Steroids: reactions and partial synthesis
James R.
Hanson
Department of Chemistry, University of Sussex, Brighton, Sussex, UK BN1 9QJ
First published on 10th January 2005
Abstract
Covering: January to December 2003
This article reviews the progress in the chemistry of the steroids that was published between January and December 2003. The reactions and partial synthesis of estrogens, androgens, pregnanes, cholic acid derivatives, cholestanes and vitamin D analogues are covered. There are 152 references.
1 Introduction
This review follows the pattern of its predecessor1 with sections on the chemistry of the major skeletal types of steroids. There has been continued progress in the development of compounds which inhibit stages in steroid metabolism, particularly the C-17(20) lyase, in defining the structural requirements for binding to the steroid receptors and in establishing structure–activity relationships for the neurosteroids that target the GABAA receptor.A review of the synthesis, physical and biological properties of enantiomeric steroids has appeared.2Ent-desmosterol and ent-cholesterol have been prepared3 from ent-androst-4-ene-3,17-dione in order to study sterol–lipid interactions. A review has appeared4 covering the use of transition metal catalysts in steroid synthesis. Although steroid total synthesis is outside the scope of this article, it is worth noting that a new strategy for a convergent steroid synthesis has been described.5 A review has also appeared6 covering chemical approaches to the study of transcription, a topic which included steroid examples.
2 Estrogens
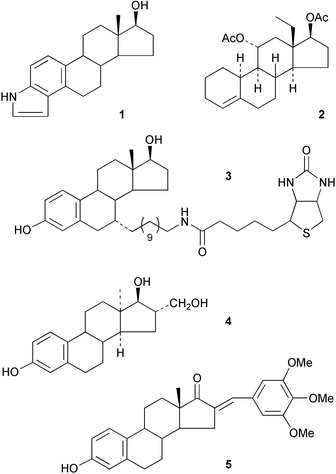
The receptor interactions involving anti-estrogens and the role of selective estrogen receptor modulators in medicine has been reviewed.7 NMR studies on the conformations adopted by estrogen receptor ligands has been reported.8 2-Methoxyestradiol has tumour growth inhibitory properties. A new short synthesis has been described9 based on the oxidation of estradiol bis-THP ether by hydrogen peroxide in the presence of a strong base. An efficient synthesis of estrieno[2,3-b]- and [3,4-c]-pyrroles 1 by a palladium-catalysed amination has been reported.10Some observations have been made11 on the hydrolytic behaviour of the ethylene ketals of 5α,10α-epoxy-9(11)-estrenes and their 5α-hydroxy-11β-alkyl counterparts. The X-ray crystal structure of the alkene 2, obtained from the reduction of the aromatic ring of 13-ethyl-3-ethoxygona-1,3,5(10)-triene-11α,17β-diol, has revealed12 the presence of products from this reaction with an unusual 10α-hydrogen atom. Some novel estratrienones have been obtained13 by oxidation at the benzylic C-6 and C-9 positions with t-butyl hydroperoxide in the presence of cobalt acetate. Estrogen esters with groups at C-7α, C-11β and C-15α have been used14 to probe the structural requirements of the estrogen receptor. The synthesis has been reported15 of a 7α-substituted estradiol–biotin chimera 3 that heterodimerizes the estrogen receptor with a streptavidin protein in a yeast.
The synthesis of a ring B aromatic 13-azasteroid has been described.16 Although they have a lower binding affinity, some normal and 13-epi-D-homoestrone derivatives are recognized by the estrogen receptor.17 The stereochemistry of the addition reactions to the 16,17-double bond of 3-methoxy-13α-estra-1,3,5(10),16-tetraene has been examined18 and the conformation of the four 16,17-diols has been established.19 A stereoselective synthesis of the isomeric trans 16-hydroxymethyl-3-methoxy-13α-estra-1,3,5(10)-trien-17-ols (e.g.4) and their halogenation, has been reported.20,21 The synthesis and anti-neoplastic activity of 16-arylidene derivatives of estrone, e.g.5, have been described.22 Syntheses have been reported of estradiol–adenosine23 and estradiol–taxol24 conjugates.
The modification of ring D of estrone has continued to be of interest in the context of its effects on estrogen metabolism and on the binding of the steroid to the estrogen receptor. Some medium-sized ring D derivatives of estrone, e.g.6, have been obtained25 by a combination of intramolecular Heck and Grignard reactions. The sulfamate 7 has been shown26 to be a potent inhibitor of a steroid sulfatase. The receptor binding activity of 17α-arylestradiols has been examined.27 The synthesis of 11C labelled compounds in the estradiol series has been reported,28 while 17α,20E/Z-[125I]-iodovinyl derivatives of 7α-cyanoestradiol29 and 7α-cyano-19-nortestosterone30 have been prepared as radioligands for the relevant steroid receptors. Some 4′-substituted cinnamylestradiol derivatives 8 have been evaluated31 as probes for the estrogen receptor binding domain. Various 17α-substituted estradiolpyridin-2-yl hydrazine complexes with rhenium have also been prepared32 as potential ligands for labelling the estrogen receptor.
The biomimetic hydroxylation of C-12 to afford 9 using a copper complex and oxygen, with the 17-pyridinyl imine as a directing group, has been examined.33 Some 17β-estradiol dimers linked via an alkyl chain between C-17α have been shown34 to have cytotoxic activity against human cancer cell lines that are responsive to estrogens.
3 Androgens
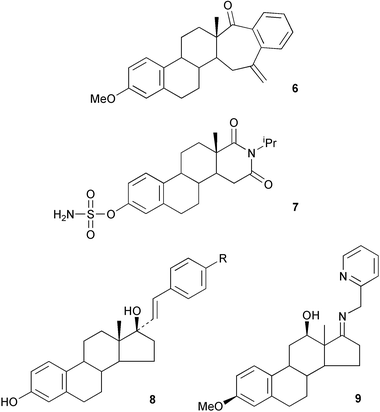
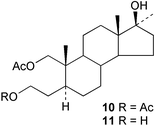
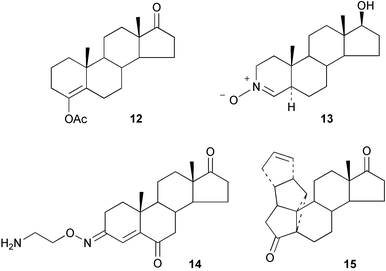
Methods have been reported for the regioselective aminolysis of steroidal 2α,3α-epoxides catalysed by gadolinium triflate35 and for the preparation of 3β-amino-5α-androstan-17-one.36 A regioselective Candida antarctica lipase catalysed hydrolysis of the diacetate 10 to the mono-ol 11 has provided37 the basis for a convenient synthesis of the ring A lactone of oxandrolone. The stereochemistry of some reactions of 2-methylene and 2-hydroxymethylene-5α-androstanes has been described.38,39The structure–activity relationships of some 3-deoxyandrogens, e.g.12, as aromatase inhibitors have been evaluated.40 The nitrone 13 has been examined41 as a transition state mimic of an enolate intermediate in the formation of 5α-dihydrotestosterone by testosterone 5α-reductase. The oxime ether 14 has been shown42 to be a novel Na+,K+-ATPase inhibitor and this compound may have application in the treatment of heart failure.
Some thermodynamic features of the isomerization of androst-5-ene-3,17-dione to androst-4-ene-3,17-dione have been examined.43 The microwave-induced enol-acetylation of steroidal ketones has been reported.44 Some unusual steroidal polyquinane hybrids, e.g.15, have been described.45 The proton chemical shifts of androstane amines have been assigned.46
Some structural requirements for binding to the androgen receptor have been determined47 using some androst-16-enes. The partial syntheses of androst-16-ene-3β,5α-diol and 3β,5α,6β-triol48 and of some cytostatic ring B lactams49 have been described. The stereochemical factors affecting the 1,2-addition of Grignard reagents to steroidal unsaturated ketones and the oxidation of 3-hydroxy-3-methyl-Δ4-steroids have been examined.50,51
Androstanes have continued to provide useful substrates for defining the structural requirements for microbiological hydroxylations. Studies on the biotransformation of 3α,17β- and 3β,17α-dihydroxy-5α-androstanes,52 4,4-dimethylandrost-5-enes,53 5β-methylestr-9-enes,54 des ring D androstanes,55 17-chloroandrosta-4,16-dien-3-one,56 and 4-formylandrostanes57 have all been reported. The extent of the double bond migration in the Westphalen rearrangement of des ring D androstenes has been examined.58
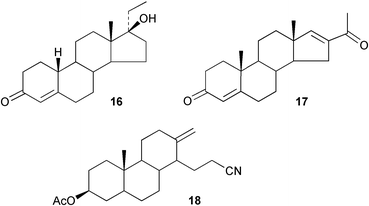
The syntheses of some equine metabolites of the anabolic steroid, norethandrolone 16, have been reported.59 Solvent effects have been shown60 to play a role in the addition of organolithium reagents to hindered C-11 ketones. The preparation and X-ray crystal structure of 16-acetylandrosta-4,16-diene-3-one 17 has been reported.61 The conditions for the abnormal Beckmann rearrangement of steroidal C-17 oximes have been optimized62 for the fragmentation of ring D to form 18. 16,17-Secosteroids with aminomethylene-2-pyridine structures have been used63 as chiral ligands for the copper ion activation of oxygen.
The palladium-catalysed aminocarbonylation of 17-iodoandrost-16-enes64 in ionic liquids and the X-ray structures of 16-amino-D-homosteroids have been reported.65 The Cu/Fe-mediated hydrogenation of 17-halo-16-enes in the presence of hydrazine has also been reported.66
Androgens containing various 17-azole substituents have been examined as inhibitors of the hydroxylase in the 17(20)-lyase and as potential agents for prostatic cancer therapy.67,68 Some 5α,13α-D-azasteroids have been prepared69 as potential precursors of GABAA receptor modulators. Improvements in the phosphorylation of alcohols with titanium t-butoxide as the catalyst have been made70 using steroidal 17β-alcohols as examples. The synthesis of two new haptens of 16α-hydroxydehydroepiandrosterone has been reported.71
4 Pregnanes
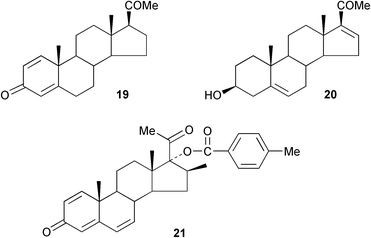
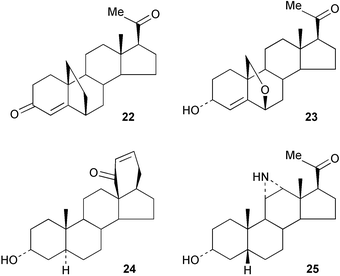
Some general patterns have been identified72 in the wavelength dependence of various photochemical reactions of pregna-1,4-diene-3,20-dione 19. An efficient process has been developed73 for the production of the key steroid intermediate, 16-dehydropregnenolone 20, from diosgenin. A synthesis has been reported74 of 5,7-dienes related to pregnane-3,17α,20-triols which are abnormal steroid metabolites. Methods have been developed75 for the synthesis of 6β-hydroxycortisol labelled with stable isotopes. Some pregna-1,4,6-trienones, e.g.21, have been evaluated76 as inhibitors of testosterone 5α-reductase. The conjugate addition of p-aminothiophenol to pregnane-4,6-dien-3-ones has been observed.77The 6,19-methanoprogesterone 22 has been prepared.78 Neurosteroids have continued to attract interest. The GABAA receptor activity of the 6,19-oxidopregnane analogue 23 has been examined.79 Some other pregnanes with conformationally constrained side chains, e.g.24, have also been prepared80 as analogues of neurosteroids with GABA modulatory activity. The synthesis of 11,12-aziridino analogues, e.g.25 of neuroactive steroids has also been reported.81 Dysfunction of glucocorticoid receptors in the brain has been associated with some neuropsychiatric disorders. Some novel arylpyrazolocorticosteroids have been developed82 for imaging these receptors.
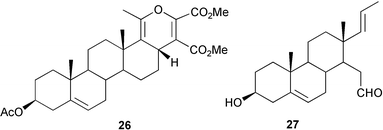
16-Dehydropregnenolone acetate has provided the starting material for the preparation of 16β-halo83 and 16α-methyl84 derivatives of cyproterone and for the formation of a pyrone 26 by a 4 + 2 cycloaddition.85
21,21-Difluoro-3β-hydroxypregna-5,20-diene and the corresponding 5,16,20-triene have been shown86 to be inhibitors of the 17(20)-lyase. The Grob fragmentation of ring D to give the aldehyde 2787 and the base-catalysed cleavage of the dihydroxyacetone side chain of the corticosteroids88 have been examined. Aerial oxidation of the corticosteroid side chain in the presence of copper acetate has been shown89 to lead to dimers based on 17,21-acetals. The preparation of 21,21-dimethylprogesterone90 and the conformation of the side chain of 21-alkylpregnanes91 have been examined.
5 Cholic acids
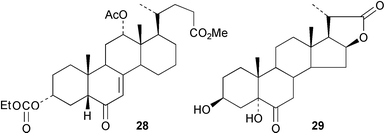
The application of residual dipolar couplings in the NMR spectrum to the assignment of conformation has been explored92 with sodium cholate. The preparation of fluorinated derivatives of cholic acids has been explored93 in the context of the synthesis of squalamine analogues. The marine sterol 3β,6α-dihydroxy-5α-cholan-23-one has been synthesized.94 The conversion of cholic acid to the unsaturated 6-ketone 2895 and the synthesis of the lactone 2996 have been described.Metal complexing has been explored97 as a tool for controlling the self assembly and gelation properties of cholic amide–phenthroline gelating agents. A review has appeared98 of steroids as the organising components in anion receptors. A cholapod quaternary ammonium salt containing pendant urea groups has been examined99 in the context of anion recognition. Desoxycholic acid–copper phosphite complexes have been explored100 as ligands for the enantioselective conjugate addition of diethyl zinc to acyclic enones.
Clathrates based on cholic acid have been used101 to form complexes with the o-, m- and p-xylenes. The X-ray structure of an inclusion complex between ursodeoxycholic acid and phenanthrene has been described.102
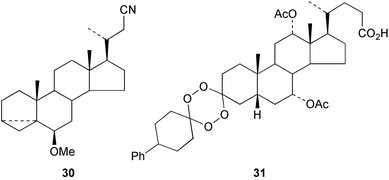
The addition of metal cyanides to the p-toluenesulfonyl hydrazones of aldehydes has been explored103 as a method of one-carbon homologation on the steroid side chain, as in the formation of 30. The properties of a model trans-membrane ionophore based on the dimer 3β,6α,7β-trihydroxy-bisnorchol-16-ene 22-terephthalate have been examined.104 The synthesis of ester-linked lithocholic acid dimers has been reported.105 Some novel steroidal dendrons have been obtained106 from 3α-trifluoroacetoxy-5β-cholan-24-oyl chloride and the benzyl ester of 2,2-bis(hydroxymethyl)propionic acid. The complexing properties of some cholane–porphyrin conjugates have been examined.107 The anti-malarial, anti-mycobacterial and anti-proliferative activity of phenyl-substituted tetraoxanes, e.g.31, have been examined.108 The photochemical rearrangement of the C-3 o-nitrobenzyl ethers of cholic acid to o-nitrosohemiacetals has been observed.109
6 Cholestanes
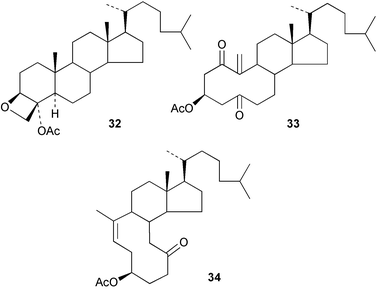
Evidence has been provided110 for the effect of cholesterol in a membrane on unravelling neighbouring phospholipids by acting as a a rigid hydrophobic template and maximizing the hydrophobic interactions. The interactions of cholesterol with cyclodextrins in solution has been studied111 by NMR methods.A formal synthesis of squalamine from desmosterol has been reported.112 The anti-viral activity of the disulfate of 23,3α-dihydroxy-5α-cholestane has been noted.113 The reactions of the oxetane 32 have been studied114 as a model for aspects of taxol chemistry. The oxidative fragmentation of 5α-hydroxy-1-oxo-cholestan-3β-yl acetate to form 33, and of some 5-hydroxy-B-norcholestan-3β-yl acetates to give 5,10-secosteroids such as 34, together with their acid-catalysed reactions, have been examined.115–117
The allylic oxidation of cholesteryl acetate has been examined using t-butylhydroperoxide and silica modified with a cobalt alkylphosphonate118 or copper iodide in acetonitrile.119 Some further epoxides have been isolated120 from the autoxidation of isotachysterol. The synthesis of deuteriated samples of 7α- and 26-hydroxycholesterol for use in studying the bile acid pathway has been described.121
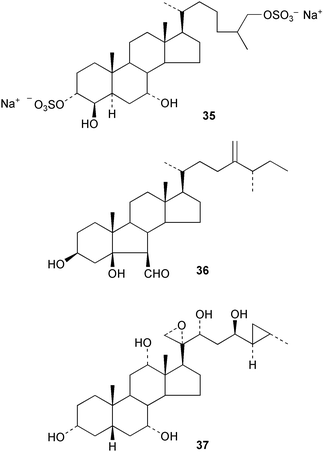
The sperm-activating factor 35 of the ascidian Ciona intestinalis, has been synthesized.122 A synthesis of the follicular fluid meiosis activating sterol, 3β-hydroxy-4,4-dimethyl-5α-cholesta-8,14,24-triene, has been reported123 in the context of improving in vitro fertilization. The synthesis of the B-noraldehyde, orostanal 36, has attracted interest124 because it induces apoptosis in HL-60 cells.
A computer program for predicting 13C NMR chemical shifts which takes into account stereochemistry has been applied125 in the ecdysone series. The brassinolide plant hormones have continued to attract interest with reports on novel methods of constructing the side chain,126 and the synthesis of B-homo analogues,127 C-28 homo analogues,128 and labelled brassinolides.129 The anti-tumour activity of the marine sterol aragusterol A has provided the stimulus for the synthesis130 of some analogues, 37, from cholic acid. The tumour-inhibiting properties of the dimeric steroids of the cephalostatin series has also continued to stimulate synthetic methodology.131,132 Methyl protodioscin133 and some rhamnosylated diosgenin glucosides134 have been synthesized from diosgenin in the course of studies on steroidal cytostatic agents.
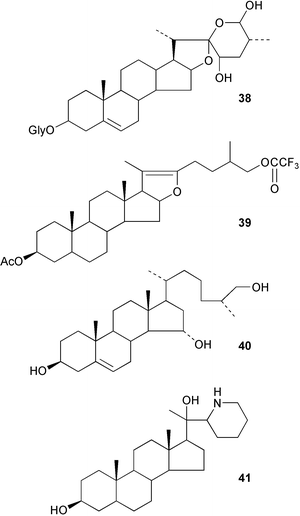
A number of modifications of the spiroketal ring system of the steroidal sapogenins such as diosgenin have been reported including the unusual cleavage of the diol 38 to the 22→16-lactone,135 the formation of C-26 dithioketals136 and the opening of the spiroketal with trifluoroacetyltrifluoromethanesulfonate to form 39137 and the cleavage with boron trifluoride–acetic anhydride.138 The synthesis of the aglycone of 26-O-deacetylpavoninin 5 40 from diosgenin has been reported.139 Distinctions between the 25R and 25S spirostanes have been made140 using their NMR spectra. The azasterol analogue 41 has been shown141 to be an inhibitor of the 24-methyl transferase in Leishmania species.
7 Vitamin D
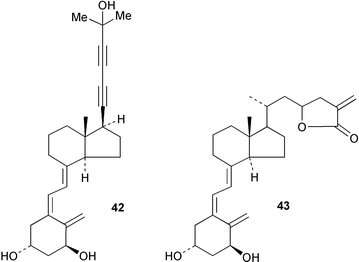
The organic chemistry of vitamin D analogues has been reviewed.142 1α,25-Dihydroxyvitamin D3 (calcitriol) may exert some of its biological activity by binding to a nuclear receptor and initiating gene transcription whilst other effects involving calcium homeostasis may involve interactions with a membrane receptor. The synthesis of analogues modified on rings C and D to differentiate between these binding sites has been reported.143 The thermal isomerization of vitamin D3144 and the photoconversion of 25-hydroxytachysterol to 25-hydroxyprevitamin D3145 have been examined.The rigid acetylenic side chain analogue 42 of calcitriol has been shown146 to induce vitamin D receptor transcriptional activity whilst 1α-hydroxyvitamin D3 26→23-lactones e.g.43, have antagonistic activity at the vitamin D receptor.147,148 Functionalization of C-12 of 1α,25-dihydroxyvitamin D3 modifies the affinity for the vitamin D receptor. The analogue with a 12-methyl substituent possessed a high affinity for the receptor.149
A number of novel hapten derivatives of 1α,25-dihydroxyvitamin D3 have been prepared.150 Compounds linked through C-16 expose both ring A and the side chain, to maximize antibody specificity. A number of new strategies for the synthesis of vitamin D analogues have been described.151,152
8 References
- Previous report: J. R. Hanson, Nat. Prod. Rep., 2004, 21, 386 Search PubMed.
- J. F. Biellmann, Chem. Rev., 2003, 103, 2019 CrossRef CAS.
- E. J. Westover and D. F. Covey, Steroids, 2003, 68, 159 CrossRef CAS.
- R. Skoda-Foeldes and L. Kollar, Chem. Rev., 2003, 103, 4095 CrossRef CAS.
- O. Lepage and P. Deslongchamps, J. Org. Chem., 2003, 68, 2183 CrossRef CAS.
- R. V. Weatherman, Org. Biomol. Chem., 2003, 1, 3257 RSC.
- V. Craig Jordan, J. Med. Chem., 2003, 46, 883 CrossRef; V. Craig Jordan, J. Med. Chem., 2003, 46, 1081 CrossRef.
- A. B. Sebag, R. N. Hanson, D. A. Forsyth and C. Y. Lee, Magn. Reson. Chem., 2003, 41, 246 CrossRef CAS.
- P. S. Kiuru and K. Wahala, Steroids, 2003, 68, 373 CrossRef CAS.
- X. Zhang and Z. Sui, Tetrahedron Lett., 2003, 44, 3071 CrossRef CAS.
- E. Franscic-Czinege, Z. Tuba, C. Molnar, J. Horvath, J. Caoergei, G. Visky, G. Balogh, M. Mak, R. Hegedues and M. Magyari, Steroids, 2003, 68, 739 CrossRef.
- E. Modica, F. Compostella, D. Colombo, F. Ronchetti, A. Scala and B. Bovio, Steroids, 2003, 68, 367 CrossRef CAS.
- E. Modica, G. Bombieri, D. Colombo, N. Marchini, F. Ronchetti, A. Scala and L. Toma, Eur. J. Org. Chem., 2003, 2964 CrossRef CAS.
- D. C. Labaree, J. Zhang, H. A. Hariis, C. O'Conner, T. Y. Reynolds and R. B. Hochberg, J. Med. Chem., 2003, 46, 1886 CrossRef CAS.
- S. L. Hussey, S. S. Muddani and B. R. Peterson, J. Am. Chem. Soc., 2003, 125, 3692 CrossRef CAS.
- C. M. Marson, J. H. Pink and C. Smith, Tetrahedron, 2003, 59, 10019 CrossRef CAS.
- J. Wolfling, E. Mernyak, E. Frank, G. Falkay, A. Marki, R. Minorica and G. Schneider, Steroids, 2003, 68, 277 CrossRef CAS.
- E. Mernyak, B. Schonecker, C. Lange, M. Kotteritzsch, H. Gorls, J. Wolfling and G. Schneider, Steroids, 2003, 68, 289 CrossRef CAS.
- S. Schwarz, B. Schonecker, K. Fritsche, A. Poser, C. Lange, W. Gunther, S. Gottke, H. Gorls and S. Basler, Steroids, 2003, 68, 113 CrossRef CAS.
- E. Mernyak, J. Wolfling, G. Bunkoczi, L. Luo, T. R. Schneider and G. Schneider, Collect. Czech. Chem. Commun., 2003, 68, 1141 CrossRef CAS.
- J. Wolfling, E. Mernyak, P. Forgo and G. Schneider, Steroids, 2003, 68, 451 CrossRef CAS.
- M. Minu, D. P. Jindal, G. Leclercq and M. Borras, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2003, 42B, 166 CAS.
- D. Poirier, R. P. Boivin, M. Berube and S.-X. Lin, Synth. Commun., 2003, 33, 3183 CrossRef CAS.
- C. Liu, J. S. Strobl, S. Bane, J. K. Schilling, M. McCracken, S. K. Chatterjee, R. Rahim-Bata and D. G. I. Kingston, J. Nat. Prod., 2003, 67, 152 CrossRef.
- L. F. Tietze, K. M. Sommer, G. Schneider, P. Tapolesanyi, J. Wolfling, P. Mueller, M. Noltemeyer and H. Terlau, Synlett., 2003, 1491.
- D. S. Fischer, L. L. W. Woo, M. F. Mahon, A. Purohit, M. J. Reed and B. V. L. Potter, Bioorg. Med. Chem., 2003, 11, 1685 CrossRef CAS.
- N. Foy, E. Stephen, A. Vessieries, E. Salomon, J. M. Heldt, M. Huche and G. Jaouen, ChemBioChem, 2003, 4, 494 CrossRef CAS.
- F. Wust, J. Zessin and B. Johannsen, J. Labelled Compd. Radiopharm., 2003, 46, 333 CrossRef CAS.
- H. Ali, J. Rousseau, B. Paquette, C. Dube, B. Marko and J. E. van Lier, Steroids, 2003, 68, 1189 CrossRef CAS.
- H. Ali, J. Rousseau, N. Ahmed, V. Guertin, R. B. Hochberg and J. E. van Lier, Steroids, 2003, 68, 1163 CrossRef CAS.
- R. N. Hanson, C. Y. Lee, C. J. Friel, R. Dilis, A. Hughes and E. R. DeSombre, J. Med. Chem., 2003, 46, 2865 CrossRef CAS.
- J. B. Arterburn, C. Corona, K. V. Rao, K. E. Carlson and J. A. Katzenllenbogen, J. Org. Chem., 2003, 68, 7063 CrossRef CAS.
- B. Schonecker, T. Zheldakova, Y. Liu, M. Kotteritzsch, W. Gunther and H. Gorls, Angew. Chem., Int. Ed., 2003, 42, 3240 CrossRef.
- D. Rabouin, V. Perron, B. N. Zamba, R. Gaudreault and G. Berube, Bioorg. Med. Chem. Lett., 2003, 13, 567 CrossRef.
- D. Thibeault and D. Poirier, Synlett, 2003, 1192 CAS.
- M. Reyes, A. Rosado, Y. M. Alvarez, J. A. Ruiz, J. Aguero and H. Velez, J. Chem. Res. (S), 2003, 234 Search PubMed.
- P. Ferraboschi, D. Colombo and P. Prestileo, Tetrahedron: Asymmetry, 2003, 4, 2781 CrossRef.
- K. Al-Fouti and J. R. Hanson, J. Chem. Res. (S), 2003, 232 Search PubMed.
- K. Al-Fouti and J. R. Hanson, J. Chem. Res. (S), 2003, 6 Search PubMed.
- M. Nagaoka, Y. Watari, H. Yajima, K. Tsukioka, Y. Muroi, K. Yamada and M. Numazawa, Steroids, 2003, 68, 533 CrossRef CAS.
- A. J. Robinson, I. DeLuca, S. Drummond and G. A. Boswell, Tetrahedron Lett., 2003, 44, 4801 CrossRef CAS.
- S. De Munari, A. Cerri, M. Gobbini, N. Almirante, L. Banfi, G. Carzana, P. Ferrari, G. Marazzi, R. Micheletti, A. Schiavone Sputore, M. Torri, M. P. Zappavigna and P. Melloni, J. Med. Chem., 2003, 46, 3644 CrossRef CAS.
- W. J. Houck and R. M. Pollack, J. Am. Chem. Soc., 2003, 125, 10206 CrossRef CAS.
- P. Marwah, A. Marwah and H. A. Lardy, Tetrahedron, 2003, 59, 2273 CrossRef CAS.
- V. Singh, S. Lahiri, V. V. Kane, T. Stey and D. Stalke, Org. Lett., 2003, 5, 2199 CrossRef CAS.
- H. Iwamoto, Y. Kondo, T. Kawatani, T. Haino and Y. Fukazawa, Tetrahedron Lett., 2003, 44, 5975 CrossRef CAS.
- J. Jyrkkarinne, J. Makinen, J. Gynther, H. Savolainen, A. Poso and P. Honkakoski, J. Med. Chem., 2003, 46, 4687 CrossRef.
- C. M. Marson, A. S. Rioja, K. E. Smith and J. M. Behan, Synthesis, 2003, 535 CrossRef CAS.
- A. I. Koutsourea, E. S. Arsenou, M. A. Fousteris and S. S. Nikolaropoulos, Steroids, 2003, 68, 659 CrossRef CAS.
- C. Uyanik, J. R. Hanson and P. B. Hitchcock, J. Chem. Res. (S), 2003, 474 Search PubMed.
- C. Uyanik, J. R. Hanson and P. B. Hitchcock, J. Chem. Res. (S), 2003, 794 Search PubMed.
- J. R. Hanson and A. C. Hunter, J. Chem. Res. (S), 2003, 216 Search PubMed.
- C. Bensasson, J. R. Hanson and A. Parvez, J. Chem. Res. (S), 2003, 218 Search PubMed.
- J. R. Hanson, P. B. Hitchcock and K. Yildirim, J. Chem. Res. (S), 2003, 214 Search PubMed.
- C. Bensasson and J. R. Hanson, J. Chem. Res. (S), 2003, 124 Search PubMed.
- J. R. Hanson and I. Kiran, J. Chem. Res. (S), 2003, 130 Search PubMed.
- J. R. Hanson and I. Kiran, J. Chem. Res. (S), 2003, 136 Search PubMed.
- J. R. Hanson, P. B. Hitchcock and I. Kiran, J. Chem. Res. (S), 2003, 222 Search PubMed.
- A. R. McKinney, D. D. Ridley and P. Turner, Aust. J. Chem., 2003, 56, 829 CrossRef CAS.
- V. Lecomte, E. Stephen, F. Le Bideau and G. Jaouen, Tetrahedron, 2003, 59, 2169 CrossRef CAS.
- J. Dalmaris, J. R. Hanson, P. B. Hitchcock and I. Kiran, J. Chem. Res. (S), 2003, 150 Search PubMed.
- C. Wang, X. Jiang, H. Shi, J. Lu, Y. Ho and H. Hu, J. Org. Chem., 2003, 68, 4579 CrossRef CAS.
- A. Magyar, B. Schonecker, J. Wolfling, G. Schneider, W. Gunther and H. Gorls, Tetrahedron: Asymmetry, 2003, 14, 2705 CrossRef CAS.
- R. Skoda-Foeldes, E. Takacs, J. Horvath, Z. Tuba and L. Kollar, Green Chem., 2003, 5, 643 RSC.
- K. M. Penov Gasi, D. A. Miljkovic, L. D. Media Mijacevic, E. A. Djurendic, S. Z. Stojanovic, M. N. Sakac, S. M. Stankovic, D. Lazar, S. Andric and R. Kovacevic, Steroids, 2003, 68, 667 CrossRef.
- K. H. Chang, M. N. Jenkins, H. R. Wu and W. S. Li, Tetrahedron Lett., 2003, 44, 1351 CrossRef CAS.
- N. Zhu, Y. Ling, X. Lei, V. Handratta and A. M. H. Brodie, Steroids, 2003, 68, 603 CrossRef CAS.
- O. O. Clement, C. M. Freeman, R. W. Hartmann, V. D. Handratta, T. S. Vasaitas, A. M. H. Brodie and V. C. O. Njar, J. Med. Chem., 2003, 46, 2345 CrossRef CAS.
- C. Wang, S. Wang, Y. Hu, Y. Xu and H. Hu, Steroids, 2003, 68, 677 CrossRef CAS.
- S. Jones, D. Selitsianos, K. J. Thompson and S. M. Toms, J. Org. Chem., 2003, 68, 5211 CrossRef CAS.
- V. Pouzar, I. Cerny, O. Lapcik, M. Hill and R. Hampl, Steroids, 2003, 68, 149 CrossRef CAS.
- A. Ricci, E. Fasani, M. Mella and A. Albini, J. Org. Chem., 2003, 68, 4361 CrossRef CAS.
- A. Goswami, R. Kotoky, R. C. Rastogi and A. C. Ghosh, Org. Process Res. Dev., 2003, 7, 306 Search PubMed.
- L.-W. Guo, W. K. Wilson, J. Pang and C. H. L. Shackleton, Steroids, 2003, 68, 31 CrossRef CAS.
- T. Furuta, A. Suzuki, M. Matsuzawa, H. Shibasaki and Y. Kasuya, Steroids, 2003, 68, 693 CrossRef CAS.
- E. Bratoeff, E. Ramirez, E. Flores, N. Valencia, M. Sanchez, I. Heuze and M. Cabeza, Chem. Pharm. Bull., 2003, 51, 1132 CrossRef CAS.
- J. H. Wynne, C. T. Lloyd and G. W. Mushrush, Synth. Commun., 2003, 33, 885 CrossRef CAS.
- M. Joselevich, A. Ghini and G. Burton, Org. Biomol. Chem., 2003, 1, 939 RSC.
- A. S. Veleiro, R. E. Rosenstein, C. O. Jaliffa, M. L. Grilli, F. Speroni and G. Burton, Bioorg. Med. Chem. Lett., 2003, 13, 466 CrossRef CAS.
- X. Jiang, B. D. Manion, A. Benz, N. P. Rath, A. S. Evers, C. F. Zorumski, S. Mennerick and D. F. Covey, J. Med. Chem., 2003, 41, 5334 CrossRef.
- P. H. Di Chenna, P. Dauban, A. Ghini, R. Baggio, M. T. Garland and G. Burton, Tetrahedron, 2003, 59, 1009 CrossRef CAS.
- F. Wust, K. E. Carlson and J. A. Katzenellenbogen, Steroids, 2003, 68, 177 CrossRef CAS.
- U. Sakee and N. Kongkathip, Synth. Commun., 2003, 33, 1695 CrossRef CAS.
- U. Sakee, B. Kongkathip and N. Kongkathip, J. Chem. Res. (S), 2003, 12 Search PubMed.
- A. Chetia, A. Saikia, C. J. Saikia and R. C. Boruah, Tetrahedron Lett., 2003, 44, 2741 CrossRef CAS.
- P. M. Weintraub, A. K. Holland, C. A. Gates, W. R. Moore, R. J. Resvick, P. Bey and N. P. Peet, Bioorg. Med. Chem., 2003, 11, 427 CrossRef CAS.
- J. Wolfling, A. Magyar and G. Schneider, Monatsch. Chem., 2003, 134, 1387.
- A. Le Pera, A. Leggio, C. Siciliano, M. L. D. Gioia, A. Napoli, G. Sindona and A. Liguori, Steroids, 2003, 68, 139 CrossRef CAS.
- C. Arbez-Gindre, V. Berl and J. P. Lepoittevin, Steroids, 2003, 68, 361 CrossRef CAS.
- J. R. Hanson, I. Kiran and C. Scarbrough, J. Chem. Res. (S), 2003, 301 Search PubMed.
- J. R. Hanson, P. B. HItchcock and J. A. R. Salvador, J. Chem. Res. (S), 2003, 556 Search PubMed.
- A. Mangoni, V. Esposito and A. Randazza, Chem. Commun., 2003, 54 RSC.
- J. Xia, Y. Chen, K. M. Liberatore and B. S. Selinsky, Tetrahedron Lett., 2003, 44, 9295 CrossRef CAS.
- L. Nahar and A. B. Turner, Tetrahedron, 2003, 59, 8623 CrossRef CAS.
- S. P. Masterson, T. A. Miller and B. E. Nassim, Steroids, 2003, 68, 253 CrossRef CAS.
- J. L. Mola Garate, L. S. Garcia, C. S. Perez Martinez, M. A. Iglesias-Arteaga, D. C. Herrera and F. C. Manchado, Synth. Commun., 2003, 33, 1203 CrossRef CAS.
- M. Dukh, D. Saman, J. Kroulik, I. Cerny, V. Pouzar, V. Kral and P. Drasar, Tetrahedron, 2003, 59, 4069 CrossRef CAS.
- A. P. Davis and J. B. Joos, Coord. Chem. Rev., 2003, 240, 143 CAS.
- A. L. Sisson, J. P. Clare, L. H. Taylor, J. P. H. Charmant and A. P. Davis, Chem. Commun., 2003, 2246 RSC.
- A. Juliano and P. Scafato, Tetrahedron: Asymmetry, 2003, 14, 611 CrossRef.
- K. Nakano, E. Mochizuki, N. Yasui, K. Morioka, Y. Yamauchi, N. Kanehisa, Y. Kai, N. Yoswathananont, N. Tohnai, K. Sada and M. Miyata, Eur. J. Org. Chem., 2003, 2428 CrossRef CAS.
- T. Fukami, K. Yamaguchi, Y. Tozuka, K. Moribe, T. Oguchi and K. Yamamoto, Chem. Pharm. Bull., 2003, 51, 227 CrossRef CAS.
- S. Marczak and J. Wicha, Synthesis, 2003, 1049 CAS.
- M. Di Filippo, I. Izzo, L. Savignano, P. Tecilla and F. De Riccardis, Tetrahedron, 2003, 59, 1711 CrossRef CAS.
- L. Nahar and A. B. Turner, Steroids, 2003, 68, 1157 CrossRef CAS.
- J. Ropponen, J. Tamminen, E. Kolehmainen and K. Rissanen, Synthesis, 2003, 2266.
- M. Dukh, D. Saman, K. Lang, V. Pouzar, I. Cerny, P. Drasar and V. Krai, Org. Biomol. Chem., 2003, 1, 3458 RSC.
- D. Opsenica, D. E. Kyle, W. K. Milhous and B. A. Soleja, J. Serb. Chem. Soc., 2003, 68, 291 CAS.
- Y. Hirayama, M. Iwamura and T. Furuta, Bioorg. Med. Chem. Lett., 2003, 13, 905 CrossRef CAS.
- H. Cao, N. Tokutake and S. L. Regen, J. Am. Chem. Soc., 2003, 125, 16183.
- T. Nishijo, S. Moriyama and S. Shiota, Chem. Pharm. Bull., 2003, 51, 1253 CrossRef.
- K. Okumura, Y. Nakamura, S. Takeuchi, I. Kato, Y. Fujimoto and N. Ikekawa, Chem. Pharm. Bull., 2003, 51, 1177 CrossRef CAS.
- G. A. G. Santos, A. P. Murray, C. A. Pujol, E. B. Damonte and M. S. Maier, Steroids, 2003, 68, 125 CrossRef CAS.
- J. L. Giner and J. A. Faraldos, Helv. Chim. Acta, 2003, 86, 3613 CrossRef CAS.
- N. M. Krstic, M. S. Bjelakovic, L. B. Lorenc and V. D. Pavlovic, J. Serb. Chem. Soc., 2003, 68, 785 CAS.
- M. S. Bjelakovic, V. D. Pavlovic, M. M. Dabovic and L. B. Lorenc, J. Serb. Chem. Soc., 2003, 68, 303 CAS.
- M. S. Bjelakovic, L. B. Lorenc, V. D. Pavlovic, B. Tinant, J. P. Declerq and J. Kalvoda, Helv. Chim. Acta, 2003, 86, 2121 CrossRef CAS.
- M. Jurado-Gonzalez, A. C. Sullivan and J. R. H. Wilson, Tetrahedron Lett., 2003, 44, 4283 CrossRef CAS.
- E. S. Arsenon, A. I. Koutsourea, M. A. Fousteria and S. S. Nikolaropoulous, Steroids, 2003, 68, 407 CrossRef.
- X. Jin, X. Yang, L. Yang, Z. Liu, F. Zhang, J. Wang and Y. Li, J. Chem. Res. (S), 2003, 477 Search PubMed.
- P. Ciuffreda, S. Casati, L. Alessandrini, G. Terraneo and E. Santaniello, Steroids, 2003, 68, 733 CrossRef CAS.
- T. Oishi, H. Tsuchikawa, M. Murata, M. Yoshida and M. Morisawa, Tetrahedron Lett., 2003, 44, 6387 CrossRef CAS.
- T. Blume, J. Kuhnke and L. Zorn, Org. Lett., 2003, 5, 1837 CrossRef CAS.
- B. Liu and W. S. Zhou, Tetrahedron, 2003, 59, 3379 CrossRef CAS.
- H. Satoh, H. Koshino, J. Uzawa and T. Nakata, Tetrahedron, 2003, 59, 4539 CrossRef CAS.
- L. Peng, Y. Li and W. Z. Li, Tetrahedron Lett., 2003, 44, 3991 CrossRef CAS.
- M. Sisa, M. Budesinsky and L. Kohout, Collect. Czech. Chem. Commun., 2003, 68, 2171 CrossRef CAS.
- A. P. Massey, V. S. Pore and B. G. Hazra, Synthesis, 2003, 426 CAS.
- A. Kolbe, A. Porzel, J. Schmidt and G. Adam, J. Labelled Compd. Radiopharm., 2003, 46, 231 CrossRef CAS.
- H. Mitome, M. Shinohara, H. Miyaoka and Y. Yamada, Chem. Pharm. Bull., 2003, 51, 640 CrossRef CAS.
- W. Li and P. L. Fuchs, Org. Lett., 2003, 5, 2853 CrossRef CAS.
- W. Li and P. L. Fuchs, Org. Lett., 2003, 5, 2849 CrossRef CAS.
- M. S. Cheng, Q. L. Wang, Q. Tian, H. Y. Song, Y. X. Liu, Q. Li, X. Xu, H. D. Miao, X. S. Yao and Z. Yang, J. Org. Chem., 2003, 68, 3658 CrossRef CAS.
- R. Suhr, P. Pfefferkorn, S. Weingarten and J. Thiem, Org. Biomol. Chem., 2003, 1, 4373 RSC.
- A. M. Nafady, M. A. El-Shanawany, M. H. Mohamed, H. A. H. Hassanean, X. H. Zhu, T. Yoshihara, M. Okawa, T. Ikeda and T. Nohara, Tetrahedron Lett., 2003, 44, 3509 CrossRef CAS.
- Q. Xu, X.-W. Peng and W. S. Tian, Tetrahedron Lett., 2003, 44, 9375 CrossRef CAS.
- J. S. Lee and P. L. Fuchs, Org. Lett., 2003, 5, 3619 CrossRef CAS.
- J. Sandoval-Ramirez, S. Meza-Reyes, R. E. del Rio, G. Hernandez-Linares, A. Suarez-Rojas, S. Rincon, N. Farfan and R. L. Santillan, Steroids, 2003, 68, 199 CrossRef CAS.
- J. R. Williams, D. Chai, J. D. Bloxton, H. Gong and W. R. Solvibile, Tetrahedron, 2003, 59, 3183 CrossRef CAS.
- P. K. Agrawal, Magn. Reson. Chem., 2003, 41, 965 CrossRef CAS.
- F. Magaraci, C. J. Jimenez, C. Rodriguez, J. C. F. Rodriguez, M. V. Braga, V. Yardley, K. de Luce-Fradley, S. L. Croft, W. de Souza, L. M. Ruiz-Perez, J. Urbana, D. Gonzalez-Pacanowska and I. H. Gilbert, J. Med. Chem., 2003, 46, 4714 CrossRef CAS.
- G. H. Posner and M. Kahraman, Eur. J. Org. Chem., 2003, 3889 CrossRef CAS.
- Y. J. Chen, L. J. Gao, I. Murad, A. Verstuyf, L. Verlinden, C. Verboven, R. Bouillon, D. Viterbo, M. Milanesio, D. van Haver, M. Vandewalle and P. J. De Clercq, Org. Biomol. Chem., 2003, 1, 257 RSC.
- X. Jin, X. Yang, L. Yang, Z. L. Liu and F. Zhang, J. Chem. Res. (S), 2003, 691 Search PubMed.
- J. Saltiel, L. Cires and A. M. Turek, J. Am. Chem. Soc., 2003, 125, 2866 CrossRef CAS.
- X. Perez-Garcia, A. Rumbo, M. J. Lariba, P. Ordonez, A. Munoz and A. Mourino, Chem. Lett., 2003, 5, 4033 CAS.
- N. Saito, H. Saito, M. Anzai, A. Yoshida, T. Fujishima, K. Takenouchi, D. Miura, S. Ishizuka, H. Takatama and A. Kittaka, Org. Lett., 2003, 5, 4859 CrossRef CAS.
- N. Saito, T. Matsunaga, T. Fujishima, M. Anzai, H. Saito, K. Takenouchi, D. Miura, S. Ishizuka, H. Takayama and A. Kittaka, Org. Biomol. Chem., 2003, 1, 4396 RSC.
- X. C. Gonzalez-Avion and A. Mourino, Org. Lett., 2003, 5, 2291 CrossRef CAS.
- L. K. A. Bilhr, F. Bjoerkling, M. J. Calverley, E. Binderup and M. Begtrup, J. Org. Chem., 2003, 68, 1367 CrossRef.
- M. Ahmed, C. E. Atkinson and A. G. M. Barrett, Org. Lett., 2003, 5, 669 CrossRef CAS.
- T. Hanazawa, A. Koyama, T. Wada, E. Morishige, S. Okamoto and F. Sato, Org. Lett., 2003, 5, 523 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |