, which enhances water-repellency.2 However, we do not know if this is just a simple effect of roughness,12,30 or if the second structure induces specific properties—such as anti-dew, for example, the largest scale being rather as anti-rain. Moreover, it would be interesting to understand if a third level would be likely to contribute as well, or if there is a sort of saturation of the effects—in other words, to which extent is it relevant to build fractal surfaces?3,12 On the other hand, we expect a loss of superhydrophobicity below some microscopic size: solids decorated with nanometric defects are not superhydrophobic. The minimum texture size which promotes water repellency is a question of obvious practical interest, in particular because textures significantly smaller than the wavelength of light allows the construction of transparent materials,31 but we do not yet know this minimum size, nor the mechanism provoking the loss of superhydrophobicity. We could for example think of van der Waals forces which act at scales smaller than 100 nm, and often favor solid/liquid contacts (and thus squeeze the air films), leading to non-desired Wenzel states. More trivially, the reduction of texture size makes the roughness decrease, which is unfavorable for water repellency.
A special pattern, namely micro- or nano-hairs, deserves a discussion. Such filaments are observed to decorate a few natural solids (such as the legs of water striders, or the leaf of Drosera) and have also been recently synthesized onto flat substrates, in both entangled and very ordered states.18,19 As mentioned by Otten and Herminghaus, hairy materials can very efficiently repel water: a deposited drop bends the fibers, whose stiffness prevents the contact with the substrate, promoting a fakir state.32 We could even imagine along this line to have a hydrophilic substrate beneath (allowing water vapor exchanges). Conversely, as also argued by Otten and Herminghaus, the fibers may themselves be hydrophilic (as they are on the leaves of a superhydrophobic plant, Lady's Mantle), because a fiber partially wetted by water has a tendency to make drops roll up on one side of the fiber, thus minimizing the solid/liquid contact.32
But these hairy materials might also have anti-dew properties. The case of forests of hydrophobic nanotubes seems particularly favorable:19 condensing a drop in this forest makes the surrounding “trees” bend, once the drop size becomes larger than the inter-tree distance (typically a few microns, in experiments). The deeper the drop, the larger the elastic force (nanotubes are particularly stiff)—from which we understand that the drop can be expelled from the inside, so that it eventually floats off the “canopy” of the forest, avoiding filling of the structures.
The latter example also emphasizes that the choice of texture may depend on the application. Let us cite a few examples. In some cases, it can be interesting to induce anisotropic wetting (in order to drive liquids in a preferred direction), and it was proved that anisotropic textures can lead to such behavior.14,23,32 Spontaneous motions might also be desired, for example for drying a particular spot on a sample: it would be interesting to look at the properties of solids decorated by micropillars of variable densities. The induced forces might be weak, but the adhesion is weak as well, so that we could expect motion from the region of low ϕs to the one of higher ϕs.
3.3. Practical applications
The last ensemble of questions concerns the practical use of water repellent surfaces. Potentially, the list of applications is impressive: waterproofing of clothes, concrete or paints, anti-rain windshields and window panes, materials of very low friction in water (boat or swimsuits coatings, plastics for microfluidics), etc. The list is even larger when considering the so-called “self-cleaning” properties attributed to these materials.2,34 This word is quite improper: these surfaces of course do not self-clean, but they are observed to be cleaner than usual ones. There are two reasons for that: firstly, this is due to a low surface energy (they are hydrophobic), so that less particles settle on them; secondly, a simple rain will wash them, taking with it the dust present at the surface, just because drops do not stick, and are easily removed (a drop which remains stuck will not carry the dust, and it will in addition concentrate it when evaporating). This is often called the lotus effect—but the efficiency of the transfer of contaminants on a moving drop remains to be quantified.In spite of all these potential applications, very few products were launched using water repellent surfaces. This is mainly due to the aging of these surfaces, which are generally fragile: the microtextures are easily destroyed by impacts, or even in some cases by simply rubbing them with a duster. In most cases, it remains a challenge to build strong microtextures, which are able to resist the different aggressions endured by the sample. A second issue concerns the contamination of these materials: although they are supposed to be self-cleaning, they will generally absorb oily substances which will condense and migrate down to the smallest scales of the texture. These oils can eventually fill the textures, from where it is extremely difficult to remove them, leading to an irreversible loss of the superhydrophobic properties. This (today) prevents the use of these materials for paints and other long-term coatings, and rather suggests temporary applications (such as cheap microfluidics devices which can be changed after a few weeks). It also suggests that other solutions might be imagined: for example, considering the issue of contamination as incurable, we could think of treatments making a surface temporarily superhydrophobic (in the spirit of silicon-based oils which are spread on windshields to improve the mobility of rain drops). Mixtures of hydrophobic particles and solvent might, for a good affinity of the particle for the substrate, build transient water repellent surfaces after solvent evaporation. A more sophisticated version of that is the case of evolving structures, for which the nanostructures constantly renew, at the speed at which they are damaged. In any case, it remains quite a challenge to build a permanent superhydrophobic surface!
4. Conclusions
The research on water repellency has been rapidly developing for about eight years. This topic is characteristic of Soft Matter: as in many other examples, it produces a state which looks ambiguous (a water drop behaves a little bit as if it were solid, standing as a marble and bouncing as a balloon); and the questions it raises are constantly at the frontier between fundamental and applied science. The main achievements concern the fabrication of the substrates, but a lot remains to be done to characterize these different materials and discriminate them from each other. This question of optimization might be the most important one from a fundamental point of view, but practical applications also require improvements: these materials are generally fragile, and their aging can be problematic. Many questions remain…Acknowledgements
It is a pleasure to thank Anne-Laure Biance, José Bico, Christophe Clanet, Aurélie Lafuma, Christian Marzolin, Ko Okumura and Denis Richard for invaluable contributions in this field, and Wilhelm Barthlott, Elisabeth Charlaix, Yong Chen, Stephan Herminghaus, Glen McHale, L. Mahadevan, Avi Marmur, Christoph Neinhuis, Neelesh Patankar and Uwe Thiele for many enlightening discussions.References
- C. V. Boys, Soap bubbles, Society for Promoting Christian Knowledge, London, 1902 Search PubMed
.
- C. Neinhuis and W. Barthlott, Ann. Botany, 1997, 79, 667–677 CrossRef
.
- T. Onda, S. Shibuichi, N. Satoh and K. Tsujii, Langmuir, 1996, 12, 2125–2127 CrossRef CAS
.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS
.
- A. Lafuma and D. Quéré, Nature Mater., 2003, 2, 457–460 CrossRef CAS
.
- J. Bico, U. Thiele and D. Quéré, Colloids Surf. A, 2002, 206, 41–46 CrossRef CAS
.
- N. Patankar, Langmuir, 2003, 19, 1249–1253 CrossRef CAS
.
- A. Marmur, Langmuir, 2003, 19, 8343–8348 CrossRef CAS
.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546–551 RSC
.
-
R. E. Johnson and R. H. Dettre, in Contact angle, Wettability and Adhesion, Advances in Chemistry Series, American Chemical Society, Washington DC, 1964, vol. 43, pp. 112–135 Search PubMed
.
- D. Öner and T. J. McCarthy, Langmuir, 2000, 16, 7777–7782 CrossRef
.
- S. Herminghaus, Europhys. Lett., 2000, 52, 165–170 CrossRef
.
- A. Nakajima, K. Hashimoto and T. Watanabe, Monatsh. Chem., 2001, 132, 31–41 CrossRef CAS
.
- J. Bico, C. Marzolin and D. Quéré, Europhys. Lett., 1999, 47, 220–226 CrossRef CAS
.
- Z. Z. Gu, H. Uetsuka, K. Takahashi, R. Nakajima, H. Onishi, A. Fujishima and O. Sato, Angew. Chem., Int. Ed., 2003, 42, 894–897 CrossRef CAS
.
- N. A. Patankar, Langmuir, 2004, 20, 7097–7102 CrossRef CAS
.
- C. Ishino, K. Okumura and D. Quéré, Europhys. Lett., 2004, 68, 419–425 CrossRef CAS
.
- H. Li, X. Wang, Y. Song, Y. Liu, Q. Li, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2001, 40, 1743–1746 CrossRef CAS
.
- K. K. S. Lau, J. Bico, K. B. K. Teo, M. Chhowalla, G. A. J. Amaratunga, W. I. Milne, G. H. McKinley and K. K. Gleason, Nano Lett., 2003, 3, 1701–1705 CrossRef CAS
.
- X. Feng, L. Feng, M. Jin, J. Zhai, L. Jiang and D. Zhu, J. Am. Chem. Soc., 2004, 126, 62–63 CrossRef CAS
.
- T. Sun, G. Wang, L. Feng, B. Liu, Y. Ma, L. Jiang and D. Zhu, Angew. Chem., Int. Ed., 2004, 43, 357–360 CrossRef CAS
.
- T. N. Krupenkin, J. A. Taylor, T. M. Schneider and S. Yang, Langmuir, 2004, 20, 3824–3827 CrossRef CAS
.
- Z. Yoshimitsu, A. Nakajima, T. Watanabe and K. Hashimoto, Langmuir, 2002, 18, 5818–5822 CrossRef
.
- J. Kim and C. J. Kim, Proc. IEEE Micro Electro Mech. Syst., 2002, 479–482 Search PubMed
.
- C. Cottin-Bizonne, J. L. Barrat, L. Bocquet and E. Charlaix, Nature Mater., 2003, 2, 237–240 CrossRef CAS
.
- J. Ou, B. Perot and J. P. Rothstein, Phys. Fluids, 2004, 16, 4635–4643 Search PubMed
.
-
G. S. Hartley and R. T. Brunskill, in Surface Phenomena in Chemistry and Biology, ed. J. F. Danielli, Pergamon Press, London, 1958, pp. 214–223 Search PubMed
.
- D. Richard and D. Quéré, Europhys. Lett., 2000, 50, 769–775 CrossRef CAS
.
- G. McHale, personal communication, 2004; F. Bouamrirene and D. Bonn, Communication at the workshop Pattern formation in thin liquid films, MPI, Dresden, 2004
.
- N. J. Shirtcliffe, G. McHale, M. I. Newton, G. Chabrol and C. C. Perry, Adv. Mater., 2004, 16, 1929–1932 CrossRef CAS
.
- K. Tadanaga, N. Katata and T. Minami, J. Am. Ceram. Soc., 1997, 80, 1040–1042 CAS
.
- A. Otten and S. Herminghaus, Langmuir, 2004, 20, 2405–2408 CrossRef CAS
.
- Y. Chen, B. He, N. A. Patankar and J. Lee, J. Colloid Interface Sci., 2005, 281, 458–464 CrossRef CAS
.
- R. Blossey, Nature Mater., 2003, 2, 301–306 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2005 |
