2,5-Dimethylphenacyl carbonates: A photoremovable protecting group for alcohols and phenols†
Jaromír
Literák
a,
Jakob
Wirz
b and
Petr
Klán
*a
aDepartment of Organic Chemistry, Faculty of Science, Masaryk University, Kotlarska 2, CZ - 611 37 Brno, Czech Republic. E-mail: klan@sci.muni.cz; Fax: +420-549492688; Tel: +420-549494856
bDepartement Chemie der Universität, Klingelbergstrasse 80, Basel, Switzerland CH-4056. E-mail: j.wirz@unibas.ch; Fax: +41-61-2673855; Tel: +41-61-2673842
First published on 20th September 2004
Abstract
Synthesis and photochemistry of a photochemically removable protecting group for alcohols and phenols, based on the 2,5-dimethylphenacyl (DMP) chromophore, is described. DMP carbonates release the corresponding hydroxy group containing molecules in high isolated yields (>70%), with quantum yields Φ = 0.1–0.2 in methanol and Φ = 0.36–0.51 in cyclohexane. The reactions proceed predominantly by the triplet pathway via E-photoenols, the lifetimes of which of approximately 2 s or 3 ms in cyclohexane or methanol, respectively, were determined by laser flash photolysis.
Introduction
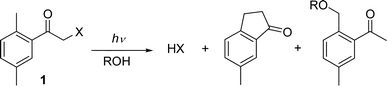
Photochemically removable protecting groups (PPGs) for various functional groups have a wide span of applications including synthetic organic chemistry,1,2 photolithography,3 biochemistry, or cell biology.4 To date the major effort has been devoted to mask phosphates, carboxylates, and alcohols as biologically the most significant functional groups.5 The hydroxy group-containing molecules can be effectively protected by a variety of PPGs, for example, as 2-nitrobenzyl,6 3,5-dimethoxybenzoin,7 phenacyl8 and anthraquinon-2-yl methyl9 carbonates, or 9-phenylxanthyl,10 2-benzoylbenzoic,11–13 quinolyl methyl,14 or hydroxystyrylsilyl15 derivatives.Investigations in our laboratories have focused on the 2,5-dimethylphenacyl (DMP) chromophore, which was found suitable as a new photoremovable protecting group for carboxylic acids,16,17 or phosphates and sulfonates.18 The photoreaction of the DMP esters is initiated by intramolecular hydrogen abstraction of ortho-methyl substituted aromatic ketones yielding a free acid along with 6-methylindanone and, in alcohol, 2-(alkoxymethyl)-5-methylacetophenone (Scheme 1). In this work, we wish to report that the DMP moiety can be used as a PPG for alcohols and phenols that are protected as mixed carbonates in order to enhance their leaving group ability.
 | ||
| Scheme 1 | ||
Experimental
Materials and methods
Benzene, hexane, and methanol were purified by distillation through a vacuum-sealed column (70 cm) packed with glass beads. Piperylene (penta-1,3-diene; a mixture of cis/trans isomers; Fluka) was distilled before use. α-Chloro-2’,5’-dimethylacetophenone was prepared according to the standard procedure.19 The main photoproducts, 2-(methoxymethyl)-5-methylacetophenone and 6-methyl-1-indanone, were available from previous work17,18 and used as analytical standards. NMR spectra were recorded on a Bruker 300 MHz spectrometer. 1H- and 13C-NMR data were measured in CDCl3 with tetramethylsilane as an internal standard. Gas chromatography (GC) was performed on a Shimadzu GC-2010 gas chromatograph equipped with an SPB-5 (15 m, 5% diphenyldimethylsiloxane) column. Melting points were measured on a Kofler hot stage VEB Wägetechnik Rapido 79/2106. UV spectra were obtained on a Shimadzu UV-1601 instrument with matched 1.0 cm quartz cells.Preparation of mixed 2,5-dimethylphenacyl carbonates
The aryl or alkyl 2,5-dimethylphenacyl carbonates were synthesized by two different methods (Scheme 2). The carbonates 1a–1c were prepared by coupling of 2,5-dimethylphenacyl alcohol (DMPA) with the corresponding esters of chloroformic acid in pyridine according to the literature8 (Scheme 2a). The second method was chosen for the synthesis of 1d and 1e and involved the reaction of an alcohol with N,N-carbonyldiimidazole (CDI) to give a carbamate, from which the desired mixed carbonate was obtained by treatment with lithium alcoholate (Scheme 2b).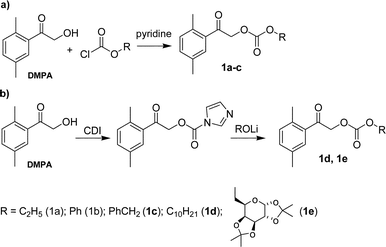 | ||
| Scheme 2 | ||
2,5-Dimethylphenacyl alcohol (DMPA)
DMPA was prepared from α-chloro-2’,5’-dimethylacetophenone (7.05 g, 38.6 mmol) according to Falvey et al.8 Yield 4.74 g (75%), white crystalline solid, mp 44.7–47.0 °C. 1H NMR (300 MHz, CDCl3): δ (ppm) 2.36 (s, 3H), 2.52 (s, 3H), 3.66 (bb, 1H), 4.74 (s, 2H), 7.17 (d, J = 7.8 Hz, 1H), 7.25 (d, J = 7.8 Hz, 1H), 7.41 (s, 1H); 13C NMR (75.5 MHz, CDCl3) δ (ppm): 21.0 (CH3), 21.3 (CH3), 66.7 (CH2), 129.0 (CH), 132.5 (CH), 133.1 (Cq), 133.7 (CH), 135.7 (Cq), 136.7 (Cq), 201.2 (Cq). MS (EI) m/z: 165, 164 (M+), 133, 119, 105, 103, 91, 79, 77, 65.2,5-Dimethylphenacyl ethyl carbonate (1a)
Ethyl chloroformate (0.93 g, 8.56 mmol) was added dropwise under stirring to a solution of 2,5-dimethylphenacyl alcohol (0.702 g, 4.28 mmol) in dry pyridine (20 ml) and the resulting mixture was stirred overnight. Dichloromethane (100 ml) was then added and the solution was washed with cold 10% HCl (2 × 100 ml) followed by cold saturated aqueous NaHCO3 and 35% aqueous NaCl (100 ml). The organic phase was dried with anhydrous MgSO4 and the solvent was removed in vacuo. The crude carbonate was purified by column chromatography on silica gel using hexane/ethyl acetate mixture (3 ∶ 1) as a mobile phase. Yield 0.761 g (75%), yellowish oil. 1H NMR (300 MHz, CDCl3): δ (ppm) 1.31 (t, J = 7.1 Hz, 3H), 2.33 (s, 2H), 2.44 (s, 3H), 4.23 (q, J = 7.1 Hz, 2H), 5.15 (s, 2H), 7.13 (d, J = 7.8 Hz, 1H), 7.20 (d, J = 7.8 Hz, 1H), 7.36 (s, 1H); 13C NMR (75.5 MHz, CDCl3): δ (ppm) 14.2 (CH3), 20.6 (CH3), 20.8 (CH3), 64.6 (CH2), 69.7 (CH2), 128.6 (Cq), 132.2 (Cq), 133.0 (Cq), 134.2 (CH), 135.4 (CH), 135.9 (CH), 155.0 (Cq), 195.5 (Cq). MS (EI) m/z: 237, 236 (M+), 191, 147, 133, 119, 105, 91, 79, 77.2,5-Dimethylphenacyl phenyl carbonate (1b)
The procedure used for 1a gave a crude carbonate that was purified by column chromatography on silica gel using CH2Cl2/hexane mixture (10 ∶ 1) as a mobile phase. Yield 0.456 g starting from 0.304 g of DMPA (87%), white crystalline solid, mp 83.6-84.4 °C. 1H NMR (300 MHz, CDCl3): δ (ppm) 2.36 (s, 3H), 2.51 (s, 3H), 5.30 (s, 2H), 7.17–7.28 (m, 5H), 7.37–7.41 (m, 3H); 13C NMR (75.5 MHz, CDCl3): δ (ppm) 20.6 (CH3), 20.8 (CH3), 70.2 (CH2), 121.0 (CH), 126.1 (CH), 128.6 (CH), 129.5 (CH), 132.2 (CH), 133.1 (CH), 133.9 (Cq), 135.5 (Cq), 136.0 (Cq), 151.2 (Cq), 153.5 (Cq), 194.9 (Cq). MS (EI) m/z: 285 (M+ +1), 191, 147, 133, 119, 105, 91, 79, 77, 65.2,5-Dimethylphenacyl benzyl carbonate (1c)
The procedure used for 1a gave a crude product that was purified by column chromatography on silica gel using CH2Cl2 as a mobile phase. Yield 0.409 g starting from 0.310 g of DMPA (73%), white crystalline solid, mp 51.2–53.2 °C. 1H NMR (300 MHz, CDCl3): δ (ppm) 2.33 (s, 3H), 2.49 (s, 3H), 5.18 (s, 2H), 5.22 (s, 2H), 7.14 (d, J = 7.6 Hz, 1H), 7.21 (d, J = 7.6 Hz, 1H), 7.34–7.38 (m, 6H); 13C NMR (75.5 MHz, CDCl3): δ (ppm) 20.4 (CH3), 20.6 (CH3), 69.7 (CH2), 69.8 (CH2), 128.0 (CH), 128.3 (CH), 128.4 (CH), 128.4 (CH), 131.9 (CH), 132.8 (CH), 133.9 (Cq), 135.0 (Cq), 135.2 (Cq), 135.6 (Cq), 154.8 (Cq), 195.1 (Cq). MS (EI) m/z: 299, 298 (M+), 280, 236, 223, 147, 133, 119, 105, 91, 79, 77, 65, 51.2,5-Dimethylphenacyl 1-decyl carbonate (1d)
N,N-Carbonyldiimidazole (0.63 g, 3.89 mmol) was added dropwise to a stirred solution 2,5-dimethylphenacyl alcohol (0.623 g, 3.79 mmol) in dry THF (25 ml). The mixture was stirred under argon overnight. 1-Decanol (0.60 g, 3.79 mmol) and 1 equivalent of LiH (0.03 g) were added and the reaction mixture was refluxed under argon atmosphere for 10 h. The mixture was washed with 35% aqueous NaCl (25 ml) and water, the organic phase was dried with anhydrous MgSO4 and the solvent was removed in vacuo. The crude carbonate was purified by column chromatography on silica gel using hexane/ethyl acetate mixture (3 ∶ 1) as a mobile phase. Yield 0.632 g (48%), yellowish oil. 1H NMR (300 MHz, CDCl3): δ (ppm) 0.86 (t, J = 5.9 Hz, 3H), 1.42–1.16 (m, 14H), 1.68–1.62 (m, 2H), 2.31 (s, 3H), 2.42 (s, 3H), 4.14 (t, J = 6.4 Hz, 2H), 5.12 (s, 2H), 7.10 (d, J = 7.6 Hz, 1H), 7.17 (d, J = 7.6 Hz, 1H), 7.35 (s, 1H); 13C NMR (75.5 MHz, CDCl3): δ (ppm) 14.0 (CH3), 20.5 (CH3), 20.7 (CH3), 22.7 (CH2), 25.6 (CH2), 28.6 (CH2), 29.2 (CH2), 29.3 (CH2), 29.4 (CH2) 29.5 (CH2), 31.9 (CH2), 68.6 (CH2), 69.6 (CH2), 128.5 (CH), 132.1 (CH), 132.9 (CH), 134.1 (CHq), 135.3 (CHq), 135.8 (CHq), 155.0 (CHq), 195.4 (CHq). MS (EI) m/z: 349, 348 (M+), 335, 319, 279, 251, 209, 190, 149, 147, 133, 119, 105, 91, 79, 77, 55, 43, 41.2,5-Dimethylphenacyl 1,2,3,4-di-O-isopropylidene-D-galactopyranosyl carbonate (1e)
The procedure used for 1d gave a crude carbonate that was purified by column chromatography on silica gel using hexane and acetone 2 ∶ 1 mixture as a mobile phase. Yield 0.384 g (34%) starting from 0.405 g of DMPA, yellowish oil. 1H NMR (300 MHz, CDCl3): δ (ppm) 1.29 (s, 6H), 1.41 (s, 3H), 1.49 (s, 3H), 2.31 (s, 3H), 2.41 (s, 3H), 4.06 (ddd, J1 = 1.3 Hz, J2 = 2.7 Hz, J3 = 6.3 Hz, 1H), 4.31–4.21 (m, 4H), 4.57 (dd, J1 = 2.3 Hz, J2 = 7.8 Hz, 1H), 5.14 (s, 2H), 5.48 (d, J = 4.7 Hz, 1H), 7.11 (d, J = 7.8 Hz, 1H), 7.18 (d, J = 7.8 Hz, 1H), 7.33 (s, 1H); 13C NMR (75.5 MHz, CDCl3): δ (ppm) 20.6 (CH3), 20.8 (CH3), 24.5 (CH3), 25.0 (CH3), 26.0 (CH3), 26.1 (CH3), 65.7 (CH), 67.0 (CH2), 69.8 (CH2), 70.5 (CH), 70.7 (CH), 70.8 (CH), 96.3 (CH), 108.8 (Cq), 109.6 (Cq), 128.6 (CH), 132.2 (CH), 133.0 (CH), 134.1 (Cq), 135.4 (Cq), 135.9 (Cq), 154.8 (Cq), 195.1 (Cq). MS (EI) m/z: 435, 393, 319, 303, 273, 245, 217, 190, 185, 147, 133, 119, 105, 86, 84, 77, 49.Preparative photolysis
The free alcohols from 1d and 1e were obtained in >70% isolated yields using a standard irradiation protocol:18 solutions (≈0.005 M) of the DMP carbonate in cyclohexane were irradiated with a 125-W medium pressure Hg lamp filtered by a Pyrex glass sleeve (λ >280 nm). The irradiation was stopped when the conversion reached at least 95% as indicated by GC (1d) or TLC (1e). In the case of 1e, the mixture was washed with dilute HCl and water, and water was subsequently evaporated in vacuo. The products were analyzed by NMR and GC.Quantum yield measurements
The experiments were carried out on an optical bench consisting of a high-pressure 200 W Hg lamp, an Oriel CornerStone 130 1/8 m monochromator with grating 200–1600 nm set to 313 ± 5 nm and a 1 cm quartz cell containing the sample solution (degassed by bubbling with argon). The light intensity was monitored by a Si photodiode detector (UV enhanced) with an Oriel OPM multifunction optical power meter controlled by TRACQ32 software. Valerophenone20 was used as an actinometer.Laser flash photolysis (LFP)
Experiments were carried out by exciting the sample solutions with absorbances of (0.5 cm−1 at 308 nm with 20 ns, ≈100 mJ pulses of a XeCl excimer laser. A pulsed Xenon arc was used as the monitoring beam (cell path length 4.5 cm, orthogonal to the excitation pulse). The detection system allowed monitoring of either the kinetics at a single wavelength using a transient digitizer, or the whole transient spectrum, with a gated diode array. To avoid interference from secondary photolysis, the samples were freshly prepared from cyclohexane or methanol stock solutions and discarded after each laser shot.Results and discussion
Photoenolization is the primary process of the ortho-methyl substituted aromatic ketones.21,22 The DMP chloride (1, X = Cl) photochemistry was investigated by Bergmark19 and later by us23 showing that HCl is released upon irradiation of with quantum yields Φ of 76 and 11% in methanol and benzene, respectively, via the major photoenol product Z-xylylene in methanol, but via E-xylylene in benzene. In addition, the release of phosphoric and sulfonic acids from the corresponding 2,5-dimethylphenacyl esters with similar quantum yields and mechanism as that of the chloride was reported recently.18 When a good leaving group was replaced by a poorer one–the carboxylate–intramolecular reketonisation of the Z-xylylene was found to be too fast so that only the E-xylylene led to the release of acid.We wished to extend the application of the DMP chromophore to the protection and photorelease of alcohols and phenols. The poor leaving ability of the hydroxy compounds was enhanced by connecting them to the chromophore through a carbonate tether, a similar strategy as, for example, used by Falvey et al.8 on the phenacyl systems.
Irradiation of 1a–1e provided the by-products 2-(methoxymethyl)-5-methylacetophenone (2) and 6-methyl-1-indanone (3) in methanol (a product concentration ratio of 3/2 ≈ 1.6), and exclusively product 3 in cyclohexane, which were the same as those obtained by irradiation of DMP chloride or carboxylate.16–18 In the photolysis of 1b and 1c, the formation of the products, phenol and benzyl alcohol, leveled off at higher conversions (≈55%), presumably caused by photoinduced reduction of the starting materials and the ketone by-products by the hydroxy compounds. Preparative irradiation of 1d and 1e in cyclohexane, followed by purification, gave the corresponding hydroxy compounds in >70% chemical yields.
Table 1 displays the quantum yields of esters disappearance in methanol and cyclohexane at 313 nm irradiation. The fact that the Φ values in methanol were significantly lower than those in the non-polar hydrocarbon parallels the tendency observed in the case of DMP carboxylates.17
| Compound | R (Scheme 2) | Φ (methanol) | Φ (cyclohexane) |
|---|---|---|---|
| a Φ were measured by reference to valerophenone (Φ = 0.30)20 at 313 ± 5 nm (optical bench). b 1,2,3,4-Di-O-isopropylidene-D-galactopyranosyl. | |||
| 1a | Et | 0.13±0.02 | 0.51±0.02 |
| 1b | Ph | 0.20±0.01 | 0.50±0.03 |
| 1c | Bn | 0.14±0.01 | 0.48±0.01 |
| 1d | Decyl | 0.09±0.01 | 0.36±0.03 |
| 1e | Galactopyranosylb | 0.16±0.01 | 0.40±0.02 |
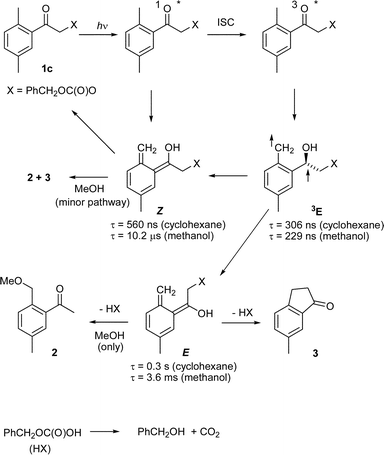
The mechanism of the photoreactions of 1b and 1c was investigated by laser flash photolysis (Table 2 and Scheme 3). The photochemistry of 1c in cyclohexane produced two types of transients (Fig. 1). Based on a previous study,18 the first one with λmax ≈ 350 nm is assigned to the triplet photoenol 3E and the second one with λmax ≈ 390 nm to both the E- and Z-ground-state photoenols. The kinetic traces contained three exponential components: three decays at 350 nm and a rise followed by a decay at 390 nm. The lifetime of the short-lived component 3E was sensitive to oxygen; it dropped from 306 ns in degassed solution to 78 ns in air-saturated solution. The fast decay of 3E was followed by the second decay with τ = 560 ns that was attributed to the Z-photoenol, which is expected to undergo fast intramolecular reketonisation in hydrocarbon solvents.21 The third component with τ = 0.3 s was assigned to the E-photoenol. The same transients were observed by LFP of 1b in methanol, only the lifetimes differed somewhat. The photolysis of compounds 1a–1e then releases a monoester of carbonic acid that eliminates carbon dioxide in a subsequent ‘dark’ reaction. The rates of decarboxylation of monocarbonates (ROCO2−) are known to be in the order of 10−3 s−1 for PhCH2OCO2− and 103 s−1 for PhOCO2− in aqueous solutions and are pH-dependent.24 The values in methanol should not be much different.
 | ||
| Scheme 3 | ||
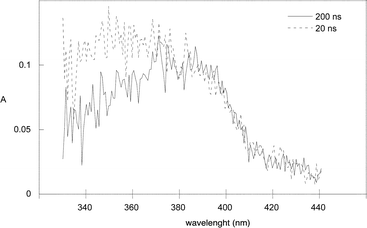 | ||
| Fig. 1 Transient absorption spectra of 1c in cyclohexane at 20 ns and 200 ns delay. | ||
Both transient and steady-state quenching studies were performed in order to investigate whether the photoproducts from DMP carbonates are formed exclusively via the triplet pathway as in the case of DMP carboxylates.17 Thus, addition of a triplet quencher (piperylene, penta-1,3-diene) had no effect on the lifetime of the transient 3E, but its amplitude decreased with increasing quencher concentration. The triplet energy of the piperylene is too high for efficient quenching of 3E, however, low enough to quench the DMP triplet. Similarly, the lifetimes of the two ground-state photoenols Z and E were not altered in the presence of piperylene but their total amount decreased with increasing quencher concentration. A Stern–Volmer analysis of the amplitudes A of both photoenols of 1c in methanol produced by flash photolysis revealed a linear dependence of A0/Aq on c(Q) for the E-photoenol (with kqτ = 13 M−1; assuming that kq ≈ 5 × 109 M−1 s−1, and the lifetime of the DMP triplet is 2.6 ns), whereas the plot for the Z-photoenol reached a plateau at A0/Aq ≈ 1.3, indicating that approximately 77% of the Z-photoenol is formed via the singlet pathway. The ratio A0(E)/Ao(Z) ≈ 0.82 provides another measure of the concentration ratio of E- and Z-enols. In cyclohexane, the formation of the Z-photoenol was not fully suppressed even at high quencher concentrations. The Stern–Volmer plot reached a plateau at A0/Aq ≈ 1.5 corresponding to 67% of Z-photoenol formed by the singlet pathway.
Consistent results were obtained by measuring the quantum yield of 1a degradation in methanol and cyclohexane as a function of quencher concentration. The results are displayed in Fig. 2. No products were observed in methanol at high piperylene concentrations. However, formation of the product was not fully quenched in cyclohexane, indicating that the singlet state pathway via the Z-enol to products is partially (<20%) available (Scheme 3), in contrast to the exclusive triplet reaction of DMP carboxylates in non-polar solvents.17
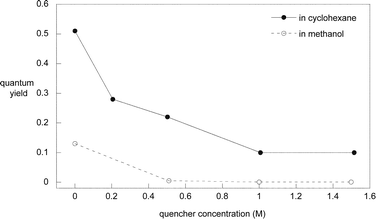 | ||
| Fig. 2 Steady-state quenching study of 1a by piperylene. | ||
In conclusion, the 2,5-dimethylphenacyl carbonate moiety was found to be suitable for an efficient photorelease of aliphatic and aromatic hydroxy-group containing molecules, being thus promising as an excellent photoremovable protecting group in organic synthesis and biochemistry.
Acknowledgements
We gratefully acknowledge financial support from the Czech Ministry of Education, Youth and Sport (CEZ: J07/98:143100005) and the Swiss National Science Foundation (Project No. 20-68087.02).References
- V. N. R. Pillai, Photo-removable protecting groups in organic synthesis, Synthesis, 1980, 1–26 CAS.
- T. W. Greene and G. M. Wuts, Protective Groups in Organic Chemistry, John Wiley and Sons, Inc., New York, 3rd edn., 1999 Search PubMed.
- M. C. Pirrung, Spatially addressable combinatorial libraries, Chem. Rev., 1997, 97, 473–488 CrossRef CAS.
- J. Abelson, M. Simon and G. Marriott, Caged Compounds, Academic Press, New York–London, 1998 Search PubMed.
- A. P. Pelliccioli and J. Wirz, Photoremovable protecting groups: reaction mechanisms and applications, Photochem. Photobiol. Sci., 2002, 1, 441–458 Search PubMed.
- C. G. Bochet, Photolabile protecting groups and linkers, J. Chem. Soc., Perkin Trans., 2002, 1, 125–142 Search PubMed.
- M. C. Pirrung and J. C. Bradley, Dimethoxybenzoin carbonates - photochemically-removable alcohol protecting groups suitable for phosphoramidite-based DNA-synthesis, J. Org. Chem., 1995, 60, 1116–1117 CrossRef CAS.
- A. Banerjee, K. Lee and D. E. Falvey, Photoreleasable protecting groups based on electron transfer chemistry. Donor sensitized release of phenacyl groups from alcohols, phosphates and diacids, Tetrahedron, 1999, 55, 12699–12710 CrossRef CAS.
- T. Furuta, Y. Hirayama and M. Iwamura, Anthraquinon-2-ylmethoxycarbonyl (Aqmoc): A new photochemically removable protecting group for alcohols, Org. Lett., 2001, 3, 1809–1812 CrossRef CAS.
- A. Misetic and M. K. Boyd, The pixyl (Px) group: a novel photocleavable protecting group for primary alcohols, Tetrahedron Lett., 1998, 39, 1653–1656 CrossRef CAS.
- P. B. Jones and N. A. Porter, 2-Aroylbenzoyl serine proteases: photoreversible inhibition or photoaffinity labeling?, J. Am. Chem. Soc., 1999, 121, 2753–2761 CrossRef CAS.
- P. B. Jones, M. P. Pollastri and N. A. Porter, 2-Benzoylbenzoic acid: A photolabile mask for alcohols and thiols, J. Org. Chem., 1996, 61, 9455–9461 CrossRef CAS.
- J. Pika, A. Konosonoks, R. M. Robinson, P. N. D. Singh and A. D. Gudmundsdottir, Photoenolization as a means to release alcohols, J. Org. Chem., 2003, 68, 1964–1972 CrossRef CAS.
- G. A. Epling and A. A. Provatas, Approaches to a photocleavable protecting group for alcohols, J. Chem. Soc., Chem. Commun., 2002, 1036–1037 Search PubMed.
- M. C. Pirrung and Y. R. Lee, Photochemically-removable silyl protecting groups, J. Org. Chem., 1993, 58, 6961–6963 CrossRef CAS.
- P. Klan, M. Zabadal and D. Heger, 2,5-dimethylphenacyl as a new photoreleasable protecting group for carboxylic acids, Org. Lett., 2000, 2, 1569–1571 CrossRef CAS.
- M. Zabadal, A. P. Pelliccioli, P. Klan and J. Wirz, 2,5-dimethylphenacyl esters: A photoremovable protecting group for carboxylic acids, J. Phys. Chem. A, 2001, 105, 10329–10333 CrossRef CAS.
- P. Klan, A. P. Pelliccioli, T. Pospisil and J. Wirz, 2,5-Dimethylphenacyl esters: A photoremovable protecting group for phosphates and sulfonic acids, Photochem. Photobiol. Sci., 2002, 1, 920–923 RSC.
- W. R. Bergmark, Photolysis of alpha-chloro-o-methylacetophenones, J. Chem. Soc., Chem. Commun., 1978, 61–62 RSC.
- P. J. Wagner, I. E. Kochevar and A. E. Kemppainen, Type II photoprocesses of phenyl ketones. Procedures for determining meaningful quantum yields and triplet lifetimes, J. Am. Chem. Soc., 1972, 94, 7489–7494 CrossRef CAS.
- R. Haag, J. Wirz and P. J. Wagner, The photoenolization of 2-methylacetophenone and related compounds, Helv. Chim. Acta, 1977, 60, 2595–2607 CrossRef CAS.
- P. G. Sammes, Photoenolization, Tetrahedron, 1976, 32, 405–422 CrossRef CAS.
- A. P. Pelliccioli, P. P. Klan, M. Zabadal and J. Wirz, Photorelease of HCl from o-methylphenacyl chloride proceeds through the Z-xylylenol, J. Am. Chem. Soc., 2001, 123, 7931-7932 CrossRef CAS.
- C. K. Sauers, W. P. Jencks and S. Groh, Alcohol-bicarbonate-water system. Structure-reactivity studies on the equilibriums for formation of alkyl monocarbonates and on the rates of their decomposition in aqueous alkali, J. Am. Chem. Soc., 1975, 97, 5546–5553.
Footnote |
| † Dedicated to Professor Hiroshi Masuhara on the occasion of his 60th birthday. |
| This journal is © The Royal Society of Chemistry and Owner Societies 2005 |
