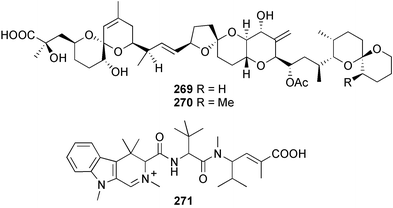
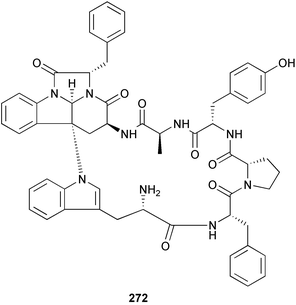
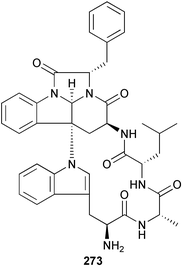
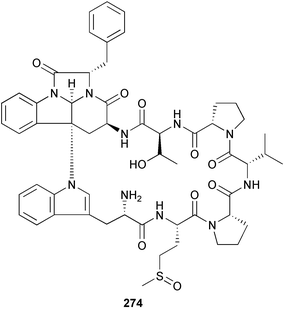
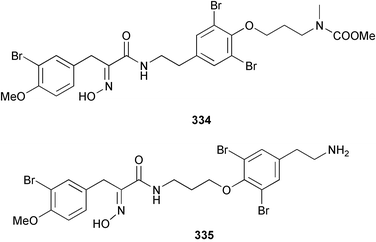
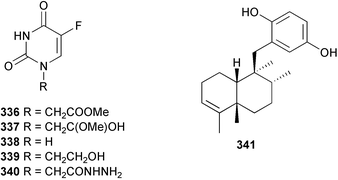
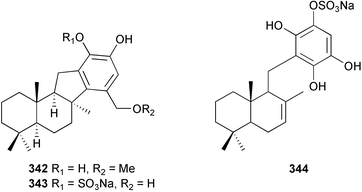
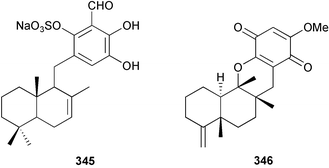
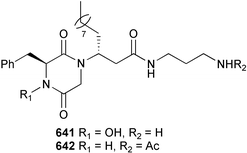
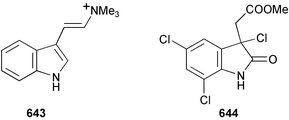
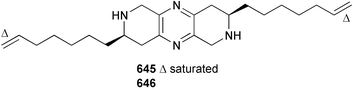
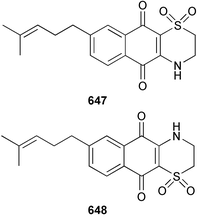
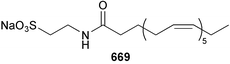
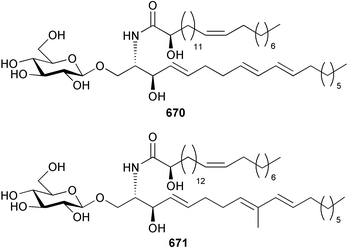
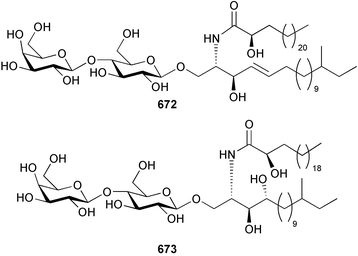
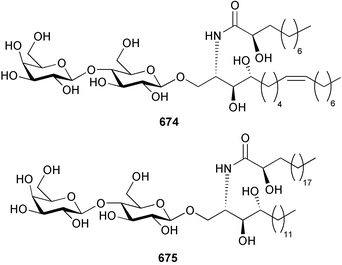
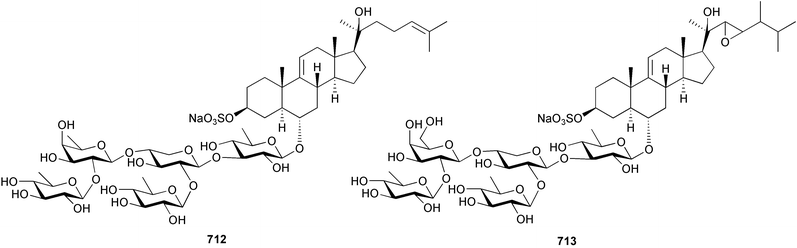
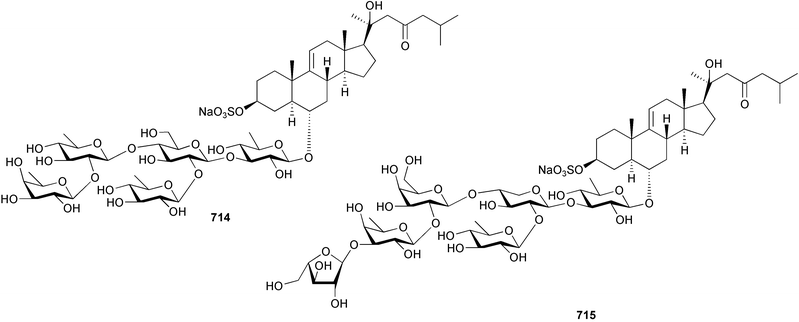
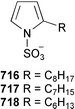
Marine natural products
John W.
Blunt
*a,
Brent R.
Copp
b,
Murray H. G.
Munro
a,
Peter T.
Northcote
c and
Michèle R.
Prinsep
d
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: john.blunt@canterbury.ac.nz
bDepartment of Chemistry, University of Auckland, Auckland, New Zealand
cSchool of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dDepartment of Chemistry, University of Waikato, Hamilton, New Zealand
First published on 19th January 2005
Abstract
Covering: 2003. Previous review: Nat. Prod. Rep., 2004, 21, 1
This review covers the literature published in 2003 for marine natural products, with 619 citations (413 for the period January to December 2003) referring to compounds isolated from marine microorganisms and phytoplankton, green algae, brown algae, red algae, sponges, coelenterates, bryozoans, molluscs, tunicates and echinoderms. The emphasis is on new compounds (656 for 2003), together with their relevant biological activities, source organisms and country of origin. Biosynthetic studies or syntheses that lead to the revision of structures or stereochemistries have been included (78), including any first total syntheses of a marine natural product.
 John W. Blunt John W. Blunt | John Blunt obtained his BSc (Hons) and PhD degrees from the University of Canterbury, followed by postdoctoral appointments in Biochemistry at the University of Wisconsin-Madison, and with Sir Ewart Jones at Oxford University. He took up a lectureship at the University of Canterbury in 1970, where he is now a Professor. His research interests are with natural products, and the application of NMR techniques to structural problems. |
 Brent R. Copp Brent R. Copp | Brent Copp received his BSc (Hons) and PhD degrees from the University of Canterbury, where he studied the isolation, structure elucidation and structure–activity relationships of biologically active marine natural products under the guidance of Professors Blunt and Munro. He undertook postdoctoral research with Jon Clardy at Cornell and Chris Ireland at the University of Utah. 1992–93 was spent working in industry as an isolation chemist with Xenova Plc, before returning to New Zealand to take a lectureship at the University of Auckland, where he is currently a Senior Lecturer. |
 Murray H. G. Munro Murray H. G. Munro | Murray Munro, a Professor in Chemistry at the University of Canterbury, Christchurch, New Zealand, has worked on natural products, mainly of New Zealand origin, for all of his professional career. A marine natural products research group was started in 1975 and in more recent years the research interests of the group have widened to include terrestrial as well as marine fungi and actinomycetes and drug delivery systems based on polymer therapeutics. |
 Peter T. Northcote Peter T. Northcote | Peter Northcote, received his BSc and PhD degrees from the University of British Columbia, Canada where he was a member of R. J. Andersen's marine natural products research group. He carried out postdoctoral research with Professors Blunt and Munro at the University of Canterbury before taking a position as a senior research scientist at Lederle Laboratories, American Cyanamid Co. He joined the faculty of the Victoria University of Wellington in 1994 where he is currently a Senior Lecturer in organic chemistry. |
 Michèle R. Prinsep Michèle R. Prinsep | Michèle Prinsep received her BSc (Hons) and PhD degrees from the University of Canterbury, where she studied the isolation and structural elucidation of biologically active secondary metabolites from sponges and bryozoans under the supervision of Professors Blunt and Munro. She undertook postdoctoral research on cyanobacteria with Richard Moore at the University of Hawaii before returning to New Zealand to take up a lectureship at the University of Waikato, where she is currently a Senior Lecturer. |
1 Introduction
This review is of the literature for 2003 and describes 656 new compounds from 243 articles. These numbers are comparable to those of the past few years. We show structures only for new compounds, or for previously reported compounds where there has been a structural revision or a newly established stereochemistry. Previously reported compounds for which first syntheses or new bioactivities are described, are referenced, but separate structures are generally not shown.2 Reviews
A number of reviews have dealt with classes of compounds: “Sterols in microorganisms”,1 “Bioactive macrolides and polyketides from marine dinoflagellates”,2 “Chemistry and biology of new marine alkaloids from the indole and annelated indole series”,3 “Brominated diterpenes of marine origin”,4 “Sulfur-containing natural products from marine invertebrates”,5 “The cerebrosides”,6 “Nonribosomal peptides from marine sponges”,7 “Bioactive polyhydroxysterols and their sapogenins from marine organisms”,8 “Sphingolipids from marine organisms”,9 “A review of research on the cyanotoxin cylindrospermopsin”,10 and “The manzamine alkaloids”.11Reviews that focus on bioactivity and development as drug candidates include: “Natural products as sources of new drugs over the period 1981–2002”,12 “Marine natural products as prototype agrochemical agents”,13 “Detection of pharmacologically active natural products using ecology”,14 “Marine pharmacology in 2000: antitumour and cytotoxic compounds”,15 “Bioactive natural products from marine invertebrates and associated fungi”,16 “Marine pyridoacridine alkaloids and synthetic analogues as antitumour agents”,17 “Drugs from the deep: marine natural products as drug candidates”,18 “Marine-derived anticancer agents in clinical trials”,19 “Marine natural products as lead anti-HIV agents”,20 “Natural products with anti-HIV activity from marine organisms”,21 “Algae, a possible source for new drugs in the treatment of HIV and other viral diseases”,22 and “Antimycobacterial natural products”.23
Chemical synthesis is the theme of a number of reviews covering specific types of compounds through to more generally applicable methodology: “Total synthesis of (+)-macrosphelides A, C, E, F and G based on enzymatic function”,24 “The total syntheses of phorboxazoles-new classes in natural product synthesis”,25 “The development of a practical total synthesis of discodermolide”,26 “Synthesis of the pyrrole-imidazole alkaloids”,27 “Chemistry of bis-spiroacetal systems: natural products, synthesis and stereochemistry”,28 “Approaches towards the synthesis of cephalostatins, ritterazines and saponins from Ornithogalum saundersiae”,29 “New and old challenges in total synthesis. From concept to practise”30 and “Microtubule-stabilizing marine metabolite laulimalide and its derivatives: synthetic approaches and antitumour activity”.31
Other more general reviews include: “Molecular biodiversity. Case study: Porifera (sponges)”,32 “Microalgal metabolites”,33 “Enhancing marine natural product structural diversity and bioactivity through semisynthesis and biocatalysis”,34 and “Marine natural products”.35 References to other reviews are more appropriately placed in the following sections. The Marinlit database36 continues to be updated and has again been used as the basis for the preparation of this present review.
3 Marine microorganisms and phytoplankton
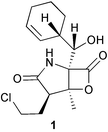
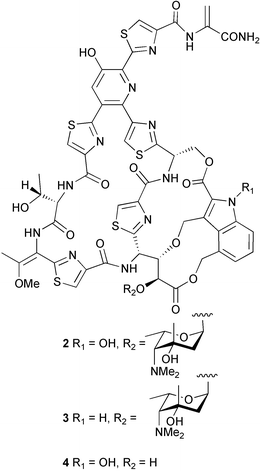
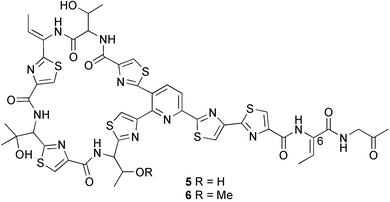
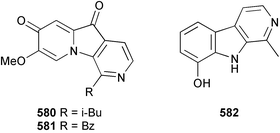
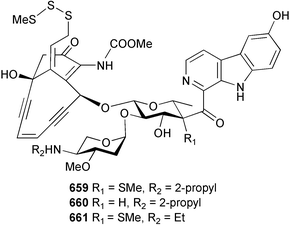
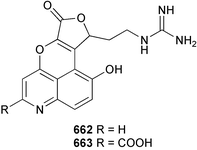
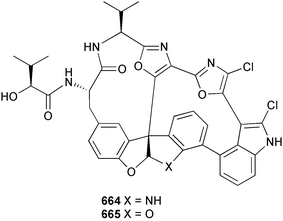
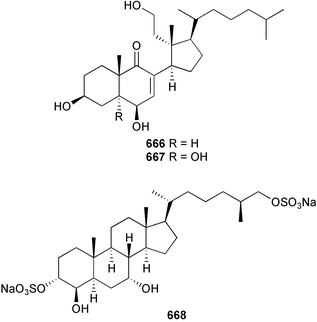
Probably the most important paper on marine microorganisms in 2003 was the first report on chemistry from the new obligate marine actinomycete taxon Salinospora.37 In excess of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 strains from this taxon have now been isolated and the potent proteasome inhibitor salinosporamide A 1 was isolated from a culture of a Salinospora sp. originating from a heat-treated marine sediment sample from the Bahamas. The structure of salinosporamide A, including the absolute stereochemistry, was deduced through spectral and X-ray analyses. Salinosporamide A displayed potent and selective in vitro cytotoxicity against cell lines in the NCI panel. Salinosporamide A also exhibited highly potent inhibition of the proteasomal chymotrypsin-like proteolytic activity of purified 20S proteasome. The unique functionalisation of the core-fused γ-lactam-β-lactone bicyclic ring structure of salinosporamide A 1 appears to contribute to its potency. The thiazolyl peptide antibiotics, nocathiacins I–III 2–4, have been isolated from the culture broth of Nocardia sp. (source not given).38 The nocathiacins exhibit potent in vitro activity against a wide range of bacteria, including several multiple-drug resistant pathogens and also exhibit excellent in vivo efficacy in a systemic Staphylococcus aureus infection mouse model.39 However, nocathiacin I 2 was found to be identical to an antibiotic isolated from Amycolatopsis sp.40 but spectral data and stereochemical details had not been originally reported for this compound. Two cyclic thiopeptides 5 and 6, obtained from a culture of Bacillus cereus isolated from the marine sponge Halichondria japonica,41 exhibited potent antibacterial activities against Staphylococci and Enterococci sp., and were active against multiple-drug resistant strains.42
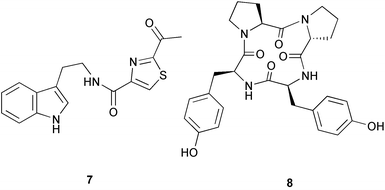
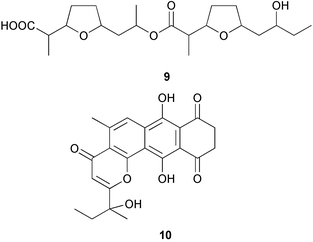
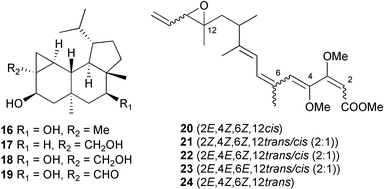
(6Z)-Geometry for these compounds was implied by ROESY correlations. 1H-15N HMBC analysis was used in determining the structure of bacillamide 7, a peptidic metabolite of an algicidal marine Bacillus sp. isolated during the termination of a bloom of Cochlodinium polykrikoides in Masan Bay, Korea.43 Bacillamide was shown to be active against a wide range of dinoflagellates and raphidophytes.44 Culture of an exocellular extract of a Pseudomonas sp. associated with Ircinia muscarum from the Bay of Naples, Italy gave the cyclotetrapeptide 8.45 The amino acid stereochemistry was established by standard methods (for example, chiral HPLC analysis of the acid hydrolysate, Marfey's method etc.). Four Streptomyces sp. of diverse origin yielded a range of metabolites. Firstly, culture of a Streptomyces sp. from a sediment sample from Oahu, Hawaii, yielded the antibacterial and antifungal metabolite bonactin 9.46 Parimycin 10, a new 1,4-anthraquinone, was isolated from a Streptomycete sediment sample from Laguna de Terminos, Gulf of Mexico. Parimycin had moderate activity against B. subtilis, Streptomyces viridochromogenes, S. aureus and E. coli, in addition to activity against a number of human tumour cell lines.47 A Streptomyces sp. cultured from an unidentified Mexican marine invertebrate yielded the cytotoxic indoles 11–13 which had moderate activity against a panel of 14 tumour cell lines.48 Finally, the anthracycline komodoquinone A 14 and the aglycone komodoquinone B 15 were isolated from a culture of a Streptomyces sp. isolated from marine sediment off Komodo Island, Indonesia. Komodoquinone A displayed dose-dependent neuritogenic activity against the neuroblastoma cell line Neuro 2A.49 A culture broth of an ATCC strain of the marine gliding bacterium Saprospira grandis yielded four neoverrucosane diterpenoids, 16–19. The relative and absolute stereochemistries of 16 were determined by standard methods50
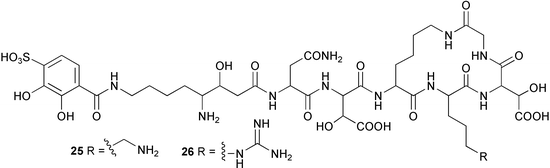
(for example, X-ray analysis, NOESY and ROESY NMR experiments, the modified Mosher method, chiral HPLC, comparison of circular dichroism (CD) or other optical data against standards or model compounds etc.). The marine myxobacterium Haliangium ochraceum,51 originally H. luteum, yielded several new isomers of the polyene antifungal antibiotic haliangicin.52,53 These are cis-haliangicin 20 and haliangicins B–D 21–23, geometrical isomers of the polyene and epoxide moieties. The stereochemistry of the epoxide in the known haliangicin 2453 has been determined as trans. All of the haliangicins were active against the phytopathogenic fungus Phytophthora capsici.54 Two siderophores, pseudoalterobactins A 25 and B 26, were isolated from a culture of the bacterium Pseudoalteromonas sp. isolated from the marine sponge Cinachyrella australiensis collected in Palau. Both compounds displayed strong binding affinity for the ferric ion in the chrome azurol S (CAS) assay.55 The bactericidal compound 27, obtained from a culture of a new marine species Pseudoalteromonas phenolica sp. nov., isolated from seawater collected off Ogasawara Island Japan,56 had potent activity against methicillin-resistant S. aureus
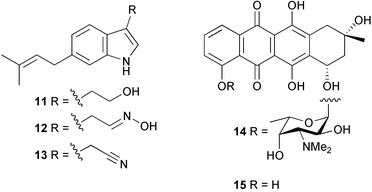
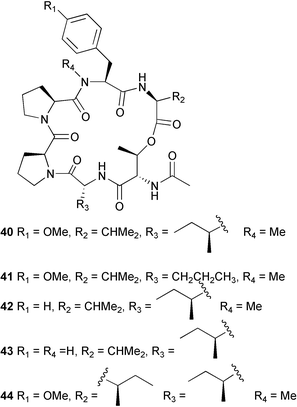
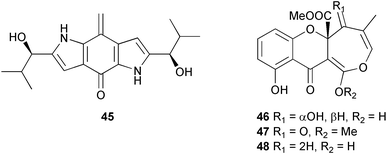
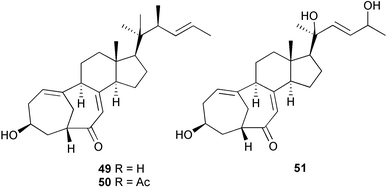
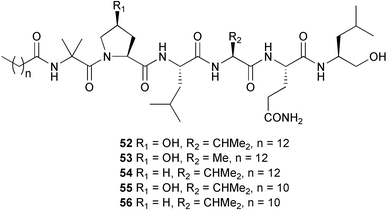
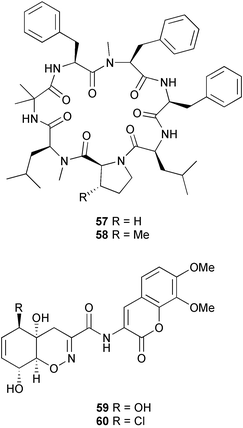
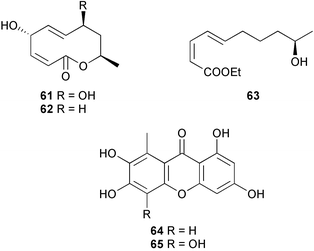
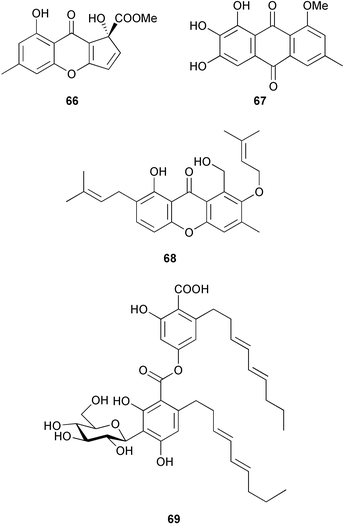
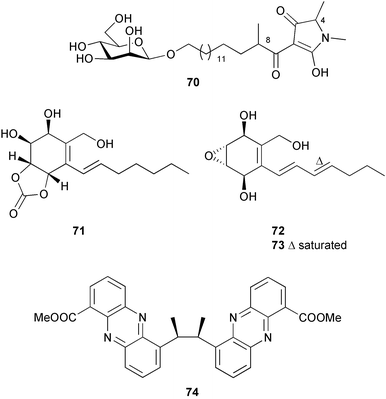
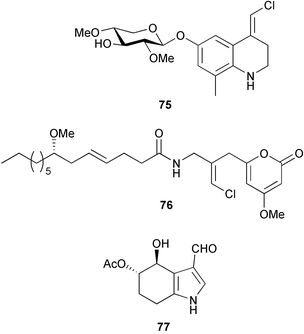
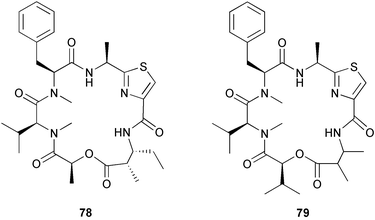
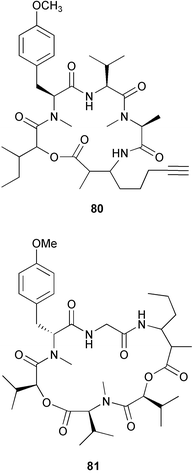
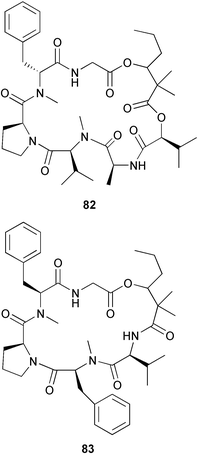
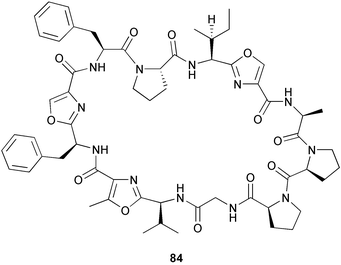
(MRSA) and was also strongly active against Enterococcus serolicida, E. faecium and E. faecalis.57 This compound is available commercially, but this is the first reported isolation as a natural product. Cultures of two marine bacterial strains isolated from cultures of Pecten maximus larvae in Galicia, Spain, led to the first reported isolation, as natural products, of a series of DD-diketopiperazines 28–31 and established them as potent inhibitors of the pathogenic marine bacterium Vibrio anguillarum. The structures were confirmed by synthesis.58 A cytotoxic polycyclic xanthone 32 has been isolated from the culture broth of the actinomycete Actinomadura sp.59 The phenoxazin-3-one antibiotics, chandrananimycins A–C 33–35, were also isolated from a culture of Actinomadura sp. derived from sediment from Jiaozhou Bay, China. Chandrananimycins A–C were active against human tumour cell lines while 35 exhibited potent activity against the fungus Mucor meihei and the bacteria B. subtilis and E. coli, and antialgal activity against the microalga, Chlorella vulgaris, C. sorokiniana and Scenedesmus suspicatus.60 The fungus Aspergillus tamarii was isolated from driftwood collected in Okinawa and cultured to yield a pentacyclic oxindole alkaloid, speradine A 36. The structure and relative stereochemistry of 36 were confirmed by X-ray analysis. Speradine A exhibited inhibitory activity against histone deacetylase and antibacterial activity against Micrococcus luteus.61 A culture of the fungus Aspergillus ostianus, isolated from an unidentified marine sponge from Pohnpei, was the source of three chlorinated antibiotics, the asperlactone derivatives 37 and 38 and the aspyrone derivative 39. Compound 37 was the most potent, inhibiting the growth of the marine bacterium Ruegeria atlantica and that of E. coli and S. aureus to a lesser extent.62 Five novel depsipeptides, aspergillicins A–E 40–44, were obtained from a culture of Aspergillus carneus collected from estuarine sediment in Tasmania, Australia. The amino acid sequences were assigned by MSn ion-trap ESI mass spectrometry and stereochemistry was assigned by standard methodology. The aspergillicins exhibited modest cytotoxicity against Haemonchus contortus.63 A chiral dipyrrolobenzoquinone derivative, terreusinone 45, has been obtained from a cultured strain of the marine algicolous fungus Aspergillus terreus isolated from the surface of the marine red alga Halymenia acuminata collected from Bijin Island, South Korea. The absolute stereochemistry was determined by a combination of Horeau's method and quantum chemistry calculations. Terreusinone has intense UV-A absorbtivity.64 A culture of Penicillium brocae from the tissue of the Fijian sponge Zyzzya sp. was the source of three novel cytotoxic polyketides, brocaenols A–C 46–48. These contain the unusual enolised oxepine lactone ring system. Structure determination included an INADEQUATE experiment on brocaenol A. The absolute stereochemistry of 46 was established by a standard method and extended to 47 and 48 by comparison of CD and optical rotation data.65 Brocaenols A–C displayed moderate activity against the HCT-116 cell line. Structures for brocaenols B and C were reversed in the original paper, but a correction has since been published.66 The steroids isocyclocitrinol A 49 and 22-acetylisocyclocitrinol A 50 were extracted from a salt water culture of Penicillium citrinum isolated from an Axinella sp. collected in Papua New Guinea.67 The absolute stereochemistry of 50 was established by standard methods, extended to 49, leading to the structural revision of cyclocitrinol, previously isolated from a terrestrial P. citrinum,68 to 51. Compounds 49 and 50 displayed weak antibacterial activity against Staphylococcus epidermidis and Enterococcus durans. The halovirs A–E 52–56, lipophilic linear peptides, are potent in vitro inhibitors of Herpes simplex viruses 1 and 2 and were isolated from a Scytalidium sp. sourced from the Caribbean seagrass Halodule wrightii.69 Two cyclic heptapeptides, scytalidamides A 57 and B 58, have been isolated from the culture broth of another Scytalidium sp. derived from the surface of the green alga Halimeda sp. collected off the Bahamas. The absolute configurations were confirmed by standard methods including CD measurements. Both scytalidamides displayed moderate cytotoxicity to the HCT-116 cell line in vitro.70 Trichodermamides A 59 and B 60, modified dipeptides, were isolated from cultures of Trichoderma virens isolated from the ascidian Didemnum molle and from the surface of a green alga of the genus Halimeda, both collected in Papua New Guinea. The ascidian-derived culture contained trichodermamide A with traces of trichodermamide B while a greater quantity of trichodermamide B was isolated from the algal-derived strain. The structure of 59 was assigned by X-ray diffraction while the absolute stereochemistry was determined using the modified Mosher method. Trichodermamide B displayed significant in vitro cytotoxicity against HCT-116 and moderate antimicrobial activity against amphoterocin-resistant C. albicans, MRSA and vancomycin-resistant E. faecium.71 Trichodermamide A is closely related to penicillazine, reported from a marine-derived Penicillium sp.72 The reported structures differ only in the translocation of ester and amide bonds, but spectral data comparison suggests that these compounds may be identical. Two macrolides, modiolides A 61 and B 62, and a linear pentaketide modiolin 63 have been isolated from the culture of Paraphaeosphaeria sp. separated from the marine horse mussel Modiolus auriculatus, collected in Okinawa. The absolute stereochemistry of 61 was determined by the exciton chirality method73 using a p-methoxycinnamoyl ester, while the absolute stereochemistry of 63 was defined by the modified Mosher method. Modiolides A and B exhibited modest antibacterial activity against Micrococcus luteus and Neurospora crassa.74 A culture of the marine fungus Wardomyces anomalus, isolated from the green alga Enteromorpha sp. collected in the Baltic Sea, yielded two xanthone derivatives, anomalins A 64 and B 65.75 The anomalins were only weakly antimicrobial, but anomalin A possessed significant tyrosine kinase p56lck enzyme inhibitor activity and antioxidative properties. Remisporine A 66, a novel cyclopentachromenone, isolated from a culture of the marine fungus Remispora maritima from an unspecified wood source, is unstable under normal conditions and autocatalytically dimerises stereospecifically, via a Diels–Alder reaction, to remosporine B.76 A new anthraquinone, evariquinone 67, and the new prenylxanthone isoemericellin 68 were isolated from a culture of the fungus Emericella variecolor derived from the marine sponge Haliclona valliculata collected at Elba, Italy. The known C-glycosidic depside stromemycin 6977 was also isolated, and the previously undescribed double bond configurations established. Evariquinone 67 showed antiproliferative activity towards KB and NCI-H460 cells.78 A culture of a marine strain of the fungus Epicoccum purpurascens, isolated from inner tissue of the jellyfish Aurelia aurita collected from the North Sea, Germany, yielded the tetramic acid derivative epicoccamide 70. Attempts to resolve the stereochemistry at C-4 and C-8 by comparision of CD spectra with those of similar compounds were ambiguous.79 Two highly oxygenated polyketides, phomoxin 71 and phomoxide 72, are metabolites from a Phoma sp. isolated from a microbial mat collected from a Bahaman hypersaline pond, along with eupenoxide 73, a previously synthesised, but unpublished fungal metabolite.80 An actinomycete, Pseudonocardia sp., isolated from littoral sediment from Mauritius, Indian Ocean, was the source of a new phenazine derivative, phenazostatin D 74 which is the meso- form of the known antibiotic phenazostatin B.81,82 Investigations of a collection of Lyngbya majuscula from Puerto Rico resulted in the isolation of three new metabolites, a quinoline alkaloid, 75, malyngamide T 76 and a tryptophan derivative 77.83 Geometries for the vinyl chloride functionalities of 75 and 76 were established as (E) by 1H-13C coupling constant measurement from HSQMBC NMR experiments.84 Six cyclic depsipeptides, guineamides A–F 78–83, were isolated from a collection of Lyngbya majuscula collected from Papua New Guinea. Absolute stereochemistries for most of the amino acids were determined by standard methods. Guineamides B and C were moderately cytotoxic to a mouse neuroblastoma cell line.85L. majuscula from Papua New Guinea was the source of the novel cyclic dodecapeptide wewakazole 84 which contains an unprecedented number of five-membered
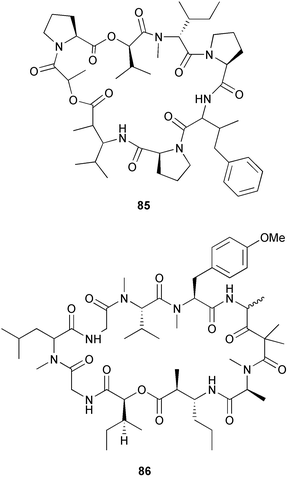
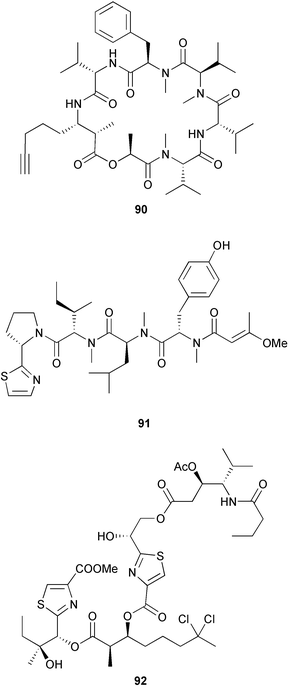
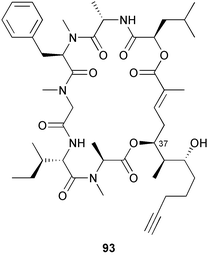
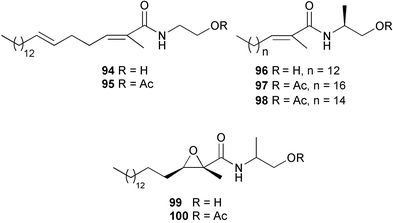
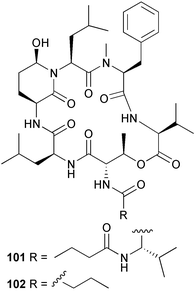
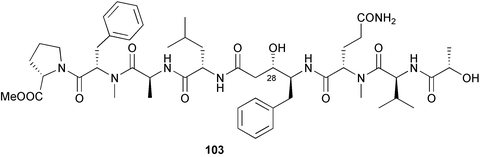
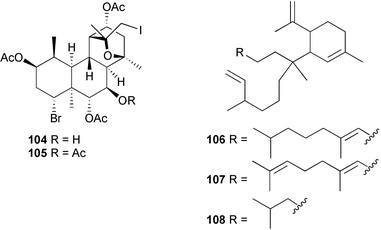
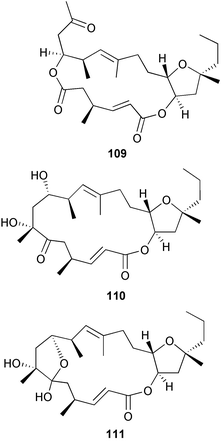
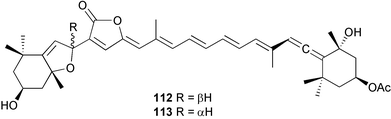
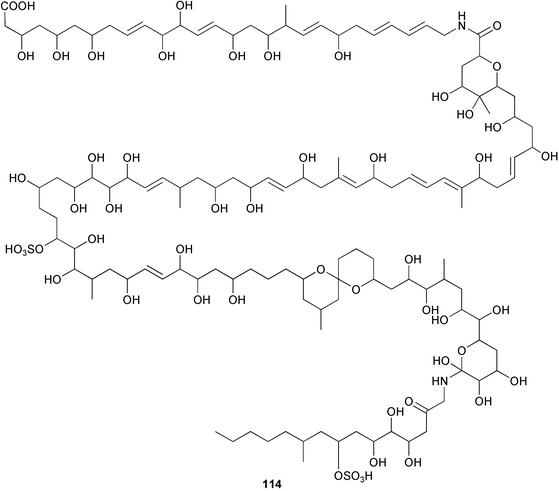
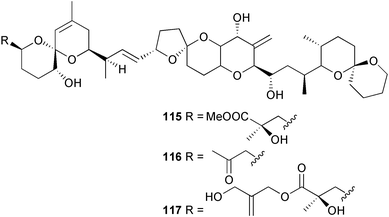
heterocyclic rings (six). Due to extensive signal overlap the structural assignment required multiple NMR and MS/MS experiments. The absolute stereochemistry was determined by standard methods.86L. majuscula from the southern Kenyan Coast was the source of the cyclic depsipeptide homodolastatin 16 85. The absolute stereochemistries of most of the amino acids in homodolastatin 16 were determined by standard methods. Homodolastatin 16 85 displayed moderate activity against oesophageal and cervical cancer cell lines.87 The cyclic peptide lyngbyastatin 3 86, isolated from L. majuscula collected from Guam, contains two unusual amino acid units, including 4-amino-2,2-dimethyl-3-oxopentanoic acid (Ibu). The configuration of the Ibu unit was established by acid hydrolysis and comparison with synthetic standards, while the absolute stereochemistries of the remaining residues were determined by standard methods. Lyngbyastatin 3, along with the previously isolated lyngbyastatin 1 and dolastatin 12,88 are in fact diastereotopic mixtures of both Ibu epimers. Lyngbyastatin 3 86 exhibited activity against KB and LoVo cell lines in vitro, but was poorly tolerated in vivo with little antitumour activity.89 Three new malyngamides, U–W 87–89, have been isolated from L. majuscula collected in Papua New Guinea. Partial relative stereochemistries only were determined.90 A collection of Lyngbya sp. from Palau yielded ulongapeptin 90, a cytotoxic cyclic depsipeptide,91 while a Lyngbya sp. from Guam yielded two new compounds, 15-norlyngbyapeptin A 91 and lyngbyabellin D 92.92 The absolute stereochemistries in each case were determined through degradative studies and/or comparison with commercially available and synthetic standards. Ulongapeptin was moderately cytotoxic against KB cells in vitro91 and lyngbyabellin D displayed activity against the KB cell line.92 Bioassay-guided fractionation of an extract from a Lyngbya sp. collected in Palau led to the isolation of palau'amide 93. Effective use was made of a band-selective HMBC experiment to unambiguously assign 13C NMR signals that were separated by only 0.1 ppm.93 Except for C-37, relative and absolute configurations were determined by standard methods. By modelling, and from NOE data, C-37 was assigned as having the (S) configuration. Palau'amide 93 exhibited potent cytotoxicity against KB cells.94 Semiplenamides A–G 94–100, anandamide-like fatty acid amides, were isolated from a collection of Lyngbya semiplena collected in Papua New Guinea. The absolute stereochemistries of the amino alcohols in semiplenamides C–E 96–98 were elucidated as all L by chemical derivatisation and chiral GCMS methods. All of the semiplenamides displayed toxicity in the brine shrimp assay, while semiplenamides A, B and G exhibited weak affinity for the rat cannabinoid CB1 receptor. Semiplenamide A was also a moderate inhibitor of the anandamide membrane transporter (AMT).95 Samples of the marine cyanobacterium Symploca sp. collected in Palau were the source of the depsipeptides tasipeptins A 101 and B 102,96 and a cytotoxic peptide, tasiamide B 103.97 The relative and absolute configurations of the tasipeptins and tasiamide B were determined by standard methods except for the configuration of C-28 in tasiamide B. This was tentatively suggested as (S) from NMR data analysis.97 Both tasipeptins exhibited moderate cytotoxicity towards KB cells in vitro. Also collected in Palau was an assemblage of a Symploca sp. cyanobacterium and an unidentified red alga. From this was isolated the iodinated diterpenes, tasihalides A 104 and B 105. These compounds possess a novel cage structure with both an oxabicyclic ring system and a cis-decalin system. These are the only examples of iodinated diterpenes in nature. Since terpenoids are almost never reported from marine cyanobacteria, but halogenated terpenes are ubiquitous in red algae, the authors speculate that the more likely source of the tasihalides is the alga and not the cyanobacterium.98 Two polyunsaturated monocyclic triterpenes 106 and 107 have been isolated from a culture of the common marine diatom Rhizosolenia setigera. The structure of a related monocyclic sesterterpene 108 was also proposed on the basis of mass spectral comparisons with compounds 106 and 107.99 Amphidinolide X 109100 and amphidinolide Y 110101 are cytotoxic 16- and 17-membered macrodiolides isolated from cultures of the marine dinoflagellate Amphidinium sp., originally separated from the inside cells of the marine acoel flatworm Amphiscolops sp. collected from Okinawa. Amphidinolide Y exists as a 9 : 1 equilibrium mixture of the 6-keto- 110 and 6(9)-hemiacetal 111 forms. Both amphidinolides X and Y were moderately cytotoxic against murine lymphoma L1210 and human epidermoid carcinoma KB cells in vitro. Feeding experiments with 13C-labelled acetates suggested that amphidinolide Y might be a precursor of amphidinolide X.101 A culture of the dinoflagellate Symbiodinium sp., a symbiont of the soft coral Clavularia viridis collected from Okinawa, yielded two diastereoisomeric norcarotenoids 112 and 113. Both compounds exhibited moderate growth-inhibitory activity in vitro against a range of human cancer cell lines.102 A culture of the free-living marine dinoflagellate Symbiodinium sp. isolated from a tide pool, Coconut Island, Hawaii,103 yielded the polyhydroxy compound zooxanthellamide A 114.104 Cultures of a strain of the dinoflagellate Prorocentrum lima105 afforded okadaic acid methyl ester 115, norokadanone 116 and an okadaic acid diol ester 117.106 Three hydroxybenzoate saxitoxin analogues, GC1–GC3 118–120, have been isolated from the cultured dinoflagellate Gymnodinium catenatum originally isolated from a planktonic bloom in Tasmania. GC1 and GC2 are the epimeric 11-hydroxysulfate derivatives of GC3, the 4-hydroxybenzoate ester derivative of decarbamoylsaxitoxin. Preliminary investigations indicate that the compounds bind to rat brain sodium channels, in keeping with known PSP toxins.107 Biosynthetic investigations using 13C-labelled precursors of the meroterpenoid neomarinone, originally isolated from culture of an unidentified marine actinomycete from sediment from Batiquitos Lagoon, California,108 led to the structural revision of neomarinone to 121.109 A correction to the text of the article describing the structure and absolute stereochemistry of phormidolide from the marine cyanobacterium Phormidium sp.110 has been published, amending two descriptors [(17R,26R) to (17S,26S)].111 The absolute configuration of the fungal metabolite phomopsidin 122, derived from a cultured strain of Phomopsis sp.,112 has been determined by the exciton chirality method. Phomopsidin exhibited potent anti-microtubule activity in a microtubule assembly assay utilising purified porcine
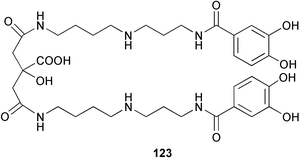
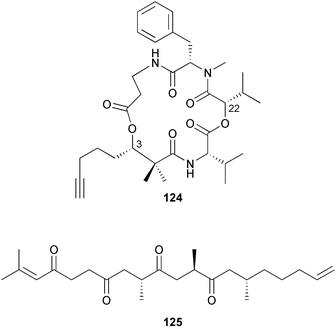
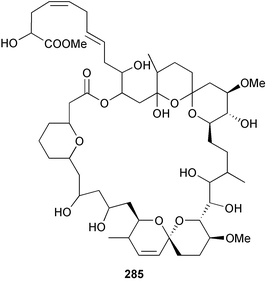
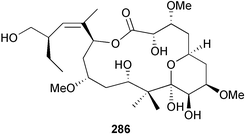
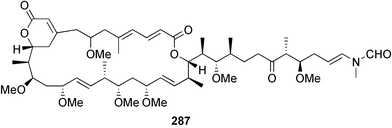
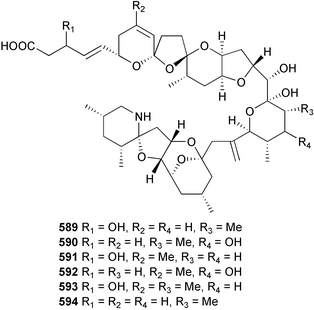
brain microtubule proteins.113 A total synthesis of petrobactin, a siderophore isolated from the marine bacterium Marinobacter hydrocarbonoclasticus has been completed. Comparison of the 1H NMR spectrum of the synthetic product with literature data for the natural product114 resulted in a structural revision of petrobactin from 2,3-dihydroxybenzoyl- to 3,4-dihydroxybenzoyl-moieties. This 3,4-dihydroxybenzoyl analogue 123 was also synthesised, giving 1H and 13C NMR spectra that were consistent with those of the natural product.115 The first total synthesis of yanucamide A 124, which was isolated from an assemblage of L. majuscula and a Schizothrix species,116 has been achieved via amide and ester coupling methods. The synthesis established the configuration at C-3, originally unassigned due to ambiguity, and revised the configuration at C-22.117 In synthetic studies towards congeners of phomactin A, total syntheses of structures isomeric to that proposed for the phomactin known as Sch 49028, also isolated from the marine fungus Phoma sp.,118 are described. None of the isomers showed spectral data consistent with those of the natural product so it is proposed that Sch 49028 does not exist and that the NMR spectral data should have been assigned as phomactin A.119 Other first total syntheses reported include that of (±)-spiroxin C, originally isolated from culture of an unidentified fungal strain from a soft coral from Vancouver Island, Canada.120 This involved a Suzuki–Miyaura cross-coupling reaction.121 Apratoxin A, a cyclodepsipeptide from Lyngbya sp. collected in both Guam122 and Palau,123 has been synthesised.124 The relative and absolute stereochemistries of amphidinoketide I 125, originally isolated from the dinoflagellate Amphidinium sp. collected in the Virgin Islands,125 have been determined by total synthesis of all four diastereoisomers. Molecular modelling was used to infer that the natural product is not the thermodynamically preferred diastereoisomer.126 Two syntheses of the 19-membered macrolide (+)-amphidinolide T1127,128 have been achieved,129,130 along with the synthesis130 of amphidinolides T3131 and T5.128 Synthesis of the structurally complex gymnocin-A, a polyether toxin with 14 contiguous rings, from the red tide dinoflagellate Karenia mikimotoi,132 has been accomplished through the use of B-alkyl Suzuki–Miyaura coupling-based methodology.133 Following the first total synthesis of gambierol, a marine polycyclic ether toxin originally isolated from the marine dinoflagellate Gambierdiscus toxicus,134 preliminary structure–activity relationship studies suggest that functionalities in the H ring and unsaturated sidechain are essential for potent murine toxicity.135 A competitive inhibition assay using the isotopically labelled brevetoxin dihydro BTX-B ([3H]PbTx-3), demonstrated that gambierol134,136 and gambieric acid-A137,138 from the dinoflagellate Gambierdiscus toxicus inhibit the binding of brevetoxins to site 5 of the voltage-gated sodium channel of excitable membranes,139 while effects of brevetoxins produced by the dinoflagellate Karenia brevis
(formerly Ptychodiscus breve and Gymnodinium breve)140 on the murine myeloma cell line SP2/O, a possible model for in vitro studies for immune cells, suggest that the brevetoxins have an aberrant effect on cell division.141
500 strains from this taxon have now been isolated and the potent proteasome inhibitor salinosporamide A 1 was isolated from a culture of a Salinospora sp. originating from a heat-treated marine sediment sample from the Bahamas. The structure of salinosporamide A, including the absolute stereochemistry, was deduced through spectral and X-ray analyses. Salinosporamide A displayed potent and selective in vitro cytotoxicity against cell lines in the NCI panel. Salinosporamide A also exhibited highly potent inhibition of the proteasomal chymotrypsin-like proteolytic activity of purified 20S proteasome. The unique functionalisation of the core-fused γ-lactam-β-lactone bicyclic ring structure of salinosporamide A 1 appears to contribute to its potency. The thiazolyl peptide antibiotics, nocathiacins I–III 2–4, have been isolated from the culture broth of Nocardia sp. (source not given).38 The nocathiacins exhibit potent in vitro activity against a wide range of bacteria, including several multiple-drug resistant pathogens and also exhibit excellent in vivo efficacy in a systemic Staphylococcus aureus infection mouse model.39 However, nocathiacin I 2 was found to be identical to an antibiotic isolated from Amycolatopsis sp.40 but spectral data and stereochemical details had not been originally reported for this compound. Two cyclic thiopeptides 5 and 6, obtained from a culture of Bacillus cereus isolated from the marine sponge Halichondria japonica,41 exhibited potent antibacterial activities against Staphylococci and Enterococci sp., and were active against multiple-drug resistant strains.42
(6Z)-Geometry for these compounds was implied by ROESY correlations. 1H-15N HMBC analysis was used in determining the structure of bacillamide 7, a peptidic metabolite of an algicidal marine Bacillus sp. isolated during the termination of a bloom of Cochlodinium polykrikoides in Masan Bay, Korea.43 Bacillamide was shown to be active against a wide range of dinoflagellates and raphidophytes.44 Culture of an exocellular extract of a Pseudomonas sp. associated with Ircinia muscarum from the Bay of Naples, Italy gave the cyclotetrapeptide 8.45 The amino acid stereochemistry was established by standard methods (for example, chiral HPLC analysis of the acid hydrolysate, Marfey's method etc.). Four Streptomyces sp. of diverse origin yielded a range of metabolites. Firstly, culture of a Streptomyces sp. from a sediment sample from Oahu, Hawaii, yielded the antibacterial and antifungal metabolite bonactin 9.46 Parimycin 10, a new 1,4-anthraquinone, was isolated from a Streptomycete sediment sample from Laguna de Terminos, Gulf of Mexico. Parimycin had moderate activity against B. subtilis, Streptomyces viridochromogenes, S. aureus and E. coli, in addition to activity against a number of human tumour cell lines.47 A Streptomyces sp. cultured from an unidentified Mexican marine invertebrate yielded the cytotoxic indoles 11–13 which had moderate activity against a panel of 14 tumour cell lines.48 Finally, the anthracycline komodoquinone A 14 and the aglycone komodoquinone B 15 were isolated from a culture of a Streptomyces sp. isolated from marine sediment off Komodo Island, Indonesia. Komodoquinone A displayed dose-dependent neuritogenic activity against the neuroblastoma cell line Neuro 2A.49 A culture broth of an ATCC strain of the marine gliding bacterium Saprospira grandis yielded four neoverrucosane diterpenoids, 16–19. The relative and absolute stereochemistries of 16 were determined by standard methods50
(for example, X-ray analysis, NOESY and ROESY NMR experiments, the modified Mosher method, chiral HPLC, comparison of circular dichroism (CD) or other optical data against standards or model compounds etc.). The marine myxobacterium Haliangium ochraceum,51 originally H. luteum, yielded several new isomers of the polyene antifungal antibiotic haliangicin.52,53 These are cis-haliangicin 20 and haliangicins B–D 21–23, geometrical isomers of the polyene and epoxide moieties. The stereochemistry of the epoxide in the known haliangicin 2453 has been determined as trans. All of the haliangicins were active against the phytopathogenic fungus Phytophthora capsici.54 Two siderophores, pseudoalterobactins A 25 and B 26, were isolated from a culture of the bacterium Pseudoalteromonas sp. isolated from the marine sponge Cinachyrella australiensis collected in Palau. Both compounds displayed strong binding affinity for the ferric ion in the chrome azurol S (CAS) assay.55 The bactericidal compound 27, obtained from a culture of a new marine species Pseudoalteromonas phenolica sp. nov., isolated from seawater collected off Ogasawara Island Japan,56 had potent activity against methicillin-resistant S. aureus
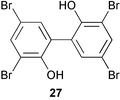
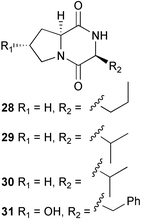
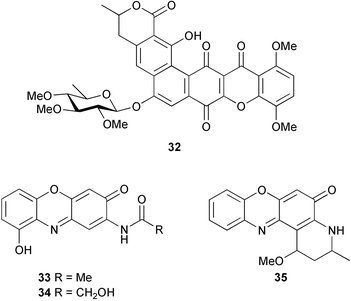
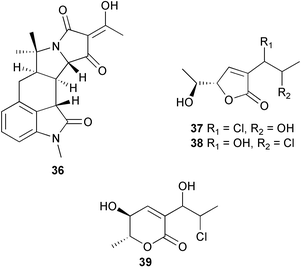
(MRSA) and was also strongly active against Enterococcus serolicida, E. faecium and E. faecalis.57 This compound is available commercially, but this is the first reported isolation as a natural product. Cultures of two marine bacterial strains isolated from cultures of Pecten maximus larvae in Galicia, Spain, led to the first reported isolation, as natural products, of a series of DD-diketopiperazines 28–31 and established them as potent inhibitors of the pathogenic marine bacterium Vibrio anguillarum. The structures were confirmed by synthesis.58 A cytotoxic polycyclic xanthone 32 has been isolated from the culture broth of the actinomycete Actinomadura sp.59 The phenoxazin-3-one antibiotics, chandrananimycins A–C 33–35, were also isolated from a culture of Actinomadura sp. derived from sediment from Jiaozhou Bay, China. Chandrananimycins A–C were active against human tumour cell lines while 35 exhibited potent activity against the fungus Mucor meihei and the bacteria B. subtilis and E. coli, and antialgal activity against the microalga, Chlorella vulgaris, C. sorokiniana and Scenedesmus suspicatus.60 The fungus Aspergillus tamarii was isolated from driftwood collected in Okinawa and cultured to yield a pentacyclic oxindole alkaloid, speradine A 36. The structure and relative stereochemistry of 36 were confirmed by X-ray analysis. Speradine A exhibited inhibitory activity against histone deacetylase and antibacterial activity against Micrococcus luteus.61 A culture of the fungus Aspergillus ostianus, isolated from an unidentified marine sponge from Pohnpei, was the source of three chlorinated antibiotics, the asperlactone derivatives 37 and 38 and the aspyrone derivative 39. Compound 37 was the most potent, inhibiting the growth of the marine bacterium Ruegeria atlantica and that of E. coli and S. aureus to a lesser extent.62 Five novel depsipeptides, aspergillicins A–E 40–44, were obtained from a culture of Aspergillus carneus collected from estuarine sediment in Tasmania, Australia. The amino acid sequences were assigned by MSn ion-trap ESI mass spectrometry and stereochemistry was assigned by standard methodology. The aspergillicins exhibited modest cytotoxicity against Haemonchus contortus.63 A chiral dipyrrolobenzoquinone derivative, terreusinone 45, has been obtained from a cultured strain of the marine algicolous fungus Aspergillus terreus isolated from the surface of the marine red alga Halymenia acuminata collected from Bijin Island, South Korea. The absolute stereochemistry was determined by a combination of Horeau's method and quantum chemistry calculations. Terreusinone has intense UV-A absorbtivity.64 A culture of Penicillium brocae from the tissue of the Fijian sponge Zyzzya sp. was the source of three novel cytotoxic polyketides, brocaenols A–C 46–48. These contain the unusual enolised oxepine lactone ring system. Structure determination included an INADEQUATE experiment on brocaenol A. The absolute stereochemistry of 46 was established by a standard method and extended to 47 and 48 by comparison of CD and optical rotation data.65 Brocaenols A–C displayed moderate activity against the HCT-116 cell line. Structures for brocaenols B and C were reversed in the original paper, but a correction has since been published.66 The steroids isocyclocitrinol A 49 and 22-acetylisocyclocitrinol A 50 were extracted from a salt water culture of Penicillium citrinum isolated from an Axinella sp. collected in Papua New Guinea.67 The absolute stereochemistry of 50 was established by standard methods, extended to 49, leading to the structural revision of cyclocitrinol, previously isolated from a terrestrial P. citrinum,68 to 51. Compounds 49 and 50 displayed weak antibacterial activity against Staphylococcus epidermidis and Enterococcus durans. The halovirs A–E 52–56, lipophilic linear peptides, are potent in vitro inhibitors of Herpes simplex viruses 1 and 2 and were isolated from a Scytalidium sp. sourced from the Caribbean seagrass Halodule wrightii.69 Two cyclic heptapeptides, scytalidamides A 57 and B 58, have been isolated from the culture broth of another Scytalidium sp. derived from the surface of the green alga Halimeda sp. collected off the Bahamas. The absolute configurations were confirmed by standard methods including CD measurements. Both scytalidamides displayed moderate cytotoxicity to the HCT-116 cell line in vitro.70 Trichodermamides A 59 and B 60, modified dipeptides, were isolated from cultures of Trichoderma virens isolated from the ascidian Didemnum molle and from the surface of a green alga of the genus Halimeda, both collected in Papua New Guinea. The ascidian-derived culture contained trichodermamide A with traces of trichodermamide B while a greater quantity of trichodermamide B was isolated from the algal-derived strain. The structure of 59 was assigned by X-ray diffraction while the absolute stereochemistry was determined using the modified Mosher method. Trichodermamide B displayed significant in vitro cytotoxicity against HCT-116 and moderate antimicrobial activity against amphoterocin-resistant C. albicans, MRSA and vancomycin-resistant E. faecium.71 Trichodermamide A is closely related to penicillazine, reported from a marine-derived Penicillium sp.72 The reported structures differ only in the translocation of ester and amide bonds, but spectral data comparison suggests that these compounds may be identical. Two macrolides, modiolides A 61 and B 62, and a linear pentaketide modiolin 63 have been isolated from the culture of Paraphaeosphaeria sp. separated from the marine horse mussel Modiolus auriculatus, collected in Okinawa. The absolute stereochemistry of 61 was determined by the exciton chirality method73 using a p-methoxycinnamoyl ester, while the absolute stereochemistry of 63 was defined by the modified Mosher method. Modiolides A and B exhibited modest antibacterial activity against Micrococcus luteus and Neurospora crassa.74 A culture of the marine fungus Wardomyces anomalus, isolated from the green alga Enteromorpha sp. collected in the Baltic Sea, yielded two xanthone derivatives, anomalins A 64 and B 65.75 The anomalins were only weakly antimicrobial, but anomalin A possessed significant tyrosine kinase p56lck enzyme inhibitor activity and antioxidative properties. Remisporine A 66, a novel cyclopentachromenone, isolated from a culture of the marine fungus Remispora maritima from an unspecified wood source, is unstable under normal conditions and autocatalytically dimerises stereospecifically, via a Diels–Alder reaction, to remosporine B.76 A new anthraquinone, evariquinone 67, and the new prenylxanthone isoemericellin 68 were isolated from a culture of the fungus Emericella variecolor derived from the marine sponge Haliclona valliculata collected at Elba, Italy. The known C-glycosidic depside stromemycin 6977 was also isolated, and the previously undescribed double bond configurations established. Evariquinone 67 showed antiproliferative activity towards KB and NCI-H460 cells.78 A culture of a marine strain of the fungus Epicoccum purpurascens, isolated from inner tissue of the jellyfish Aurelia aurita collected from the North Sea, Germany, yielded the tetramic acid derivative epicoccamide 70. Attempts to resolve the stereochemistry at C-4 and C-8 by comparision of CD spectra with those of similar compounds were ambiguous.79 Two highly oxygenated polyketides, phomoxin 71 and phomoxide 72, are metabolites from a Phoma sp. isolated from a microbial mat collected from a Bahaman hypersaline pond, along with eupenoxide 73, a previously synthesised, but unpublished fungal metabolite.80 An actinomycete, Pseudonocardia sp., isolated from littoral sediment from Mauritius, Indian Ocean, was the source of a new phenazine derivative, phenazostatin D 74 which is the meso- form of the known antibiotic phenazostatin B.81,82 Investigations of a collection of Lyngbya majuscula from Puerto Rico resulted in the isolation of three new metabolites, a quinoline alkaloid, 75, malyngamide T 76 and a tryptophan derivative 77.83 Geometries for the vinyl chloride functionalities of 75 and 76 were established as (E) by 1H-13C coupling constant measurement from HSQMBC NMR experiments.84 Six cyclic depsipeptides, guineamides A–F 78–83, were isolated from a collection of Lyngbya majuscula collected from Papua New Guinea. Absolute stereochemistries for most of the amino acids were determined by standard methods. Guineamides B and C were moderately cytotoxic to a mouse neuroblastoma cell line.85L. majuscula from Papua New Guinea was the source of the novel cyclic dodecapeptide wewakazole 84 which contains an unprecedented number of five-membered
heterocyclic rings (six). Due to extensive signal overlap the structural assignment required multiple NMR and MS/MS experiments. The absolute stereochemistry was determined by standard methods.86L. majuscula from the southern Kenyan Coast was the source of the cyclic depsipeptide homodolastatin 16 85. The absolute stereochemistries of most of the amino acids in homodolastatin 16 were determined by standard methods. Homodolastatin 16 85 displayed moderate activity against oesophageal and cervical cancer cell lines.87 The cyclic peptide lyngbyastatin 3 86, isolated from L. majuscula collected from Guam, contains two unusual amino acid units, including 4-amino-2,2-dimethyl-3-oxopentanoic acid (Ibu). The configuration of the Ibu unit was established by acid hydrolysis and comparison with synthetic standards, while the absolute stereochemistries of the remaining residues were determined by standard methods. Lyngbyastatin 3, along with the previously isolated lyngbyastatin 1 and dolastatin 12,88 are in fact diastereotopic mixtures of both Ibu epimers. Lyngbyastatin 3 86 exhibited activity against KB and LoVo cell lines in vitro, but was poorly tolerated in vivo with little antitumour activity.89 Three new malyngamides, U–W 87–89, have been isolated from L. majuscula collected in Papua New Guinea. Partial relative stereochemistries only were determined.90 A collection of Lyngbya sp. from Palau yielded ulongapeptin 90, a cytotoxic cyclic depsipeptide,91 while a Lyngbya sp. from Guam yielded two new compounds, 15-norlyngbyapeptin A 91 and lyngbyabellin D 92.92 The absolute stereochemistries in each case were determined through degradative studies and/or comparison with commercially available and synthetic standards. Ulongapeptin was moderately cytotoxic against KB cells in vitro91 and lyngbyabellin D displayed activity against the KB cell line.92 Bioassay-guided fractionation of an extract from a Lyngbya sp. collected in Palau led to the isolation of palau'amide 93. Effective use was made of a band-selective HMBC experiment to unambiguously assign 13C NMR signals that were separated by only 0.1 ppm.93 Except for C-37, relative and absolute configurations were determined by standard methods. By modelling, and from NOE data, C-37 was assigned as having the (S) configuration. Palau'amide 93 exhibited potent cytotoxicity against KB cells.94 Semiplenamides A–G 94–100, anandamide-like fatty acid amides, were isolated from a collection of Lyngbya semiplena collected in Papua New Guinea. The absolute stereochemistries of the amino alcohols in semiplenamides C–E 96–98 were elucidated as all L by chemical derivatisation and chiral GCMS methods. All of the semiplenamides displayed toxicity in the brine shrimp assay, while semiplenamides A, B and G exhibited weak affinity for the rat cannabinoid CB1 receptor. Semiplenamide A was also a moderate inhibitor of the anandamide membrane transporter (AMT).95 Samples of the marine cyanobacterium Symploca sp. collected in Palau were the source of the depsipeptides tasipeptins A 101 and B 102,96 and a cytotoxic peptide, tasiamide B 103.97 The relative and absolute configurations of the tasipeptins and tasiamide B were determined by standard methods except for the configuration of C-28 in tasiamide B. This was tentatively suggested as (S) from NMR data analysis.97 Both tasipeptins exhibited moderate cytotoxicity towards KB cells in vitro. Also collected in Palau was an assemblage of a Symploca sp. cyanobacterium and an unidentified red alga. From this was isolated the iodinated diterpenes, tasihalides A 104 and B 105. These compounds possess a novel cage structure with both an oxabicyclic ring system and a cis-decalin system. These are the only examples of iodinated diterpenes in nature. Since terpenoids are almost never reported from marine cyanobacteria, but halogenated terpenes are ubiquitous in red algae, the authors speculate that the more likely source of the tasihalides is the alga and not the cyanobacterium.98 Two polyunsaturated monocyclic triterpenes 106 and 107 have been isolated from a culture of the common marine diatom Rhizosolenia setigera. The structure of a related monocyclic sesterterpene 108 was also proposed on the basis of mass spectral comparisons with compounds 106 and 107.99 Amphidinolide X 109100 and amphidinolide Y 110101 are cytotoxic 16- and 17-membered macrodiolides isolated from cultures of the marine dinoflagellate Amphidinium sp., originally separated from the inside cells of the marine acoel flatworm Amphiscolops sp. collected from Okinawa. Amphidinolide Y exists as a 9 : 1 equilibrium mixture of the 6-keto- 110 and 6(9)-hemiacetal 111 forms. Both amphidinolides X and Y were moderately cytotoxic against murine lymphoma L1210 and human epidermoid carcinoma KB cells in vitro. Feeding experiments with 13C-labelled acetates suggested that amphidinolide Y might be a precursor of amphidinolide X.101 A culture of the dinoflagellate Symbiodinium sp., a symbiont of the soft coral Clavularia viridis collected from Okinawa, yielded two diastereoisomeric norcarotenoids 112 and 113. Both compounds exhibited moderate growth-inhibitory activity in vitro against a range of human cancer cell lines.102 A culture of the free-living marine dinoflagellate Symbiodinium sp. isolated from a tide pool, Coconut Island, Hawaii,103 yielded the polyhydroxy compound zooxanthellamide A 114.104 Cultures of a strain of the dinoflagellate Prorocentrum lima105 afforded okadaic acid methyl ester 115, norokadanone 116 and an okadaic acid diol ester 117.106 Three hydroxybenzoate saxitoxin analogues, GC1–GC3 118–120, have been isolated from the cultured dinoflagellate Gymnodinium catenatum originally isolated from a planktonic bloom in Tasmania. GC1 and GC2 are the epimeric 11-hydroxysulfate derivatives of GC3, the 4-hydroxybenzoate ester derivative of decarbamoylsaxitoxin. Preliminary investigations indicate that the compounds bind to rat brain sodium channels, in keeping with known PSP toxins.107 Biosynthetic investigations using 13C-labelled precursors of the meroterpenoid neomarinone, originally isolated from culture of an unidentified marine actinomycete from sediment from Batiquitos Lagoon, California,108 led to the structural revision of neomarinone to 121.109 A correction to the text of the article describing the structure and absolute stereochemistry of phormidolide from the marine cyanobacterium Phormidium sp.110 has been published, amending two descriptors [(17R,26R) to (17S,26S)].111 The absolute configuration of the fungal metabolite phomopsidin 122, derived from a cultured strain of Phomopsis sp.,112 has been determined by the exciton chirality method. Phomopsidin exhibited potent anti-microtubule activity in a microtubule assembly assay utilising purified porcine
brain microtubule proteins.113 A total synthesis of petrobactin, a siderophore isolated from the marine bacterium Marinobacter hydrocarbonoclasticus has been completed. Comparison of the 1H NMR spectrum of the synthetic product with literature data for the natural product114 resulted in a structural revision of petrobactin from 2,3-dihydroxybenzoyl- to 3,4-dihydroxybenzoyl-moieties. This 3,4-dihydroxybenzoyl analogue 123 was also synthesised, giving 1H and 13C NMR spectra that were consistent with those of the natural product.115 The first total synthesis of yanucamide A 124, which was isolated from an assemblage of L. majuscula and a Schizothrix species,116 has been achieved via amide and ester coupling methods. The synthesis established the configuration at C-3, originally unassigned due to ambiguity, and revised the configuration at C-22.117 In synthetic studies towards congeners of phomactin A, total syntheses of structures isomeric to that proposed for the phomactin known as Sch 49028, also isolated from the marine fungus Phoma sp.,118 are described. None of the isomers showed spectral data consistent with those of the natural product so it is proposed that Sch 49028 does not exist and that the NMR spectral data should have been assigned as phomactin A.119 Other first total syntheses reported include that of (±)-spiroxin C, originally isolated from culture of an unidentified fungal strain from a soft coral from Vancouver Island, Canada.120 This involved a Suzuki–Miyaura cross-coupling reaction.121 Apratoxin A, a cyclodepsipeptide from Lyngbya sp. collected in both Guam122 and Palau,123 has been synthesised.124 The relative and absolute stereochemistries of amphidinoketide I 125, originally isolated from the dinoflagellate Amphidinium sp. collected in the Virgin Islands,125 have been determined by total synthesis of all four diastereoisomers. Molecular modelling was used to infer that the natural product is not the thermodynamically preferred diastereoisomer.126 Two syntheses of the 19-membered macrolide (+)-amphidinolide T1127,128 have been achieved,129,130 along with the synthesis130 of amphidinolides T3131 and T5.128 Synthesis of the structurally complex gymnocin-A, a polyether toxin with 14 contiguous rings, from the red tide dinoflagellate Karenia mikimotoi,132 has been accomplished through the use of B-alkyl Suzuki–Miyaura coupling-based methodology.133 Following the first total synthesis of gambierol, a marine polycyclic ether toxin originally isolated from the marine dinoflagellate Gambierdiscus toxicus,134 preliminary structure–activity relationship studies suggest that functionalities in the H ring and unsaturated sidechain are essential for potent murine toxicity.135 A competitive inhibition assay using the isotopically labelled brevetoxin dihydro BTX-B ([3H]PbTx-3), demonstrated that gambierol134,136 and gambieric acid-A137,138 from the dinoflagellate Gambierdiscus toxicus inhibit the binding of brevetoxins to site 5 of the voltage-gated sodium channel of excitable membranes,139 while effects of brevetoxins produced by the dinoflagellate Karenia brevis
(formerly Ptychodiscus breve and Gymnodinium breve)140 on the murine myeloma cell line SP2/O, a possible model for in vitro studies for immune cells, suggest that the brevetoxins have an aberrant effect on cell division.1414 Green algae
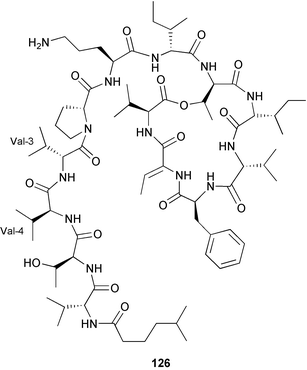
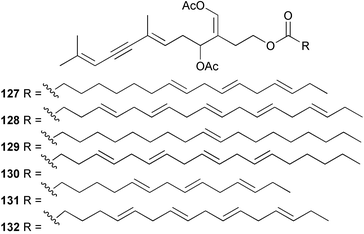
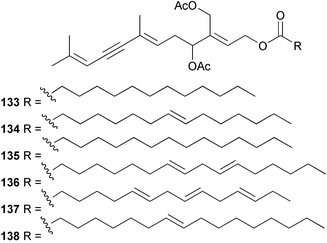
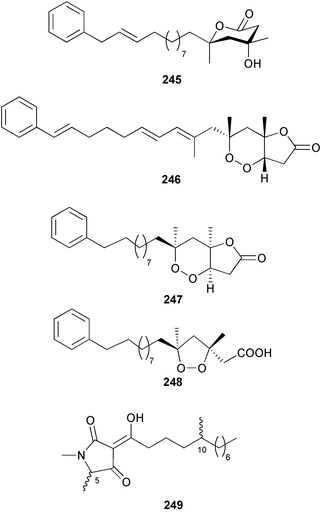
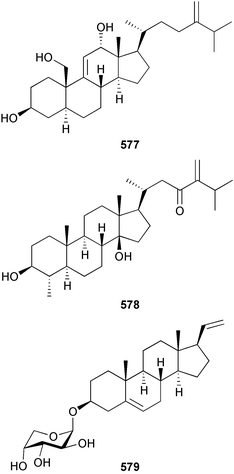
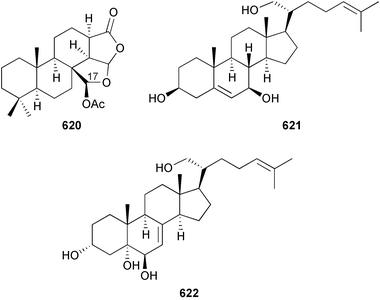
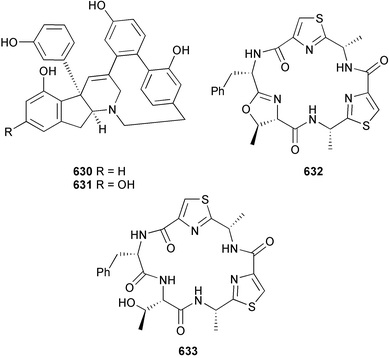
As in 2002, very few new compounds have been reported from green algae. The cyclic depsipeptide kahalalide F 126, originally isolated from both the mollusc Elysia rufescens and from the dietary source, the green alga Bryopsis sp.,142 was introduced into Phase I trials by Pharma Mar SA as a lead compound against prostate cancer. The structure of kahalalide F has been corrected based on a series of degradation reactions. The planar structure only was originally defined and the stereochemistry subsequently assigned.143 The degradation results indicate that the correct structure is a stereoisomer 126, in which the original assignments for Val-3 and Val-4 have been reversed. This stereochemistry is crucial for the observed bioactivity.144 Twelve new terpene esters, 127–138 have been isolated from the green alga Caulerpa prolifera collected from Saronicos Gulf, Greece. The C. prolifera extract exhibited moderate to significant activity against three unidentified strains of marine bacteria, in addition to strong growth inhibitory effects on the fouling microalga Phaeodactylum tricornutum.145 The first total synthesis of (±)-dihydrorhipocephalin, a bioactive sesquiterpene isolated from Caribbean marine green algae of the genera Penicillus and Udotea,146 has been reported.1475 Brown algae
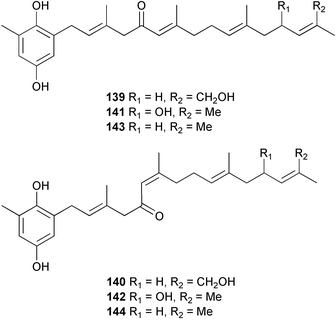
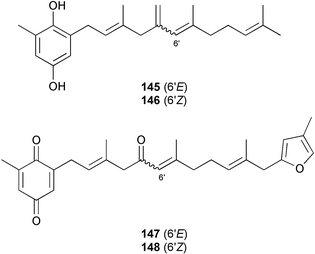
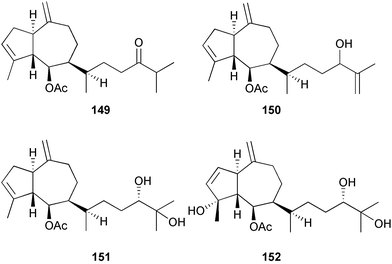
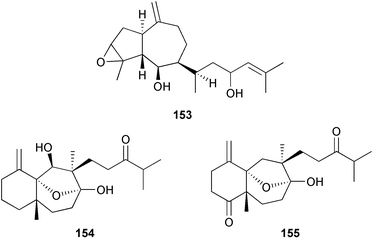
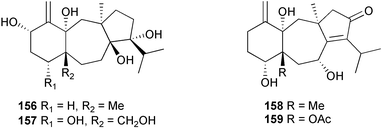
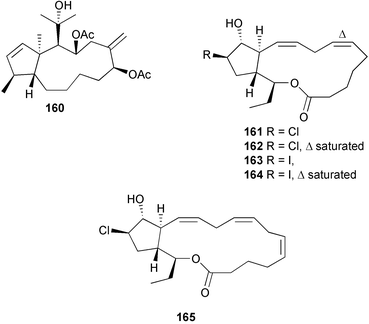
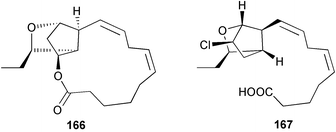
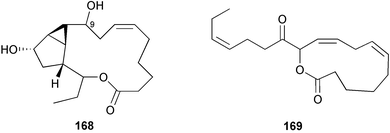
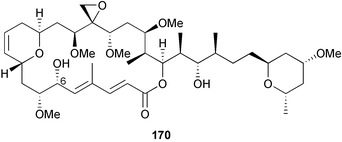
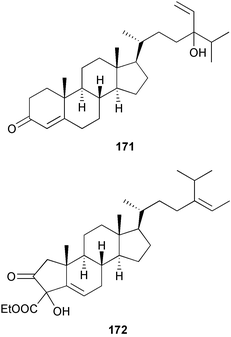
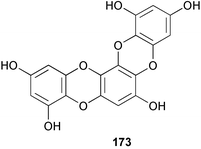
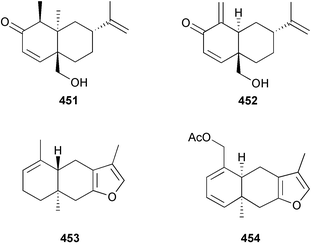
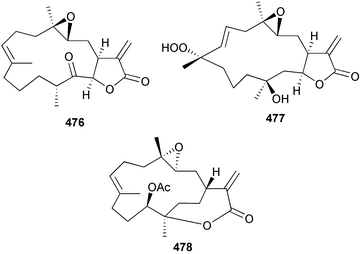
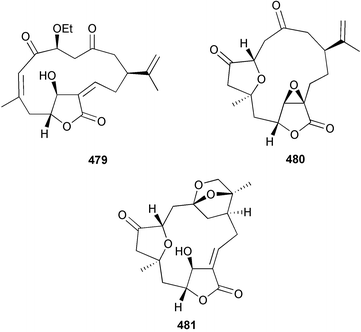
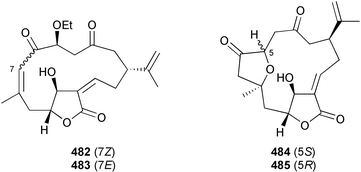
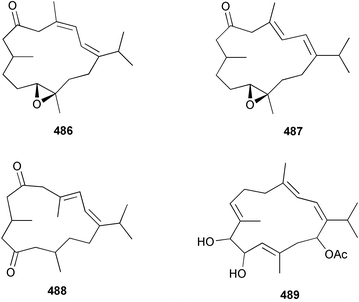
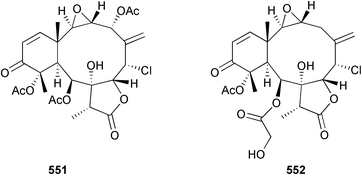
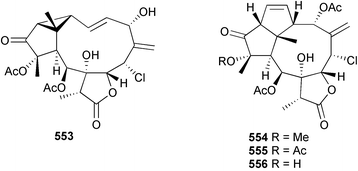
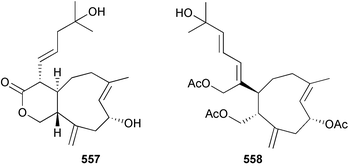
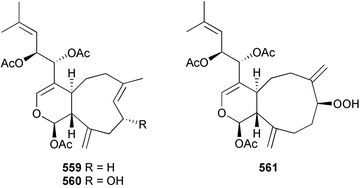
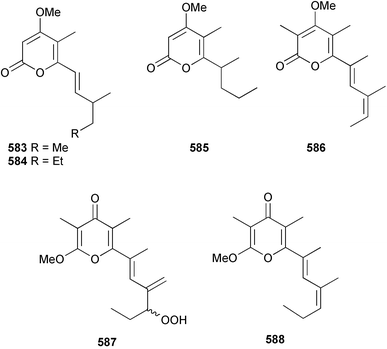
A wider range of compounds has been reported from brown algae in 2003 than in 2002, when terpenes and steroids were the predominantly reported compound classes. Six tetraprenyltoluquinols 139–144, two triprenyltoluquinols 145 and 146 and two tetraprenyltoluquinones 147 and 148 were isolated from the brown alga Cystoseira crinita collected from the south coast of Sardinia. All compounds were tested for antioxidative properties in the α,α-diphenyl-β-picrylhydrazyl radical (DPPH) and thiobarbituric acid reactive substances (TBARS) assay systems. Compounds 139–146 exhibited potent radical-scavenging effects while 147 and 148 were significantly less active, but still comparable to that of butylated hydroxytoluene (BHT). The radical scavenging activity of compounds 142, 144 and 148 was further assessed using the Trolox equivalent antioxidant capacity (TEAC) and photochemiluminescence (PCL) assays that confirmed the potent radical scavenging ability. Compounds 139 and 140 were moderately cytotoxic against several carcinoma cell lines.148 Four hydroazulene diterpenes, dictyone acetate 149, dictyol F monoacetate 150, isodictytriol monoacetate 151 and cystoseirol monoacetate 152, were isolated from the brown alga Cystoseira myrica collected in the Gulf of Suez. All four compounds exhibited moderate cytotoxicity against the murine cancer cell line KA3IT, but reduced cytotoxicity against normal NIH3T3 cells.149 Dictyone acetate along with a pachydictyol A derivative 153 (incorrect structures shown in original reference) were also isolated from the brown alga Dictyota dichotoma collected from the Red Sea.150D. dichotoma from the Arabian Sea was the source of two seco-dolastanes dichotone 154 and dichotodione 155,151 two dolastane diterpenoids, dichototetraol 156 and dichopentaol 157,152 and the related dichotenones A 158 and B 159, two enone dolastane diterpenoids.153 The configurations of 154 and 155 were determined by comparison of spectral data against those of known compounds. The new diterpene dictyocrenulol 160 was isolated from the brown alga Dictyota crenulata collected from Easter Island.154Eisenia bicyclis collected at Johgashima Island, Japan, was the source of nine novel oxylipin compounds 161–169.155 Five of these, eiseniachlorides A–C 161–163 and eiseniaiodides A 164 and B 165, are ecklonialactone derivatives and two more, 166 and 167, are cymathere type oxylipins. Stereochemistries of compounds 161–165 and 169 were elucidated by NMR analyses, but the relative stereochemistry at C-9 in 168 could not be determined unambiguously. Olefin geometry in 166 was ambiguous, but considered to be (Z) on biosynthetic grounds, and at least one olefin in compound 167 was (Z). A 22-membered cyclic lactone, lobophorolide 170, was isolated from the common brown alga Lobophora variegata, collected at several reef locations in the Bahamas and from the Red Sea. The structure was elucidated by spectral data analysis and comparison against data published for tolytoxin156 and swinholide A.157,158 It is proposed that lobophorolide and tolytoxin share the same relative configuration at all stereogenic centres in the macrolide portion of the molecule, while a (6R) configuration is suggested for both compounds rather than the (6S) configuration proposed previously for tolytoxin.156 The absolute configuration of lobophorolide is proposed to be the same as that of tolytoxin based on optical rotation. Lobophorolide 170 displayed potent and highly specific activity against the marine filamentous fungi Dendryphiella salina and Lindra thalassiae in addition to potent activity against C. albicans and antineoplastic activity against the HCT-116 cell line.159 The brown alga Sargassum asperfolium, collected in the Suez Gulf, was the source of the steroidal metabolite saringosterone 171,160 while a novel steroid 172 has been isolated from the brown alga S. carpophyllum from the South China Sea.161Ecklonia stolonifera collected from S. Korea yielded a new phlorotannin, eckstolonol 173, which possessed potent DPPH radical scavenging activity.162 Dolabellane 1, originally isolated from the opistobranch mollusc Dolabella californica,163 has been characterised as the major secondary metabolite and active chemical defense agent against herbivores (sea urchins and fish) in the brown alga Dictyota pfaffi.164 (±)-Hedaol B, a bisnorditerpene isolated from the Japanese brown alga Sargassum sp.,165 has been synthesised with geranyl acetone as a starting material and alkylation of silyl cyanide as the key step in the synthesis.1666 Red algae
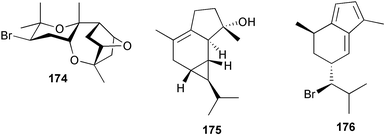
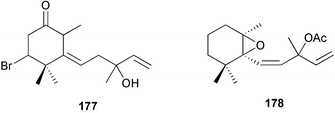
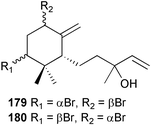
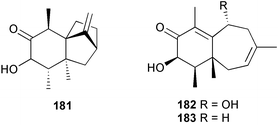
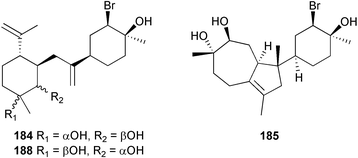
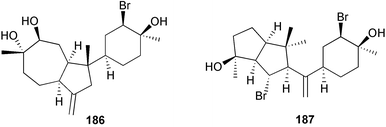
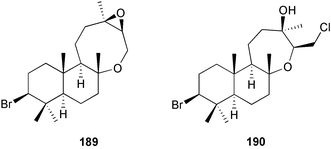
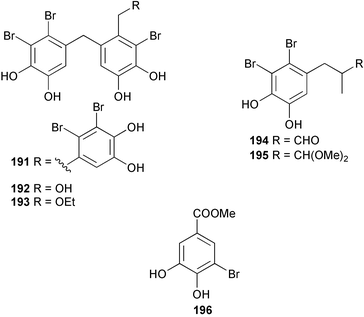
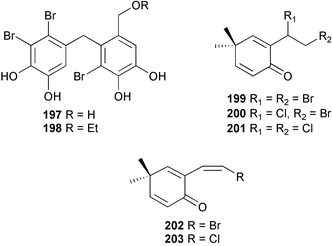
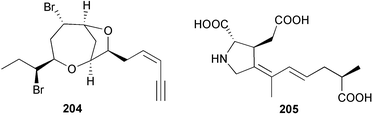
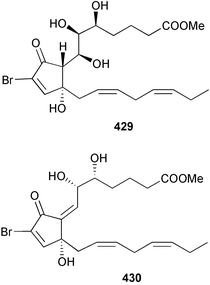
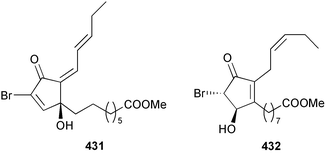
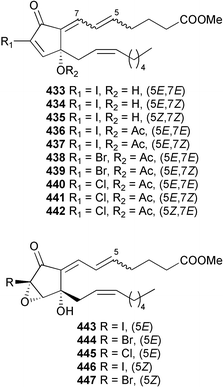
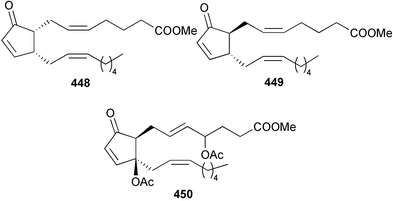
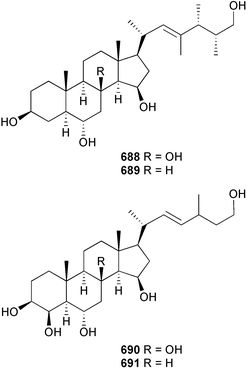
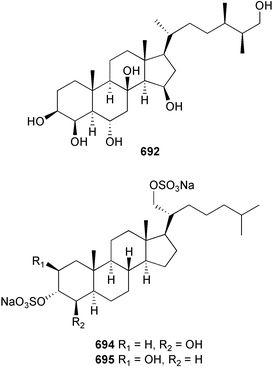
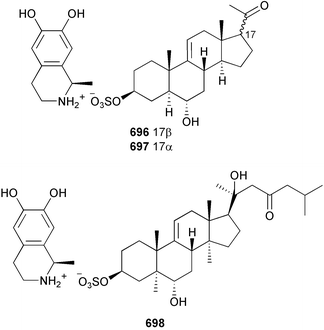
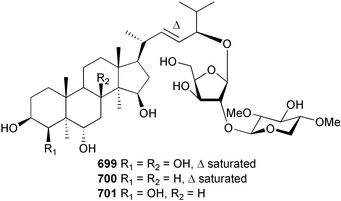
The genus Laurencia continues to be a prolific source of new metabolites. A brominated bisabolene derivative, aldingenin A 174, was isolated from Laurencia aldingensis collected from Brazil. Biogenetic considerations were of value in the structural assignment.167 From L. microcladia from Elba Island, a calenzanane sesquiterpene, debromoisocalenzanol 175 and an indene-type sesquiterpene 176 were isolated,168 while four new sesquiterpenes, 177–180 including the snyderol derivatives 179 and 180, have been isolated from L. obtusa collected from Bademli, Turkey. Compound 179 was active against D6 and W2 clones of the malaria parasite Plasmodium falciparum.169Laurencia perforata, collected from the Great Barrier Reef, Australia, was the source of the sesquiterpenes 4-hydroxy-1,8-epi-isotenerone 181 and two 3-epi-perforenone A derivatives, 182 and 183.170 A collection of L. obtusa from Greece yielded four new brominated diterpenes,171 prevezols C–E 184–186, and neorogioldiol B 187, together with the known prevezol B 188, whose structure has been revised from that reported originally.172 Prevezol B and neorogioldiol displayed significant cytotoxicity against the human tumour cell lines MCF7, PC3, HeLa, A431 and K562 while prevezol C only exhibited significant cytotoxicity against HeLa and A431 cell lines. Prevezol D was moderately active against all cell lines.171 Two labdane type brominated diterpenes 189 and 190 have been isolated from L. obtusa from Greece. These structures contain unprecedented eight- and seven-membered ether rings respectively.173 Six new bromophenols, 191–196 were isolated from Rhodomela confervoides collected from the coast of Qingdao, China.174 Compounds 193 and 195 may be artifacts of the extraction and isolation processes.174 Compounds 194 and 195 were also reported in another paper by the same authors, along with the isolation of the known 3-bromo-4,5-dihydroxybenzoic acid methyl ester (but new as a natural product) from the same source (R. confervoides).175 This benzoyl ester has previously been synthesised176 but the spectral data were not reported. R. confervoides from Qingdao was also the source of bromophenols, 197 and 198. The phenol 198, which might also be derived from 197 during isolation,177 exhibited moderate activity against five strains of bacteria.178 Five monoterpenes 199–203 of the ochtodane class have been isolated from the red alga Portieria hornemanni (source not given).179 The marine polyether triterpenoid dehydrothyrsiferol, originally isolated from the red alga Laurencia pinnatifida,180 was shown to induce apoptosis in estrogen-dependent and independent breast cancer cells.181 Elatol, a halogenated sesquiterpene alcohol from the red alga L. elata182 inhibited six species of human pathogenic bacteria, with significant antibacterial activities against Staphylococcus epidermis, Klebsiella pneumonia and Salmonella sp.183 Iso-obtusol from the red alga Laurencia obtusa184,185 exhibited antibacterial activity against four bacterial species with significant activity against K. pneumonia and Salmonella sp. Further tests indicated that both compounds were bacteriostatic rather than bacteriocidal against the bacteria tested.183 Glutathione transferase specific activity in Katharina tunicata (black chiton) was shown to be affected by the brominated phenol lanosol,186 which is prevalent among filamentous red algae of the Rhodomelaceae, and frequently consumed by K. tunicata.187 The first asymmetric total syntheses of (+)-3-(E)- and (+)-3-(Z)-pinnatifidenyne, originally isolated from Laurencia pinnatifida,188,189 have been reported and utilise an “olefin geometry-dependent” internal alkylation to give excellent stereoselectivity.190 The seven-membered ring ether (+)-neoisoprelaurefucin 204, originally isolated from L. nipponica,191 has also been synthesised, allowing the assignment of the absolute stereochemistry of the natural product.192 A nickel-catalysed coupling reaction of an alkynyl enone and an alkenylzirconium were the key steps in the synthesis of isodomoic acid G 205, originally isolated from the red alga Chondria armata from Kyushu Island.193 The sidechain stereochemistry was established as (5′R) by comparison of CD spectra of the natural and synthetic products.1947 Sponges
Sponges continue to be an important source of novel secondary metabolites and a notable growing trend is the characterisation of compounds from bacteria and fungi that have been isolated from sponges. Such compounds have been included in Section 3 of this review. There has also been increased interest in fatty acid derivatives, many of which have biological activities. An unusual galactofuranosylceramide, ectyoceramide 206, was isolated from the Bahaman sponge Ectyoplasia ferox,195 while a Jaspis species collected in Vanuatu was found to contain the cytotoxic sphingosine derivatives jaspines A 207 and B 208.196 The Korean sponge Erylus nobilus was the source of the taurine derivative 209.197 Another Korean sponge, a Stelletta species, has yielded two cytotoxic compounds, glycerol ether 210198 and cyclitol derivative norsarcotride A 211.199 Plakevulin A 212, found to inhibit DNA polymerases α and γ, was isolated from the Okinawan sponge Plakortis sp.200Latrunculia corticata, collected in the Gulf of Aqaba, Israel, was found to contain decalactone glycosides latrunculinoside A 213 and B 214, which have anti-feedant activity against goldfish.201 An inhibitor of membrane type 1 matrix metalloproteinase (MT1-MMP), callysponginol sulfate A 215, was isolated from Callyspongia truncata collected in Japan.202 An undescribed Korean species of Stelletta was found to contain cytotoxic acetylenic acids: stellettic acid A 216, (Z)- and (E)-stellettic acid B 217 and 218, and stellettic acid C 219 that exhibited marginal to moderate toxicity to five human tumour cell lines.203 Interestingly, the same sponge also yielded the glycerol derivatives of 217, the mildly cytotoxic 220 and 221 (inactive), along with other lysophosphatidylcholines and monoglycerides 222–225.204 From a seemingly identical Stelletta species, collected at a different Korean location, a similar series of acetylenic acids was isolated including 216, a dimeric anhydride 226 and a desmethoxy analogue 227; all were mildly cytotoxic to human leukemia cells.205 The Indonesian sponge Callyspongia pseudoreticulata yielded the diyne 228, which was found to be toxic in the brine shrimp assay.206 A Diplastrella species, collected in the Philippines, yielded a series of polyacetylenic diols, the diplynes A–E 229–233 and corresponding sulfates 234–236.207 Three new chlorinated polyacetylenes 237–239 were isolated from the Californian sponge Haliclona lunisimilis208 along with known compounds originally isolated from the Haliclona’s nudibranch predator, Diaulula sandiegensis.209 The moderately cytotoxic polyacetylenic amide, callyspongamide A 240, was obtained from Callyspongia fistularis collected in the Red Sea.210 Three new amides, 241–243, along with the previously reported clathrynamide A 244,211 were isolated from an Okinawan Psammoclemma species. 212 The stereochemistry of 244 was determined (Mosher method). All four compounds were found to be antifungal. The absolute stereochemistry of the amino alcohol xestoaminol C, originally isolated from a Fijian Xestospongia species,213 has been established as (2S,3R) by the synthesis of the N,O-diacetyl derivative from (S)-alanine.214 A racemic synthesis of 2-methoxy-13-methyltetradecanoic acid, isolated from a Puerto Rican specimen of Amphimedon complanata,215 has been reported.216 (R)-Strongylodiol B, originally isolated from a Strongylophora species,217 was synthesised enantioselectively using a Zn(II) acetylide addition to an aldehyde.218 Callyberynes A and B, also known as callypentaynes, obtained from Japanese specimens of Callyspongia truncata219 and Callyspongia sp.,220 were synthesised using sequential Cadiot–Chodkiewicz cross-coupling reactions.221Erylus trisphaerus, collected in Dominica, was found to contain the mildly cytotoxic polyketide lactone, trisphaerolide A 245.222 A Madagascar specimen of Plakortis aff. simplex yielded three cyclic peroxides, the plakortolides H 246 and I 247 and andavadoic acid 248, all of which were cytotoxic against a range of human tumour cell lines.223 The antimicrobial tetramic acid, melophlin C 249, from an Indonesian specimen of Melophlus sarassinorum, was isolated as an inseparable mixture of four stereoisomers arising from the stereogenic centres at C-5 and C-10 (as evidenced by NMR and modified Marfey's method). A further twelve, less active tetramic acids, melophlins D–O 250–261, were also isolated from the same sponge.224 Both plakortides M 262 and N 263, isolated from a collection of Plakortis halichondrioides from Puerto Rico, exhibited potent cytotoxicity to an array of human tumour cell lines.225 A Japanese specimen of Monotria japonica yielded the monotriajaponides A–D 264–267 which can lyse starfish oocytes without disruption of nuclear structure.226 Interestingly, the absolute stereochemistries of 265–267, as determined by reduction and a modified Mosher method, were opposite to those determined for the plakortides 262 and 263. The asymmetric synthesis of (+)-rottnestol, originally isolated from a Haliclona species,227 using a Stille coupling firmly established the absolute stereochemistry as (12R). Similarly, syntheses of (+)-raspailol A and (+)-raspailol B, originally obtained from a Raspailia species,228 have established a (12R) configuration for these two metabolites also.229 An unusual bis-dimedone thioether with strong UV A and B absorption, benzylthiocrellidone 268, was isolated from a Great Barrier Reef collection of Crella spinulata; the structure was reported in 2002,230 but was omitted from the 2002 review.231 Okadaic acid, originally isolated from Halichondria okadai,232 and subsequently found to be a dinoflagelate and shellfish toxin,233,234 has been investigated for potential as a defense molecule for the Adriatic sponge Suberites domuncula. Use of an ELISA assay established that okadaic acid was localised in the epithelium of the lacunae and water channels of the sponge, as well as in bacteria located in the sponge tissue. It was postulated that okadaic acid acts as a stimulant of the sponge immune system to the presence of bacteria, but in higher concentrations causes apoptosis.235 Two analogues of okadaic acid, 27-O-acetylokadaic acid 269 and 27-O-acetyldinophysistoxin 1 270, were isolated from a British Columbian sponge Merrianum oxeato and found to be potent G2 checkpoint inhibitors and highly cytotoxic.236 A Papua New Guinean sponge, Cymbastela sp., was found to contain the cytotoxic peptide milnamide D 271 along with the related peptides hemiasterlin237 and milnamide A.238 All three compounds were inhibitors of tubulin polymerisation.239 Three unusual new cyclic peptides, the kapakahines E–G 272–274, have been isolated from a Micronesian collection of Cribrochalina olemda and reported as cytotoxic to P388 murine leukemia cells.240 The previously described sulfoxide, waiakeamide 275, and a new sulfone analogue 276 were isolated from a Haliclona sp. collected in Palau. The sulfone 276 was found to inhibit the settlement of larvae of the blue mussel (Mytilus edulis galloprovincialis).241 The myriastramides A–C 277–279 were isolated from the same Philippine collection of Myriastra clavosa that had previously yielded the clavoside macrolides.242,243 Leucamide A, originally isolated from the Australian sponge Leucetta microraphis,244 has been synthesised.245 Due to differences in biological activity, the cis,cis- 280 and reputed trans,trans- 281 isomers of ceratospongamide, originally isolated from the Indonesian symbiotic pairing of the red alga Ceratodictyon spongiosum and the sponge Sigmadocia symbiotica,246 continue to attract considerable attention from synthetic chemists. Although both rotamers had been synthesised previously,247 slight differences in the NMR spectra of the synthetic trans,trans isomer 281 and the isolated natural product were noted. Suspecting a possible epimerisation the trans,trans-[D-allo-Ile] isomer, 282 was synthesised, by two separate routes, to produce a compound that is identical in all respects to the natural isomerisation product.248 Phakellistatins 1249 and 10,250 have been synthesised.251 Phakellistatin 1 was found to exist as the all-cis rotamer at the proline residues, while phakellistatin 10 was determined to be all-trans. Interestingly, both synthetic products were more than 100-fold less cytotoxic than the natural product.251 A large (500 kg) collection of a Phakellia species from Chuuk, Micronesia, yielded the growth inhibitory phakellistatin 12 283,252 while a Chinese collection of Phakellia fusca yielded the very cytotoxic phakellistatin 13 284.253 The macrolide spirastrellolide A was isolated as its methyl ester 285 from the Caribbean sponge Spirastrella coccinea. Unlike many other sponge-derived antimitotic macrolides, 285 does not effect tubulin polymerisation.254 An asymmetric synthesis of (−)-peloruside A, the antipode of the natural product 286 originally isolated from the New Zealand sponge Mycale hentscheli,255 has been achieved via a Mitsunobu-type lactonisation.256 The synthetic antipode proved to be biologically inactive in cytotoxicity assays, but established the absolute stereochemistry of the natural (+)-enantiomer 286 as drawn. The relative and absolute stereochemistries of the C23–C35 portion of reidispongiolide A 287, isolated from the New Caledonean sponge Reidispongia coerulea,257 have been established by synthesis of an ozonolysis fragment of the natural product.258 The total synthesis of (+)-13-deoxytedanolide, originally isolated from the Japanese sponge Mycale adherens,259 has been accomplished.260 The natural enantiomer of lasonolide A, isolated from a Caribbean Forcepia species,261 has also been synthesised and found to be bioactive.262 The hexabromobiphenylether from Dysidea herbacea263 has been synthesised and found to be a potent aldose reductase (ALR2) inhibitor.264 The Micronesian sponge Cribrochalina olemda was found to contain a new N-methyl-D-aspartate (NMDA) receptor ligand, cribronic acid 288, which has potent convulsant activity in mice.265 The known antioxidant amino acid L-5-hydroxytryptophan was found to be a major constituent of the NW Atlantic intertidal sponge Hymeniacidon heliophila and was observed to suppress apoptosis in human lymphocytes at concentrations similar to those found in the sponge tissue. Since UV light induces apoptosis, it is proposed that the high concentrations of L-5-hydroxytryptophan act to protect this sponge species from sunlight UV damage.266 The pyridinium alkaloid simplakidine A 289 was isolated from the Caribbean sponge Plakortis simplex.267 The rather remarkable tris-pyridinium alkaloid viscosamine 290 has been isolated from the Arctic sponge Haliclona viscosa. The trimeric nature of this alkaloid was deduced from a series of ions in the mass spectrum.268 Halitulin 291, isolated from a South African collection of Haliclona tulearensis,269 has been synthesised, establishing C-15 as (S).270 Clathryimine, originally isolated from Clathria basilana,271 has been synthesised using palladium-catalyzed cross-coupling reactions.272 Hachijodines F and G, isolated originally from Xestospongia and Amphimedon species,273 have been synthesised. The N-oxide moieties were introduced using modified Mukiyama conditions.274 Pyrinodemin A 292, isolated from a Okinawan collection of an Amphimedon species,275 continues to attract considerable attention from synthetic organic chemists.231 The position of the cis double bond has been contentious, with the originally published structure 292 being modified to 293276 and 294277 respectively. The structure 294 has now been synthesised asymmetrically by two independent groups establishing the absolute stereochemistry of the bicyclic core.278,279 One group was also able to compare the spectral data to the original spectra of the natural product and confirm the structure as 294.278 Petrosin and petrosin A, originally isolated from Petrosia seriata,280,281 were found to inhibit HIV-1 replication and HIV-1 reverse transcriptase.282 The total synthesis of the (+)-antipode of nakadomarin A 295, originally isolated from an Amphimedon species,283 has established the absolute stereochemistry of the (−)-natural enantiomer as (RRRR).284 Three new manzamine alkaloids 296–298, the related harman-1-one 299, and des-N-methylxestomanzamine A 300 were isolated from an Indonesian sponge.285 Three β-carbolines, 3-bromofascaplysin 301, 14-bromoreticulatine 302 and 14-bromoreticulatate 303, have been reported as metabolites of Fascaplysinopsis reticulata from Indonesia and Fiji. 3-Bromofascaplysin was also reported as a metabolite of the tunicate Didemnum sp.286 Three iodine-containing indole alkaloids, plakohypaphorines A–C 304–306, were also obtained from the same Caribbean Plakortis simplex collection that yielded simplakidine (vide supra). This is the first report of naturally occurring iodoindole alkaloids.287 Damirones A and B288 have been prepared from the corresponding makaluvamines by alkaline hydrolysis, suggesting that the damirones may be artifacts of isolation and not naturally-occurring compounds.289 The Indonesian sponge Biemna fortis yielded the pyridoacridine alkaloid labuanine 307, which along with two related synthetic pyridoacridine alkaloids and the previously isolated biemnadin,290 were found to be inducers of neuronal differentiation.291 Several new antimicrobial aaptamine type alkaloids 308–312 were isolated from an Indonesian Xestospongia species,292 while from a Japanese Neopetrosia sp. a further tetrahydroisoquinoline alkaloid, renieramycin J 313, was reported.293 The dark blue, cytostatic and antimicrobial metabolite, cribrostatin 6 314, was isolated from a species of Cribrochalina from the Maldives.294 The dictyodendrins A–E 315–319, isolated from the Japanese sponge Dictyodendrilla verongiformis were found to inhibit telomerase activity.295 Phloeodictine A1, originally isolated from a New Caledonian sponge of the genus Phloeodictyon,296 has been synthesised.297N,N-Dimethylnaamine D 320 and leucettamine C 321 are reported as new, mildly antimicrobial metabolites of two Fijian Leucetta species.298 The same research group has also isolated three further imidazole-containing alkaloids, calcaridine A 322 spirocalcaridine A 323 and spirocalcaridine C 324, from one of the two Leucetta collections.299 Isonaamidines A and C, originally isolated from an Indo-Pacific Leucetta species,300 have been synthesised.301 Sventrin, isolated from Agelas sventes,302 has been synthesised by a Red-Al reduction of an alkyne.303 An MT1-MMP inhibitor, ageladine A 325, was isolated from a Japanese Agelas nakamuri collection.304 Oroidin-type alkaloids with novel skeletons, the latonduines A 326 and B 327, were obtained from an Indonesian Stylissa carteri collection.305 A Stylissa aff. massa, obtained from Japanese waters, was found to contain a geranylgeranyltransferase type I inhibitor, massadine 328.306 Crambescidin 826 329, isolated from a Monanchora sp. collected in Palau, was found to be a potent inhibitor of HIV-1 envelope-mediated fusion, along with the known compounds crambescidin 800307 and fromiamycalin,308 while dehydrocrambine A 330, also isolated from this sponge, was found to be a weak inhibitor only.309 A related antibacterial guanidine alkaloid, Sch 575948 331, was isolated from a Ptilocaulis spiculifer (Crambe crambe) specimen.310 Two antimitotic guanidine/bromotyrosine alkaloids, ceratamines A 332 and B 333, were isolated from a Papua New Guinean Pseudoceratina sp.311 An Indian collection of Psammaplysilla purpurea was found to contain the antibacterial bromotyrosine-derived alkaloids purpuramine K 334 and L 335.312 Aerothionin, originally isolated from Verongia aerophoba,313 has been found to be active against drug-resistant strains of Mycobacterium tuberculosis and several other Mycobacterium sp.314 A Chinese collection of the sponge Phakellia fusca yielded a remarkable series of fluorinated uracil derivatives 336–340. The presence of fluorine was confirmed by X-ray diffraction and 19F NMR studies. This is the first report of fluorine-containing marine natural products.315 Sponge-derived merosequiterpenoids continue to be a fruitful area of research for both natural product and synthetic chemists. Isoarenarol 341, isolated from a Papua New Guinean collection of Dysidea arenaria, was found to be a potent protein kinase inhibitor.316 Spongiaquinone, isolated from Stelospongia conulata,317 has been prepared in an asymmetric synthesis. The absolute stereochemistry was assigned based on comparison of the optical rotation of the synthetic methyl ether with that of the natural compound.318 A Micronesian Aka species has yielded three new sesquiterpenoid quinols, akaol A 342, 343, and the tentatively assigned siphonodictyol I 344.319 Also isolated was siphonodictyal C 345, originally isolated from Siphonodictyon coralliphagum320 and previously described as a free phenol. However the sample isolated from the Aka sp. had identical NMR spectra and clearly shows the presence of SO3Na by ESIMS.319 The sulfate group is lost in EIMS, the technique used for characterisation in the original isolation procedure.320 Siphonodictyal C was a modest inhibitor of complexation in the CDK4/cyclin D1 assay.320 The moderately cytotoxic neodactyloquinone 346 and the dactylolactones A–D 347–350 were obtained from an Okinawan collection of Dactylospongia elegans.321 A Great Barrier Reef species of Spongia yielded the sesquiterpenoid aminoquinone cyclosmenospongine 351, which was found to be moderately cytotoxic to murine Ehrlich carcinoma cells.322 Methanolic extracts of an Indonesian sponge of the genus Hyrtios yielded three new puupehenone derivatives 352–354, but which are proposed to be artifacts of isolation from puupehenone.323 The biosynthesis of the sesquiterpenoid dichloroimines, stylotellanes A and B,324 was investigated. Incorporation of labelled farnesyl isocyanide and farnesyl isothiocyanate demonstrated the role of these compounds as intermediates in the formation of the stylotellanes.325 10-Formamido-4-cadinene 355, isolated from the Japanese sponge Acanthella cavernosa, was found to inhibit the settling of the cyprid (barnacle) larvae Balanus ainphitrite.326 The Indonesian sponge Axinyssa aculeata and its nudibranch predator Phyllidia varicosa were both found to contain the moderately antifungal 9-thiocyanatopupukeanane sesquiterpenoids 356 and 357.327 2-Thiocyanatoneopupukeanane 358, originally isolated from the sponge Phycopsis terpnis,328 was subsequently revised to the endo stereochemistry on the basis of long-range 1H-1H coupling and NOE correlations.329 Both enantiomers have been synthesised from (R)-carvone via the corresponding alcohols330 and the stereochemistry of 358 has now been fully established via an X-ray structure of the nitrobenzoate derivative of the corresponding alcohol.331 A Japanese Axynissa species yielded the mildly cytotoxic diterpene, axinyssene 359.332 An enantioselective synthesis of (−)-nakamurol, originally isolated from the Okinawan sponge Ageles nakamuri,333 established the relative and absolute stereochemistries of the naturally-occurring 360 enantiomer.334 Synthesis of the proposed structure of aplyroseol-14 361, originally isolated from the New Zealand sponge Aplysilla rosea,335 did not yield spectra similar to those of the natural product. The revised structure, 362, was synthesised and found to be spectrally identical with aplyroseol-14.336 Six cycloamphilectenes isolated from an Axinella species collected in Vanuatu were found to be potent inhibitors of nitric oxide production by murine macrophages.337 Only one (N-formyl-7-amino-11-cyclocamphilectene) of the six compounds in this study has had a structure determination published.338 The C-25 sesterterpenoids and related nor-compounds are characteristic of sponges, especially those of Dictyoceratid origin. A cytotoxic norsesterterpenoid, mycaleperoxide 363, was isolated from a Mycale species collected in Thailand. The relative and absolute stereochemistries were established by standard methodology, including chemical interconversions.339 Two moderately cytotoxic norsesterterpenoids, sarcotins N 364 and O 365, along with a sesterterpenoid 366, four pyrrolosesterterpenoids 367–370 and ent-kurospongin 371 were isolated from two Korean Sarcotragus species.340 The previously reported sarcotin I 372341 was found to have the (21R) configuration.340 Three norsesterterpenoids 373–375 and two sesterterpenoids 376 and 377, isolated from an Okinawan Ircinia species, were found to be moderately cytotoxic.342Darwinella australensis collected from NW Australia contained sesterterpenoid sulfates 378–380 that inhibited the cell division of sea urchin eggs, but were not cytotoxic to human leukemia cells.343 An Ircinia species collected at -70 m by dredging in the Gulf of Mexico contained a tricyclic sesterterpenoid, Sch 599473 381,344 while the Antarctic sponge, Suberites caminatus yielded the rearranged sesterterpenoid aldehyde caminatal 382.345 An asymmetric synthesis of (−)-cacospongionolide F, isolated from Fasciospongia cavernosa,346 confirmed the original stereochemical assignments.347 The bicyclic lactone astakolactin 383 and the pentacyclic diacetate 16-acetoxy-dihydrodeoxoscalarin 384 were obtained from specimens of Cacospongia scalaris collected in Greece.348 A Spongia species collected in Japan yielded three cytotoxic pentacyclic sesterterpenoids 385–387.349 Seven new polyhydroxy sterols 388–394 were isolated from a Japanese Acanthodendrilla species along with three known agosterols. These were found to be proteasome inhibitors.350 Clathriol B 395, isolated from the New Zealand sponge Clathria lissosclera, was found to inhibit the production of superoxide from human neutrophils.351 A sterol sulfate, Sch 572423 396, along with the previously described halistanol sulfate,352 isolated from a Topsentia species collected in the Bahamas, were found to bind to P2Y12 receptors.353 Another deep-water Bahaman sponge, belonging to the family Astroscleridae, yielded the trisulfated sterol Sch 575867 397,354 while a series of steroidal oligoglycosides, the mycalosides B–I 398–405, have been isolated from the Cuban sponge Mycale laxissima. The mycalosides are inhibitors of the fertilisation of sea urchin eggs.355 Four significantly cytotoxic steroidal alkaloids, plakinamines I–K 406–408 and dihydroplakinamine K 409, were isolated from a Philippine sponge Corticium niger.356 The halogenated and rearranged norsteroid, nakiterpiosin 410, isolated from the Okinawan Terpios hoshinota, was found to be cytotoxic to murine P388 leukemia cells.357 Hippospongic acid A, originally isolated from a Japanese Hippospongia species,358 inhibits all classes of vertebrate DNA polymerases and human topoisomerases I and II, but is inactive towards DNA polymerases from plants, insects and prokaryotes.359 Two mildly cytotoxic polyoxygenated triterpenes, yardenones A 411 and B 412 were isolated from a Yemenese collection of Axinella cf. bidderi.3608 Coelenterates
The number of new metabolites reported annually from coelenterates has remained relatively constant over the 2002–2003 period. A new sphingosine derivative 413 was reported from a soft coral Nephthea sp. collected at the Andaman and Nicobar Islands, Indian Ocean,361 while investigations of Sinularia grandilobata and Sinularia sp. specimens from the same location afforded 414–416 as antimicrobial metabolites.362 The absolute stereochemistry of the N-palmitate 417, isolated from a Bay of Bengal collection of Nephthea sp., was deduced by analysis of 1H-1H coupling constants of the acetonide derivative and comparison of optical properties with known compounds.363 Acylspermidines 418–420, isolated from an Okinawan collection of Sinularia sp. soft coral,364 were all potently cytotoxic towards A431 cells. In a separate study 419 and 420 were found to be potent inhibitors of plant vacuolar H+-pyrophosphatase.365 The phenol 421 was isolated from a Taiwanese collection of Isis hippuris,366 while investigation of a Japanese collection of the stony coral Tubastraea sp. afforded bisindole alkaloids 422–424.367 From Israel, eight new oxylipin derivatives were reported from Gulf of Aqaba collections of Dendronephthya sp. (425–428), Tubipora musica (429 and 430) and Dendrophyllia sp. (431 and 432) coelenterates.368 Stereochemical configurations were secured by standard methods. All eight metabolites exhibited biological activity towards bacteria, brine shrimp, sea urchin egg development and crown gall potato tumours. Fifteen new members of the clavulone family of prostanoids 433–447 were reported from an Okinawan collection of Clavularia viridis.369 The absolute configurations of 433–443, 445 and 446 were secured by analysis of CD data while those of 444 and 447 were proposed based upon biogenetic considerations. Prostanoids 448–450, possible biosynthetic precursors to the clavulones, were also isolated from an Okinawan collection of C. viridis.370 By utilising protease and detergent fractionation methodology, clavulones and arachidonic acid have been located in host C. viridis membranes, as opposed to the closely associated symbiont Symbiodinium sp.371 Sesquiterpenes ainigmaptilones A 451 and B 452 were isolated from a Weddell Sea, Antarctica, collection of Ainigmaptilon antarcticus.372 Ainigmaptilone A demonstrated activity in a number of ecologically-relevant assays, including antibiotic and feeding deterrence properties. Furanosesquiterpene 453, reported from the Antarctic gorgonian Dasystenella acanthina, bears a trans-ring junction as determined by NOESY NMR experiments and comparison with related cis-fused isomers.373 Asymmetric synthesis of both enantiomers of acetoxytubipofuran 454, originally isolated from a Japanese collection of Tubipora musica,374 defined the absolute stereochemistry as shown,375 while the structure of echinofuran376 has been confirmed by racemic synthesis.377 Confertol 455 and nephalbidol 456 were isolated from the soft corals Sinularia conferta and Nephthea albida respectively,378 while cladioxazole 457 was isolated from an Andaman Island, Indian Ocean, collection of Cladiella sp.379 A full account of the synthesis of the dolabellane diterpene claenone, previously reported from Clavularia sp.,380 the first synthesis of palominol, from Eunicea laciniata,381 and a new route to dolabellatrienone, also from E. laciniata,381,382 have also been reported.383 Stereoselective synthesis of (+)-4,5-deoxyneodolabelline, a metabolite of an Australian collection of Cespitularia sp.,384 has been reported.385 The structure of kallosin A 458, a rearranged pseudopterane diterpenoid isolated from a Caribbean collection of Pseudopterogorgia kallos, was secured by spectroscopic and X-ray analyses.386 Elisabethin A, isolated from P. elisabethae,387 has been synthesised utilising intramolecular [4 + 2] cyclisation under biomimetic conditions.388 The first synthesis of the related diterpene elisapterosin B and a new route to colombiasin A, also isolated from P. elisabethae,389,390 have been achieved based on [5 + 2] and [4 + 2] intramolecular cyclisations of a common diene intermediate.391 New members of the elisapterosin family, D 459 and E 460, were reported from a Caribbean collection of the same organism.392P. elisabethae is also a well recognised source of anti-inflammatory diterpenes, new examples of which include elisabethadione 461, elisabethol 462, pseudopterosins M–O 463–465 and seco-pseudopterosins E–G 466–468.393 Of the eight diterpenes, 461, 464 and 466 were the most potent in the mouse ear edema assay. The chemical steps involved in the biosynthesis of the pseudopterosins in P. elisabethae have been studied using 3H-labelled precursors,394 with a subsequent study showing that diterpene production is occurring within the dinoflagellate symbiont Symbiodinium sp.395 Preparation of all four C-1 and C-7 stereoisomers of pseudopteroxazole 469, a mildly antimycobacterial diterpene isolated from P. elisabethae,396 required a revision of assigned stereochemistry to that shown,397 while a new bioactive congener, homopseudopteroxazole 470, has been reported from the same organism collected near San Andrés Island, Colombia.398 The structures of the P. elisabethae metabolites, elisabatins B399 and C,400 have been confirmed by X-ray studies.401 Investigation of a Great Barrier Reef collection of Sarcophyton cherbonnieri yielded furano-cembranoids 471–473, while the same study402 also reported new seco-cembranoids 474 and 475 from a Fijian collection of Nephthea sp. in addition to the known cembrane decaryiol.403 Modest cytotoxicity towards a panel of tumour cell lines was exhibited by 471, 473 and decaryiol while the latter was shown to arrest the cell cycle at G2/M. Structures of sarcocrassolide B 476404 and sarcophyocrassolide A 477,405 cytotoxic cembrane diterpenes isolated from a Chinese collection of Sarcophyton crassocaule, were secured by X-ray studies,406 as was that of 11-epi-sinulariolide acetate 478,407 previously reported from gorgonians collected from the Gulf of Elat. 11-epi-Sinulariolide acetate was found to exhibit moderate cytotoxicity towards a range of tumour cell lines. In addition to a number of known metabolites, new nor-cembrane diterpenes leptocladolides A 479, B 480 and C 481 were isolated from a Taiwanese collection of Sinularia leptoclados, while 479 and related compounds 1-epi-leptocladolide A 482 and (7E)-leptocladolide A 483 were isolated from an ethanolic extract of S. parva.408 Both 479 and 483 exhibited modest cytotoxicity towards two tumour cell lines, but 482 was less active. Two known diterpenes, sinuleptolide 484409 and norcembrenolide 485,410 inhibit LPS-induced TNF-α production by murine macrophage-like cells in a dose-dependent manner.411 Note that while the characterisation data for the two diterpenes reported in the reference agree with original and recent reports,408 the structures are represented with incorrect relative stereochemistry at C-11. Cembranes 486–489 were isolated from an eastern Caribbean collection of Eunicea tourniforti.409 The structure and relative stereochemistry of the highly oxygenated diterpene providencin 490, purified from Caribbean collections of Pseudopterogorgia kallos, was secured by X-ray analysis.410 Mild cytotoxicity towards human tumour cell lines was observed for 490. In addition to the known metabolites stolonidiol 491 and stolonidiol monoacetate 492, two new dolabellane diterpenes, clavinflols A 493 and B 494, were isolated from a Taiwanese collection of Clavularia inflata.411 While 491, 492 and 494 exhibited selective cytotoxicity towards the KB cell line, 493 was selective towards the Hepa cell line. In contrast, the acetoxy derivatives 495 and 496 were essentially inactive in the same assays. Pachyclavulariolides M–R 497–502 were isolated from a Taiwanese collection of Pachyclavularia violacea.412 P388 cell line growth inhibition was observed for 497. (Z)-Sarcodictyin A 503 is a potently cytotoxic diterpenoid isolated from a Japanese collection of Bellonella albiflora.413 The absolute stereochemistry of 503 was related to sarcodictyin A 504 by transesterification and comparison of CD spectra. Spectroscopic discrepancies observed for the enantioselectively synthesised structure originally proposed for alcyonin 505, isolated from the Okinawan soft coral Sinularia flexibilis,414 have led to the proposal that the correct structure of the natural product is the allylic peroxide 506.415 The structures of briarellins E 507 and F 508, isolated from a Puerto Rican collection of Briareum asbestinum,416 were confirmed by enantioselective total synthesis, which also established the absolute configuration of the diterpenes.417 In addition to a number of known compounds, new briarellins J–P 509–515, two unnamed congeners 516 and 517 and polyanthellin A 518 were reported from a Puerto Rican collection of Briareum polyanthes.418 Spectroscopic evidence was also presented for revision of the structure of briarellin A from 519419 to peroxide 520, and reformulation of the structures of 521 and 522, isolated from an Australian collection of Briareum sp. in 1989,420 to the enantiomers of 518 and 523 respectively. Antimalarial testing against Plasmodium falciparum indicated 511, 516 and 517 to be the most active. Two investigations of the chemistry of Junceella juncea, one using specimens collected from the Tuticorin coast of the Indian Ocean, yielded juncins I–M 524–528,421 while a Taiwanese collection of the same organism afforded juncin N 529.422 Additional studies of J. juncea from Taiwan afforded juncenolides B–D 530–532423 and juncenolide E 533,424 of which 531 exhibited mild cytotoxicity towards Hepa and KB cell lines.423 A different diterpene structure 534, isolated from an Indian Ocean collection of J. juncea, was also given the trivial name juncenolide B.425 A Taiwanese collection of Junceella fragilis yielded 9-O-deacetylumbraculolide A 535.426 The structurally related epoxides briaexcavatolides S–V 536–539 were isolated from Taiwanese specimens of Briareum excavatum,427 while a Taiwanese collection of J. fragilis was the source of junceellolide H 540.428 Briarlides A–H 541–548, obtained from Amami Oshima, Kagoshima Prefecture collections of Briareum sp., were evaluated for cytotoxicity towards Vero and MDCK cell lines where modest activity was observed for 541, 544–546, weak activity for 542, 543 and 547 while 548 was inactive.429 In addition to a number of known metabolites, seven new briaranes, erythrolides R–U 549–552, an erythrane, erythrolide V 553, and two aquariane-skeletoned diterpenes, aquariolides B 554 and C 555, were reported from a Caribbean collection of Erythropodium caribaeorum.430 Aquariolide A 556, previously isolated from aquarium-grown specimens of E. caribaeorum,431 was also identified from the organism collected in the wild. The relative stereochemistries of 549–555 were determined either by conversion to known related derivatives, or by interpretation of ROESY NMR data, while for erythrolide S 550, Mosher methodology established the absolute configuration of the 3-hydroxybutanoyl side chain as (3′S). The biosynthetic relationships between a number of erythrolide diterpenes, involving possible enzymatic-mediated di-π-methane and vinyl-propane rearrangements were discussed. The study also reported that the known metabolites erythrolides P432 and J433 exhibited modest cytotoxicity towards the MCF7 tumour cell line. An Okinawan collection of Xenia sp. yielded the known metabolite xeniolide A434 as well as new xenicane diterpenes dihydroxeniolide A 557 and isoxeniatriacetate 558.435 The absolute configuration of 557 was established (Mosher method), while the absolute configuration of 558 was determined by synthesis from the stereochemically-defined xeniolide A.436 13-Epi-9-deacetoxyxenicin 559 was isolated as a cytotoxic component of Asterospicularia laurae collected on the Great Barrier Reef, Australia.437 Good activity was observed for 559 against P388D1 cells, while the known metabolite 13-epi-9-deacetylxenicin 560438 was less active. DCM or ether solutions of 559 readily underwent autoxidation to afford the hydroperoxide 561, while 560 was found to be resistant to further reaction. The stereochemistries of sesterterpenes cladocorans A 562 and B 563, isolated from Mediterranean collections of Cladocora cespitosa,439 have been revised by total synthesis,440 while preparation and testing of related stereoisomers indicated the series exhibits cytotoxicity towards a panel of human tumour cell lines.441 Pregnane acetal 564 was isolated from an ethanol extract of Subergorgia suberosa, collected off the Mandapam coast, Indian Ocean,442 while a Taiwanese collection of Isis hippuris afforded the polyoxygenated steroids hippuristerones E–I 565–569.443 New gorgosterol and ergosterol derivatives 570–574 were isolated from a Great Barrier Reef collection of Capnella lacertiliensis.444 All compounds exhibited weak antifungal activity while 573 and 574 also weakly inhibited tyrosine kinase p56lck. The spiroketal steroid 575 was isolated from a Tuticorin coast, Indian Ocean collection of Gorgonella umbraculum,445 while the mildly cytotoxic gibberoketosterol 576 was isolated from a Taiwanese collection of Sinularia gibberosa.446 A South China Sea collection of Nephthea chabroli afforded the weakly cytotoxic sterols 577 and 578,447 and the arabinopyranosylsterol 579 was isolated from Cladiella krempfi, also collected in Chinese waters.448 APETx1, a 4,552 Da 42-amino acid peptide cross-linked by three disulfide bonds, was isolated from the sea anemone Anthopleura elegantissima.449 The toxin inhibits HERG voltage-dependent K+ channels via gating modification rather than channel pore occlusion. Pore formation by equinatoxin II, a protein toxin isolated from the Mediterranean sea anemone Actinia equina,450 has been examined using combinations of 31P NMR, 31P MAS NMR, electron microscopy,451 FTIR452 and toxin mutagenesis.453 The ability of surface plasmon resonance to study membrane binding processes of pore forming toxins has been reviewed.4549 Bryozoans
Once again, few new compounds have been reported from bryozoans. The structural determination of the alkaloids pterocellins A 580 and B 581, isolated from the marine bryozoan Pterocella vesiculosa collected in New Zealand, relied in part on an X-ray diffraction study of pterocellin A 580. Both pterocellins A and B exhibit potent antimicrobial and antitumour activity in vitro, but only displayed modest activity in an in vivo hollow fibre assay.455 The β-carboline alkaloid 8-hydroxyharman 582 was isolated from a sample of the New Zealand marine bryozoan Cribricellina cribraria.456 A number of brominated alkaloids and a diterpene from the North Sea bryozoan Flustra foliacea457,458,459,460,461 were tested against bacteria derived from marine and terrestrial environments. These compounds exhibited significant activities against one or more marine bacterial strains originally isolated from F. foliacea, but only weak activities against the terrestrial bacteria. Dihydroflustramine C462 and flustramine D461 exhibited N-acyl-homoserine lactone (AHL)-antagonistic activity as determined by using the biosensors Pseudomonas putida (pKR-C12), P. putida (pAS-C8) and E. coli (pSB403).458 A synthesis of the cytotoxic isoquinoline alkaloid perfragilin A, originally isolated from the bryozoan Membranipora fragilis,463 has been reported.46410 Molluscs
There was a slight increase in new chemistry identified from molluscs in 2003 over that reported for the time frame of the previous review. Irregular polypropionates placidenes C–F 583–586 and hydroperoxide 587 were isolated from a Mediterranean collection of Placida dendritica.465 It is likely that 587 is derived from the known metabolite placidene A 588,466 but whether the hydroperoxide is an artifact of isolation, or a true natural product is unclear. The first synthesis (racemic) of the unsaturated polypropionate photodeoxytridachione, isolated from Placobranchus ocellatus467 and other molluscs,468 has been reported.469 Five new azaspiracid analogues 589–593, identified using tandem mass spectrometric techniques, were isolated from Mytilus edulis collected off the west coast of Ireland.470 The stereochemistries of the new azaspiracid analogues are arbitrarily shown as matching that of azaspiracid-1 594,471 the structure and stereochemistry of which has been called in to question by stereoselective synthetic studies.472,473 The isolation of N-methyl-D-glutamic acid 595 from the Japanese mollusc Scapharca broughtonii is the first report of this amino acid derivative as a natural product.474 Monterey Bay, California, collections of Calliostoma canaliculatum afforded the disulfide-linked dimer of 6-bromo-2-mercaptotryptamine 596 as a channel-gating antagonist of voltage-gated potassium channels.475 6-Bromoindirubin 597, isolated from the Mediterranean mollusc Hexaplex trunculus, and the synthetic oxime 598 were found to be potent inhibitors of glycogen synthase kinase-3 (GSK-3).476 The molecular geometry of GSK-3β inhibition by 598 was determined by a co-crystallisation X-ray study. Radio- and stable isotope incorporation studies have identified nicotinic acid and acetate as biosynthetic precursors of haminol-2,477 a de novo biosynthesised metabolite of the Mediterranean mollusc Haminoea orbignyana.478 The ability of the fungal alkaloid gliotoxin to act as a bioaccumulated toxin of shellfish has been examined using Mytilus edulis.479 Lamellarin D, a polycyclic alkaloid first isolated from molluscs of the genus Lamellaria,480 has been found to be a potent inhibitor of the DNA-processing enzyme topoisomerase I.481 Japanese and US collections of Aplysia kurodai and A. californica were sources of the gut and vasculature contraction inhibitory pentapeptide Pro-Arg-Gln-Phe-Val-amide (PRQFVa).482 Precursoral peptide cDNA was successfully cloned while PRQFVa-positive neuron distribution in CNS and peripheral tissue was mapped using in situ hybridisation and immunocytochemistry. Five excitatory peptides, r11a–e 599–603 were isolated from the venom of the fish-hunting cone snail Conus radiatus collected in the Philippines.483 Further molecular analysis of cDNA clones defined the isolated peptides as belonging to a new class, the I-superfamily, of conotoxins, which contain a scaffold with four disulfide bonds (linkages not defined). The solution conformation of αA-conotoxin EIVA 604, originally isolated from the Atlantic cone shell C. ermineus,484 was determined by NMR experiments and restrained molecular dynamics calculations.485 A South China Sea collection of Conus betulinus yielded κ-conotoxin BtX 605, a 31 residue four disulfide bond-containing K+ channel up-modulator.486 As noted in Section 4, the revised structure487 of kahalalide F 126, a potently cytotoxic488 depsipeptide isolated from the mollusc Elysia rufescens and the algal dietary source Bryopsis sp.,142 has been confirmed by careful analysis of degradation products and chiral derivatisation.144 The mechanism of biological action of dolastatin 11, a cytotoxic depsipeptide isolated from the sea hare Dolabella auricularia,489 involves stabilisation of F-actin, which has been studied using X-ray fibre diffraction of oriented filament sols.490 Also isolated from a Japanese collection of the sea hare D. auricularia, dolabellanin B2, a 33 amino acid residue peptide, exhibits a broad spectrum of antimicrobial activity.491 The solution structure of attractin, a 58-residue water-borne protein pheromone isolated from Aplysia californica has been determined by NMR methods.492 Austrodoral 606 and austrodoric acid 607 are new nor-sesquiterpenes isolated from the Antarctic nudibranch Austrodoris kerguelenensis, but with 607 most likely being an artifact of isolation.493 As noted in Section 7, the thiocyanatopupukeanane sesquiterpenes 356 and 357 were isolated as an epimeric mixture from the nudibranch Phyllidia varicosa and the nudibranch's dietary sponge Axinyssa aculeata.327 While both compounds were isolated from the digestive gland of the nudibranch, epimer 357 was found to accumulate in the mantle, suggestive of a role in chemical defense. Both compounds exhibited mild toxicity towards brine shrimp and antimicrobial activity with 357 being more potent. De novo biosynthesis, via mevalonic acid, of fatty acid ester derivatives of drimane 608 and sesquiterpene 609494 in the nudibranch Doriopsilla areolata has been determined by feeding studies utilising [1-13C]glucose, [1,2-13C2]glucose and [1,2-13C2]acetate.495 Investigation of the diterpenoid acylglycerol fraction of an extract of the mantle of the Antarctic nudibranch Austrodoris kerguelenensis afforded the acylglycerols 610 and 611.496 Also isolated were two known 1,2-diacylglyceryl esters, previously reported from the same organism,497,498 the structures of which were corrected to 612 and 613 based upon interpretation of HMBC NMR correlations. The de novo biosynthesis of the structurally related diterpenoid glyceride verrucosin A499,500 by the Mediterranean nudibranch Doris verrucosa has been investigated using both 13C- and 14C-labelled precursors.501 Four new labdane diterpenes 614–617 were isolated from the pulmonate Trimusculus peruvianus, collected near the Antofagasta Coast of Chile.502 Absolute stereochemistry was secured by standard methods. Compounds 616 and 617 exhibited mild cytotoxicity towards human tumour cell lines in vitro. The structure of aplysiallene 618, deduced for a metabolite isolated from a Japanese collection of the sea hare Aplysia kurodai,503 has been retracted504 and corrected to the known bromoallene algal metabolite 619.505 The first diastereoselective synthesis of (−)-spongian-16-oxo-17-al, originally isolated from the nudibranch Ceratosoma brevicaudatum,506 has confirmed the absolute stereochemistry of the metabolite, while synthesis of the related compound (−)-acetyldendrillol-1 620, isolated from the nudibranch Cadlina luteomarginata,507 has led to correction of stereochemistry at C-17.336 A further collection of Trimusculus peruvianus, again from the Antofagasta Coast of Chile, yielded two mildly cytotoxic polyhydroxylated steroids 621 and 622.508 The stereochemistries of 621 and 622 were determined by interpretation of NOESY NMR data and comparison of chemical shifts with stereochemically-defined related compounds.11 Tunicates (ascidians)
The number of new secondary metabolites reported from ascidians has remained essentially static for each of 2002 and 2003. Three new glycosphingolipid molecular species, the major component of each being represented by 623–625, were isolated from a Mediterranean collection of Microcosmus sulcatus.509 A full account of the synthesis of lobatamide C, a cytotoxic macrolide isolated from Aplidium lobatum collected off the southwestern coast of Australia,510 has been reported.511 In addition, preliminary V-ATPase inhibition structure–activity data was reported indicating the importance of the salicylate ring and enamide moieties for activity. The absolute configuration of iejimalide B 626, a cytotoxic 24-membered macrolide isolated from a Japanese collection of Eudistoma cf. rigida,512 has been defined by analysis of 1H-1H and 1H-13C coupling constants, distance geometry calculations and analysis of oxidative degradation products.513 During the study the gross structure was also corrected to that shown (13Z). Floresolides A 627, B 628 and C 629 are moderately cytotoxic cyclofarnesylated hydroquinones isolated from an Aplidium sp. ascidian collected at Flores Island.514 The structures and absolute configurations of all three metabolites were secured by X-ray analysis of 629. The structures of the 3-aza-[7]-paracyclophane-containing alkaloids haouamines A 630 and B 631, isolated from Aplidium haouarianum collected off Tarifa Island, Cádiz, were also secured by X-ray analysis.515 Both haouamines exhibited two sets of NMR signals, attributed to the presence of isomers resulting from either atropisomerism or slow pyramidal inversion of the bridgehead amine. Of the two compounds, haouamine A was the more potent antitumour agent. Ascidians are a well-established source of cyclic peptides, many of which exhibit cytotoxicity. Didmolamides A 632 and B 633 are cyclic hexapeptides containing all (S)-configuration amino acids isolated from Didemnum molle collected in Madagascar.516 Both compounds exhibited modest cytotoxicity towards a panel of tumour cell lines. Six new congeners of the bistratamide family of cyclic hexapeptides, E–J 634–639, were reported from a Tablas Island, Philippines collection of Lissoclinum bistratum.517 All six compounds showed weak to moderate activity towards the HCT-116 tumour cell line. A full account of the synthesis of mollamide, a cytotoxic cycloheptapeptide isolated from an Australian collection of Didemnum molle,518 has been reported.519 The solution structure of the cytotoxic cycloheptapeptide trunkamide A 640520,521 has been determined using 2D-NMR data and simulated annealing methods.522 Fluorescent analogues of ascidian-derived depsipeptides didemnin B and tamandarin A have been used to study short-term predator-prey relationships between fish and marine invertebrate larvae.523 Plicatamide, a modified octapeptide isolated from the blood of a San Diego Bay specimen of Styela plicata,524 and several synthetic analogues have been found to exhibit potent antimicrobial activity, to cause K+ efflux in Staphylococcus aureus, were potently hemolytic for human red blood cells, and formed cation-selective channels in model lipid bilayers.525 Structure–activity studies of halocidin, an antimicrobial peptide (3443 Da) isolated from hemocytes of the solitary ascidian Halocynthia aurantium,526 identified one congener with potent antimicrobial activity, but reduced hemolytic activity.527 Further biological investigation of the cytotoxic depsipeptide aplidine, isolated from Aplidium albicans,528 indicates that the compound inhibits the growth and induces apoptosis in MOLT-4 cells through inhibition of vascular endothelial growth factor (VEGF) secretion which blocks the VEGF-VEGFR-1 autocrine loop necessary for growth of these cells.529 In addition, aplidine prevents the in vitro aggregation of the prion peptide PrP 106–126.530 EPR studies of vanadium-binding proteins, isolated from the vanadocytes of the ascidian Ascidia sydneiensis samea, indicate that up to 24 vanadium ions bind per protein molecule in a mononuclear state and that coordination is through amine nitrogens.531 The absolute configuration of etzionin 641, an antifungal diketopiperazine hydroxamate originally isolated from an unidentified Red Sea ascidian,532 has been secured by synthesis of all four stereoisomers of derivative 642, and direct comparison of optical rotation values with the same natural derivative.533 An initial attempt at expanding the structure–activity relationship of the cytotoxic quinolizidine alkaloid clavepictine B isolated from the Bermudian ascidian Clavelina picta,534 has indicated the importance of sidechain unsaturation, and that relative stereochemistry about the ring system does not seem to be important for cytotoxicity.535 Two full accounts of the stereoselective synthesis of lepadiformine, a biologically active alkaloid isolated from the ascidians Clavelina lepadiformis and C. moluccensis,536,537 have been reported.538,539 The structurally related ascidian alkaloids (+)-cylindricines C–E, isolated from an Australian collection of Clavelina cylindrica,540 were prepared using ruthenium-catalysed hydrative diyne cyclisation methodology.541 The quaternised indole-enamine conicamin 643 was isolated as a histamine antagonist from a Mediterranean collection of Aplidium conicum.542 Cynthichlorine 644, previously known as a synthetic product from the chlorination of methylindolyl methylester,543 was isolated from a Moroccan collection of Cynthia savignyi.544 The alkaloid exhibited antifungal activity towards two tomato pathogenic fungi and bacteria and was also cytotoxic in the brine shrimp lethality assay. Studies of an unidentified ascidian collected in Madagascar have afforded the mildly cytotoxic alkaloids barrenazine A 645 and B 646.545 The structures of the barrenazines were secured by use of 1H-15N HMBC NMR experiments, while the observance of optical rotatory properties for 645 suggested the (R*,R*) configuration. Further investigation of the Mediterranean collection of Aplidium conicum yielded conicaquinones A 647 and B 648, both of which exhibited cytotoxicity towards a rat glioma cell line.546 Kottamide E 649, the first example of a natural product bearing the amino acid 4-amino-1,2-dithiolane-4-carboxylic acid (Adt), was isolated from the New Zealand ascidian Pycnoclavella kottae.547 Benzotrithioles related to the cytotoxic pentathiepin ascidian alkaloids varacin548 and lissonclinotoxin A549,550 have been prepared and optical rotatory properties and crystal structures investigated.551 Lissoclinotoxins E 650 and F 651 were isolated as mildly cytotoxic components of a Philippine didemnid ascidian.552 The relative orientation of the aromatic rings of 650 and 651 were deduced, as shown, based upon molecular modeling studies. New members of the rigidin family of pyrrolopyrimidine alkaloids, rigidins B–D 652–654, were isolated from an Okinawan collection of Cystodytes sp.,553 while rigidin E 655 was isolated from a Papua New Guinea collection of a Eudistoma species.554 Rigidins B–D were mildly cytotoxic towards the L1210 murine leukemia cell line553 while rigidin 656555 and rigidin E were not cytotoxic towards A431 and wild-type and p53 deficient HCT-116 human tumour cell lines.554 Two β-carboline alkaloids, eudistomins W 657 and X 658, were isolated from Chuuk Atoll, Micronesia collections of a Eudistoma species.556 The absolute stereochemistry of 657 was ascertained (Mosher method), and 658 was found to be more potent in antimicrobial assays. Shishijimicins A–C 659–661 are extraordinarily potent cytotoxic enediyne antibiotics isolated from a South Japan collection of Didemnum proliferum.557 Relative and absolute stereochemistries were determined by standard methods and by comparison of CD data with that reported for the calicheamicins, terrestrial microbe-derived enediyne antibiotics. Distomadines A 662 and B 663 are new 6-hydroxyquinoline alkaloids from the New Zealand ascidian Pseudodistoma aureum.558 The structure of styelsamine C, an hydroxylpyridoacridine alkaloid isolated from the Indonesian ascidian Eusynstyela latericius,559 has been confirmed by synthesis.560 As noted in Section 7, 3-bromofascaplysin 301 was isolated from extracts of a Didemnum species ascidian collected at Chuuk Atoll, Micronesia, as well as from Fijian collections of Fascaplysinopsis sponges.286 The structure of sebastianine A, a pentacyclic alkaloid isolated from a Brazilian collection of Cystodytes dellechiajei,561 has been confirmed by total synthesis.562 Continued study of ascididemin, isolated from a Japanese collection of a Didemnum sp.,563 indicates that derivatives are also active in antiparasitic assays,564 that the antitumour activity can be varied somewhat predictably,565,566 and that a mechanism of reductive activation to form reactive oxygen species also contributes to the cytotoxicity of the parent alkaloid.567 The structure of bengacarboline, a cytotoxic alkaloid isolated from a Fijian collection of a Didemnum sp.,568 has been confirmed by total racemic synthesis.569 A convenient solid-phase synthesis of the ascidian metabolites lamellarin L570 and U571 has been reported.572 New improved syntheses of (−)-diazonamide A 664 have been reported,573,574 and investigation of the mechanism of action of 664 and analogue 665 indicate that the alkaloids are potent inhibitors of microtubule assembly, possibly at a unique site.575 Efficient syntheses of the naturally occurring cytotoxic ecteinascidins ET-729, -745, -759B, -736, -637 and -594576,577,578,579,580 from the fermentation product cyanosafracin B have been reported.581 The parent compound, ET-743, continues to progress through clinical trials.582,583,584 Ritterazine B, a dimeric steroidal alkaloid isolated from Ritterella tokioka,585 induces apoptosis in HL-60 cells and causes cell cycle accumulation at G2/M, but has no caspase activation effect nor does it alter phosphorylation of bcl-2.586 Aplidiasterols A 666 and B 667 are new cytotoxic secosterols isolated from a Mediterranean collection of Aplidium conicum.587 The structure and absolute stereochemistry of a steroidal sperm-activating and attracting factor 668 isolated from the ascidian Ciona intestinalis588 has been unambiguously determined by total synthesis.58912 Echinoderms
A similar number of new compounds were reported from echinoderms in 2003 compared with 2002. This field continues to be dominated by glycosylated ceramides and saponins. Taurine derivative 669 was isolated from a Gomun Island, Korea, collection of the starfish Certonardoa semiregularis.197 Investigation of the Patagonian starfish Anasterias minuta afforded a range of metabolites including the new glucosylceramide anasterocerebroside A 670.590 The known ceramide 671591,592 was also characterised for the first time. A Japanese collection of the starfish Luidia maculata yielded four ceramide lactosides, luidialactosides A–D 672–675.593 The position of the olefin in the long chain base of 674 was deduced by FABMS analysis of a dimethyl disulfide derivative. Three ganglioside molecular species, SCG-1–3, the major species of which are represented by 676–678, were isolated from the Japanese sea cucumber Stichopus chloronotus.594 All three species displayed neuritogenic activity against PC12 cells in the presence of nerve growth factor. A structurally more complex ganglioside molecular species SJG-2 679, isolated from a Japanese collection of Stichopus japonicus, also exhibited neuritogenic activity.595 Brine shrimp lethality assay-directed fractionation of the starfish Certonardoa semiregularis, collected off Komun Island, Korea, afforded thirteen new polyhydroxysterols. These were certonardosterols A–M 680–692,596 as well as the known 693.597 Side chain configurations at C-24 (for 686 and 693), C-25 (for 680) and both C-24 and C-25 (for 688) were determined (Mosher method). All of the sterols, with the exception of 692, exhibited modest in vitro cytotoxicity towards a panel of human tumour cell lines. A range of hemolytic steroid disulfates, including new examples 694 and 695, were reported from the starfish Pteraster pulvillus collected by trawling in the Sea of Okhotsk in the Far East.598 Unusual alkaloid cation and steroidal anion compounds 696–698 were isolated from the starfish Lethasterias nanimensis chelifera collected by trawling near the Kuril Islands in the Far East.599 Comparison of optical rotation values identified the cation as being the (R)-isomer of salsolinol. Steroid glycosides (saponins), commonly isolated from echinoderms, present challenges in structural elucidation and exhibit a diverse range of biological activities, both aspects of which have been reviewed.600,601 Four new saponins, certonardosides K–N 699–702, isolated from the starfish Certonardoa semiregularis collected off Komun Island, Korea, exhibited varied biological activity towards a range of tumour cell lines and bacteria.602 Configuration at C-24 in 699, 701 and 702 was secured by methanolysis and analysis of MTPA ester derivatives. The polyhydroxylated steroid ketone 703 and monoglycosylated steroid 704 were reported from collections of the Far Eastern starfish Henricia sanguinolenta and H. leviuscula leviuscula.603 Both compounds mildly inhibited division of fertilised sea urchin eggs. A South China Sea collection of the sea cucumber Mensamaria intercedens yielded intercedensides A–C 705–707, novel triterpene glycosides that exhibited in vitro cytotoxicity towards a panel of human tumour cell lines.604 Intercedenside A also exhibited in vivo activity towards Lewis lung and mouse S180 sarcoma tumour models. A Sea of Japan collection of the sea cucumber Cucumaria conicospermium also afforded triterpene glycosides, cucumariosides A2-5 708, A3-2 709, A3-3 710 and isokoreoside A 711, all of which contain the same pentasaccharide moiety, but differ in the number and position of the sulfate groups and the aglycone.605 Limited quantities of two new saponins ruberoside E 712 and F 713 were isolated from specimens of the starfish Asterias rubens collected in the Baltic Sea.606 The structures of both compounds were secured using a cryogenic NMR probe in an LC-NMR-MS configuration. Two mildly cytotoxic saponins, luidiaquinoside 714 and psilasteroside 715, were reported from collections of the starfish Luidia quinaria collected at Sendai (Japan) and Psilaster cassiope collected in the northern Gulf of Mexico respectively.607 The pathological effects of sea urchin toxins has been reviewed.60813 Miscellaneous
Three alkylpyrrole sulfamates 716–718 were isolated as fish-feeding deterrent metabolites from the annelid Cirriformia tentaculata, collected in Florida.609 Close to forty years after the structure of tetrodotoxin was elucidated,610,611,612 the first asymmetric syntheses of the alkaloid have been reported.613,61414 Conclusion
In the early years of marine natural products research there was less emphasis on biological testing, but increasingly there has been a focus on the biological properties of these compounds. In the first of the Faulkner reviews (1977),615 mention was made of the antibiotic properties of only a handful of compounds and reference made to the P388 activity of some Dolabella auricularia metabolites. In this review of the literature for 2003, over 720 compounds are included with biological activities being reported for 354 of these. The distribution of biological activities and source phyla for these compounds in 2003 is shown graphically in Figs. 1 and 2. The sponges and coelenterates continue to dominate as source phyla of new compounds, with microorganisms being the other major source. The relative incidence of bioactivity detected was greatest from the green alga followed by tunicates, echinoderms and sponges, but in absolute numbers the sponges dominated. The reported biological testing has been grouped into five categories, but is dominated by various tests for anticancer and antimicrobial/antiinfective properties. Tunicates, echinoderms and sponges were prime sources for the detection of potential anti-cancer properties. This combination of source and biological activity is very much in keeping with the data presented in the timely review on marine natural products and related compounds in clinical and advanced clinical trials.616 A graphical representation of the tabular data presented in that review is shown in Fig. 3. Progress towards marine anticancer drugs dominates with the prime source phyla being sponges followed by microorganisms, tunicates and molluscs. The other categories where marine natural products are progressing are in drugs for pain and asthmatic conditions where the interest is centered on Conus toxins and analogues of sponge sterols respectively.616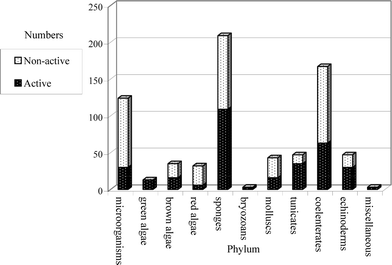 | ||
| Fig. 1 Distribution of biologically-active and non-active marine natural products by phylum, 2003. (Non-active–compounds for which no biological activity has been reported; Active–compounds that are active in at least one bioassay). | ||
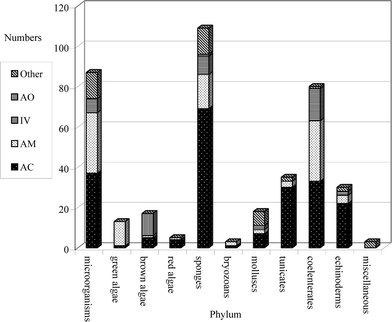 | ||
| Fig. 2 Distribution of biological activity by phylum. (AC–cancer related assays including cytotoxicity, antimitotic, histone deacetylase, proteasome, TNF, a range of kinases, DNA binding and matrix metalloproteinase; AM–antimicrobial, antiinfective, antiTb, antimalarial assays; AO–antioxidant assays; IV–in vivo assays such as brine shrimp and sea urchin eggs; Other–includes antiviral assays, assays based on central nervous system responses, feeding deterrent assays, ion channel assays, antifouling assays and assays for Fe siderophores, neuronal differentiation, oocyte lysis, sperm attractant and UV-A activity). | ||
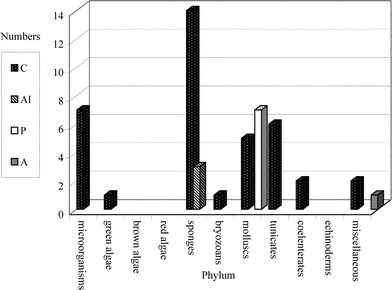 | ||
| Fig. 3 Numbers and distribution of marine and marine-derived compounds in clinical and pre-clinical trials. (Data extracted from Table 1 in reference 616) (C–anticancer drugs; AI–antiinflammatory drugs; P–drugs for intractable pain; A–Alzheimers). | ||
Since the discovery of the arabinose-based nucleosides by Bergman over 50 years ago,617–619 the explosion of interest in alternative nucleoside compositions and the subsequent development of Ara-C and Ara-A as drugs with obvious linkages to later antiviral drugs such as acyclovir and AZT, there has been a tacit assumption that marine-based drugs would soon be forthcoming. That has not yet happened, but the first truly marine drugs should be licensed within the next two years.616 Yondelis, better known as ecteinascidin 743, is in Phase II and III trials in Europe and the USA against soft tissue sarcoma, while the Conus toxin known as Ziconotide or Prialt is in Phase III clinical trials for intractable pain with plans for launching as a new drug in 2005. Despite problems in 2003 with the European Agency for the Evaluation of Medicinal Products, Yondelis will probably also be launched in 2005.616
Acknowledgements
We thank Ekkehard Unger for the collection of data for this review.References
- J. K. Volkman, Appl. Microbiol. Biotechnol., 2003, 60, 495 Search PubMed.
- J. Kobayashi, K. Shimbo, T. Kubota and M. Tsuda, Pure Appl. Chem., 2003, 75, 337 CrossRef CAS.
- A. Aygün and U. Pindur, Curr. Med. Chem., 2003, 10, 1113 CAS.
- J.-M. Kornprobst and H.-S. Al-Easa, Curr. Org. Chem., 2003, 7, 1181 CAS.
- M. R. Prinsep, Stud. Nat. Prod. Chem., 2003, 28, 617 Search PubMed.
- R. X. Tan and J. H. Chen, Nat. Prod. Rep., 2003, 20, 509 RSC.
- S. Matsunaga and N. Fusetani, Curr. Org. Chem., 2003, 7, 945 CAS.
- L. D. Han, J. G. Cui and C. S. Huang, Chin. J. Org. Chem., 2003, 23, 305 CAS.
- P. Muralidhar, P. Radhika, N. Krishna, D. V. Rao and Ch. B. Rao, Nat. Prod. Sci., 2003, 9, 117 CAS.
- D. J. Griffiths and M. L. Saker, Environ. Toxicol., 2003, 18, 78 CrossRef CAS.
- J.-F. Hu, M. T. Hamann, R. Hill and M. Kelly, Alkaloids, 2003, 60, 207 Search PubMed.
- D. J. Newman, G. M. Cragg and K. M. Snader, J. Nat. Prod., 2003, 66, 1022 CrossRef CAS.
- J. Peng, X. Shen, K. A. El Sayed, D. C. Dunbar, T. L. Perry, S. P. Wilkins, M. T. Hamann, S. Bobzin, J. Huesing, R. Camp, M. Prinsen, D. Krupa and M. A. Wideman, J. Agric. Food Chem., 2003, 51, 2246 CrossRef CAS.
- P. Proksch, R. Ebel, R. A. Edrada, P. Schupp, W. H. Lin, Sudarsono, V. Wray and K. Steube, Pure Appl. Chem., 2003, 75, 343 CrossRef CAS.
- A. M. S. Mayer and K. R. Gustafson, Int. J. Cancer, 2003, 105, 291 CrossRef CAS.
- P. Proksch, R. Ebel, R. A. Edrada, V. Wray and K. Steube, Sponges, 2003, 117 Search PubMed.
- E. Delfourne and J. Bastide, Med. Res. Rev., 2003, 23, 234 CrossRef CAS.
- B. Haefner, Drug Discovery Today, 2003, 8, 536 CrossRef CAS.
- G. Schwartsmann, A. Brondani da Rocha, J. Mattei and R. M. Lopes, Expert Opin. Invest. Drugs, 2003, 12, 1367 Search PubMed.
- D. J. Gochfeld, K. A. El Sayed, M. Yousaf, J. F. Hu, P. Bartyzel, D. C. Dunbar, S. P. Wilkins, J. K. Zjawiony, R. F. Schinazi, S. S. Wirtz, P. M. Tharnish and M. T. Hamann, Mini-Rev. Med. Chem., 2003, 3, 401 Search PubMed.
- L.-A. Tziveleka, C. Vagias and V. Roussis, Curr. Top. Med. Chem., 2003, 3, 1512 Search PubMed.
- M. Luescher-Mattli, Curr. Med. Chem.: Anti-Infect. Agents, 2003, 2, 219 Search PubMed.
- B. R. Copp, Nat. Prod. Rep., 2003, 20, 535 RSC.
- H. Akita, H. Nakamura and M. Ono, Chirality, 2003, 15, 352 CrossRef CAS.
- L. O. Haustedt, I. V. Hartung and H. M. R. Hoffmann, Angew. Chem., Int. Ed., 2003, 42, 2711 CrossRef CAS.
- I. Paterson and G. J. Florence, Eur. J. Org. Chem., 2003, 2193 CrossRef CAS.
- H. Hoffmann and T. Lindel, Synthesis, 2003, 12, 1753.
- M. A. Brimble and D. P. Furkert, Curr. Org. Chem., 2003, 7, 1461 CAS.
- A. Gryszkiewicz-Wojtkielewicz, I. Jastrzebska, J. W. Morzycki and D. B. Romanowska, Curr. Org. Chem., 2003, 7, 1257 CAS.
- S. Hanessian, R. Margarita, A. Hall, S. Johnstone, M. Tremblay and L. Parlanti, Pure Appl. Chem., 2003, 75, 209 CrossRef CAS.
- J. Mulzer and E. Öhler, Chem. Rev., 2003, 103, 3753 CrossRef CAS.
- W. E. G. Müller, F. Brümmer, R. Batel, I. M. Müller and H. C. Schröder, Naturwissenschaften, 2003, 90, 103.
- Y. Shimizu, Curr. Opin. Microbiol., 2003, 6, 236 CrossRef CAS.
- M. T. Hamann, Curr. Pharm. Des., 2003, 9, 879 Search PubMed.
- R. A. Hill, Annu. Rep. Prog. Chem., Sect. B, 2003, 99, 183 RSC.
- MarinLit database, Department of Chemistry, University of Canterbury: .
- R. H. Feling, G. O. Buchanan, T. J. Mincer, C. A. Kauffman, P. R. Jensen and W. Fenical, Angew. Chem., Int. Ed., 2003, 42, 355 CrossRef CAS.
- J. E. Leet, W. Li, H. A. Ax, J. A. Matson, S. Huang, R. Huang, J. L. Cantone, D. Drexler, R. A. Dalterio and K. S. Lam, J. Antibiot., 2003, 56, 232 CAS.
- W. Li, J. E. Leet, H. A. Ax, D. R. Gustavson, D. M. Brown, L. Turner, K. Brown, J. Clark, H. Yang, J. Fung-Tomc and K. S. Lam, J. Antibiot., 2003, 56, 226 CAS.
- T. Sasaki, T. Ohtani, H. Matsumoto, N. Unemi, M. Hamada, T. Takeuchi and M. Hori, J. Antibiot., 1998, 51, 715 CAS.
- K. Nagai, K. Kamigiri, N. Arao, K.-I. Suzumura, Y. Kawano, M. Yamaoka, H. Zhang, M. Watanabe and K. Suzuki, J. Antibiot., 2003, 56, 123 CAS.
- K.-I. Suzumura, T. Yokoi, M. Funatsu, K. Nagai, K. Tanaka, H. Zhang and K. Suzuki, J. Antibiot., 2003, 56, 129 CAS.
- Y. T. Park, J. B. Park, S. Y. Jeong, B. C. Song, W. A. Lim, C. H. Kim and W. J. Lee, Han’guk Susan Hakhoechi, 1998, 31, 767 Search PubMed.
- S.-Y. Jeong, K. Ishida, Y. Ito, S. Okada and M. Murakami, Tetrahedron Lett., 2003, 44, 8005 CrossRef CAS.
- M. Mitova, G. Tommonaro and S. De Rosa, Z. Naturforsch., C: Biosci., 2003, 58, 740 CAS.
- R. W. Schumacher, S. C. Talmage, S. A. Miller, K. E. Sarris, B. S. Davidson and A. Goldberg, J. Nat. Prod., 2003, 66, 1291 CrossRef.
- R. P. Maskey, E. Helmke, H.-H. Fiebig and H. Laatsch, J. Antibiot., 2003, 55, 1031.
- J. M. Sánchez López, M. Martinez Insua, J. Pérez Baz, J. L. Fernández Puentes and L. M. Cañedo Hernández, J. Nat. Prod., 2003, 66, 863 CrossRef.
- T. Itoh, M. Kinoshita, S. Aoki and M. Kobayashi, J. Nat. Prod., 2003, 66, 1373 CrossRef CAS.
- A. Spyere, D. C. Rowley, P. R. Jensen and W. Fenical, J. Nat. Prod., 2003, 66, 818 CrossRef.
- R. Fudou, Y. Jojima, T. Iizuka and S. Yamanaka, J. Gen. Appl. Microbiol., 2002, 48, 109 Search PubMed.
- R. Fudou, T. Iizuka and S. Yamanaka, J. Antibiot., 2001, 54, 149 CAS.
- R. Fudou, T. Iizuka, S. Sato, T. Ando, N. Shimba and S. Yamanaka, J. Antibiot., 2001, 54, 153 CAS.
- B. A. Kundim, Y. Itou, Y. Sakagami, R. Fudou, T. Iizuka, S. Yamanaka and M. Ojika, J. Antibiot., 2003, 56, 630 CAS.
- K. Kanoh, K. Kamino, G. Leleo, K. Adachi and Y. Shizuri, J. Antibiot., 2003, 56, 871 CAS.
- A. Isnansetyo and Y. Kamei, Int. J. Syst. Evol. Microbiol., 2003, 53, 583 Search PubMed.
- A. Isnansetyo and Y. Kamei, Antimicrob. Agents Chemother., 2003, 47, 480 CrossRef CAS.
- F. Fdhila, V. Vázquez, J. L. Sánchez and R. Riguera, J. Nat. Prod., 2003, 66, 1299 CrossRef CAS.
- J. C. Rodriguez, J. L. Fernández Puentes, J. Pérez Baz and L. M. Cañedo, J. Antibiot., 2003, 56, 318 CAS.
- R. P. Maskey, F. C. Li, S. Qin, H. H. Fiebig and H. Laatsch, J. Antibiot., 2003, 56, 622 CAS.
- M. Tsuda, T. Mugishima, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami, M. Shiro, M. Hirai, Y. Ohizumi and J. Kobayashi, Tetrahedron, 2003, 59, 3227 CrossRef CAS.
- M. Namikoshi, R. Negishi, H. Nagai, A. Dmitrenok and H. Kobayashi, J. Antibiot., 2003, 56, 755 CAS.
- R. J. Capon, C. Skene, M. Stewart, J. Ford, R. A. J. O'Hair, L. Williams, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, Org. Biomol. Chem., 2003, 1, 1856 RSC.
- S. M. Lee, X. F. Li, H. Jiang, J. G. Cheng, S. Seong, H. D. Choi and B. W. Son, Tetrahedron Lett., 2003, 44, 7707 CrossRef CAS.
- T. S. Bugni, V. S. Bernan, M. Greenstein, J. E. Janso, W. M. Maiese, C. L. Mayne and C. M. Ireland, J. Org. Chem., 2003, 68, 2014 CrossRef CAS.
- T. S. Bugni, V. S. Bernan, M. Greenstein, J. E. Janso, W. M. Maiese, C. L. Mayne and C. M. Ireland, J. Org. Chem., 2003, 68, 6846 CrossRef CAS.
- T. Amagata, A. Amagata, K. Tenney, F. A. Valeriote, E. Lobkovsky, J. Clardy and P. Crews, Org. Lett., 2003, 5, 4393 CrossRef CAS.
- A. G. Kozlovsky, V. P. Zhelifonova, S. M. Ozerskaya, N. G. Vinokurova, V. M. Adanin and U. Grafe, Pharmazie, 2000, 55, 470 CAS.
- D. C. Rowley, C. A. Kauffman, P. R. Jensen and W. Fenical, Bioorg. Med. Chem., 2003, 11, 4263 CrossRef CAS.
- L. T. Tan, X. C. Cheng, P. R. Jensen and W. Fenical, J. Org. Chem., 2003, 68, 8767 CrossRef CAS.
- E. Garo, C. M. Starks, P. R. Jensen, W. Fenical, E. Lobkovsky and J. Clardy, J. Nat. Prod., 2003, 66, 423 CrossRef CAS.
- Y. Lin, Z. Shao, G. Jiang, S. Zhou, J. Cai, L. L. P. Vrijmoed and E. B. G. Jones, Tetrahedron, 2000, 56, 9607 CrossRef CAS.
- N. Harada, K. Nakanishi, Circular Dichroic Spectrometry-Exciton Coupling in Organic Stereochemistry, University Science Books: Milly Valley, California, 1983 Search PubMed.
- M. Tsuda, T. Mugishima, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami and J. Kobayashi, J. Nat. Prod., 2003, 66, 412 CrossRef CAS.
- A. Abdel-Lateff, C. Klemke, G. M. König and A. D. Wright, J. Nat. Prod., 2003, 66, 706 CrossRef CAS.
- F. Kong and G. T. Carter, Tetrahedron Lett., 2003, 44, 3119 CrossRef CAS.
- C. Hopmann, M. A. Knauf, K. Weithmann and J. Wink, PCT Int. Appl., WO 01/44264 A2, 2001.
- G. Bringmann, G. Lang, S. Steffens, E. Günther and K. Schaumann, Phytochemistry, 2003, 63, 437 CrossRef CAS.
- A. D. Wright, C. Osterhage and G. M. König, Org. Biomol. Chem., 2003, 1, 507 RSC.
- Z. Liu, P. R. Jensen and W. Fenical, Phytochemistry, 2003, 64, 571 CrossRef CAS.
- R. P. Maskey, I. Kock, E. Helmke and H. Laatsch, Z. Naturforsch., B: Chem. Sci., 2003, 58, 692 CAS.
- B.-S. Yun, I.-J. Ryoo, W.-G. Kim, J.-P. Kim, H. Koshino, H. Seto and I.-D. Yoo, Tetrahedron Lett., 1996, 37, 8529 CrossRef CAS.
- L. M. Nogle and W. H. Gerwick, J. Nat. Prod., 2003, 66, 217 CrossRef CAS.
- R. T. Williamson, B. L. Marquez, W. H. Gerwick and K. E. Kover, Magn. Reson. Chem., 2000, 38, 265 CrossRef CAS.
- L. T. Tan, N. Sitachitta and W. H. Gerwick, J. Nat. Prod., 2003, 66, 764 CrossRef CAS.
- L. M. Nogle, B. L. Marquez and W. H. Gerwick, Org. Lett., 2003, 5, 3 CrossRef CAS.
- M. T. Davies-Coleman, T. M. Dzeha, C. A. Gray, S. Hess, L. K. Pannell, D. T. Hendricks and C. E. Arendse, J. Nat. Prod., 2003, 66, 712 CrossRef CAS.
- G. G. Harrigan, W. Y. Yoshida, R. E. Moore, D. G. Nagle, P. U. Park, J. Biggs, V. J. Paul, S. L. Mooberry, T. H. Corbett and F. A. Valeriote, J. Nat. Prod., 1998, 61, 1221 CrossRef.
- P. G. Williams, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 1356 CrossRef CAS.
- K. L. McPhail and W. H. Gerwick, J. Nat. Prod., 2003, 66, 132 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, M. K. Quon, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 651 CrossRef CAS.
- P. G. Williams, H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 595 CrossRef CAS.
- C. Gaillet, C. Lequart, P. Debeire and J. Nuzillard, J. Magn. Reson., 1999, 139, 454 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, M. K. Quon, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 1545 CrossRef CAS.
- B. Han, K. L. McPhail, A. Ligresti, V. Di Marzo and W. H. Gerwick, J. Nat. Prod., 2003, 66, 1364 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 620 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 1006 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, Org. Lett., 2003, 5, 4167 CrossRef CAS.
- S. T. Belt, G. Massé, W. G. Allard, J.-M. Robert and S. J. Rowland, Tetrahedron Lett., 2003, 44, 9103 CrossRef CAS.
- M. Tsuda, N. Izui, K. Shimbo, M. Sato, E. Fukushi, J. Kawabata, K. Katsumata, T. Horiguchi and J. Kobayashi, J. Org. Chem., 2003, 68, 5339 CrossRef CAS.
- M. Tsuda, N. Izui, K. Shimbo, M. Sato, E. Fukushi, J. Kawabata and J. Kobayashi, J. Org. Chem., 2003, 68, 9109 CrossRef CAS.
- M. Suzuki, K. Watanabe, S. Fujiwara, T. Kurasawa, T. Wakabayashi, M. Tsuzuki, K. Iguchi and T. Yamori, Chem. Pharm. Bull., 2003, 51, 724 CrossRef CAS.
- A. A. Carlos, B. K. Baillie, M. Kawachi and T. Maruyama, J. Phycol., 1999, 35, 1054 CrossRef CAS.
- K. Onodera, H. Nakamura, Y. Oba and M. Ojika, Tetrahedron, 2003, 59, 1067 CrossRef CAS.
- B. Suárez-Gómez, M. L. Souto, M. Norte and J. J. Fernández, J. Nat. Prod., 2001, 64, 1363 CrossRef CAS.
- J. J. Fernández, B. Suárez-Gómez, M. L. Souto and M. Norte, J. Nat. Prod., 2003, 66, 1294 CrossRef CAS.
- A. Negri, D. Stirling, M. Quilliam, S. Blackburn, C. Bolch, I. Burton, G. Eaglesham, K. Thomas, J. Walter and R. Willis, Chem. Res. Toxicol., 2003, 16, 1029 CrossRef CAS.
- I. H. Hardt, P. R. Jensen and W. Fenical, Tetrahedron Lett., 2000, 41, 2073 CrossRef CAS.
- J. A. Kalaitzis, Y. Hamano, G. Nilsen and B. S. Moore, Org. Lett., 2003, 5, 4449 CrossRef CAS.
- R. T. Williamson, A. Boulanger, A. Vulpanovici, M. A. Roberts and W. H. Gerwick, J. Org. Chem., 2002, 67, 7927 CrossRef CAS.
- R. T. Williamson, A. Boulanger, A. Vulpanovici, M. A. Roberts and W. H. Gerwick, J. Org. Chem., 2003, 68, 2060 CrossRef CAS.
- M. Namikoshi, H. Kobayashi, T. Yoshimoto and T. Hosoya, J. Antibiot., 1997, 50, 890 CAS.
- H. Kobayashi, S. Meguro, T. Yoshimoto and M. Namikoshi, Tetrahedron, 2003, 59, 455 CrossRef CAS.
- K. Barbeau, G. Zhang, D. H. Live and A. Butler, J. Am. Chem. Soc., 2002, 124, 378 CrossRef CAS.
- R. J. Bergeron, G. Huang, R. E. Smith, N. Bharti, J. S. McManis and A. Butler, Tetrahedron, 2003, 59, 2007 CrossRef CAS.
- N. Sitachitta, R. T. Williamson and W. H. Gerwick, J. Nat. Prod., 2000, 63, 197 CrossRef CAS.
- Z. Xu, Y. Peng and T. Ye, Org. Lett., 2003, 5, 2821 CrossRef CAS.
- M. Chu, I. Truumees, I. Gunnarsson, W. R. Bishop, W. Kreutner, A. C. Horan, M. G. Patel, V. P. Gullo and M. S. Puar, J. Antibiot., 1993, 46, 554 CAS.
- J. W. C. Cheing, W. P. D. Goldring and G. Pattenden, Chem. Commun., 2003, 2788 RSC.
- L. A. McDonald, D. R. Abbanat, L. R. Barbieri, V. S. Bernan, C. M. Discafani, M. Greenstein, K. Janota, J. D. Korshalla, P. Lassota, M. Tischler and G. T. Carter, Tetrahedron Lett., 1999, 40, 2489 CrossRef CAS.
- K. Miyashita, T. Sakai and T. Imanishi, Org. Lett., 2003, 5, 2683 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore, V. J. Paul and T. H. Corbett, J. Am. Chem. Soc., 2001, 123, 5418 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore, V. J. Paul and T. H. Corbett, Bioorg. Med. Chem., 2002, 10, 1973 CrossRef CAS.
- J. Chen and C. J. Forsyth, J. Am. Chem. Soc., 2003, 125, 8734 CrossRef CAS.
- I. Bauer, L. Maranda, K. A. Young, Y. Shimizu and S. Huang, Tetrahedron Lett., 1995, 36, 991 CrossRef CAS.
- L. M. Walsh and J. M. Goodman, Chem. Commun., 2003, 2616 RSC.
- J. Kobayashi, T. Kubota, M. Tsuda and T. Endo, J. Org. Chem., 2000, 65, 1349 CrossRef CAS.
- T. Kubota, T. Endo, M. Tsuda, M. Shiro and J. Kobayashi, Tetrahedron, 2001, 57, 6175 CrossRef CAS.
- A. K. Gosh and C. Liu, J. Am. Chem. Soc., 2003, 125, 2374 CrossRef CAS.
- C. Aïssa, R. Riveiros, J. Ragot and A. Fürstner, J. Am. Chem. Soc., 2003, 125, 15512 CrossRef.
- J. Kobayashi, T. Kubota, T. Endo and M. Tsuda, J. Org. Chem., 2001, 66, 134 CrossRef CAS.
- M. Satake, M. Shoji, Y. Oshima, H. Naoki, T. Fujita and T. Yasumoto, Tetrahedron Lett., 2002, 43, 5829 CrossRef CAS.
- C. Tsukano and M. Sasaki, J. Am. Chem. Soc., 2003, 125, 14294 CrossRef CAS.
- Satake, M. Murata and T. Yasumoto, J. Am. Chem. Soc., 1993, 115, 361 CrossRef CAS.
- H. Fuwa and M. Sasaki, J. Synth. Org. Chem. Jpn., 2003, 61, 742 CAS.
- A. Morohashi, M. Satake and T. Yasumoto, Tetrahedron Lett., 1999, 40, 97 CrossRef CAS.
- H. Nagai, M. Murata, K. Torigoe, M. Satake and T. Yasumoto, J. Org. Chem., 1992, 57, 5448 CrossRef CAS.
- A. Morohashi, M. Satake, H. Nagai, Y. Oshima and T. Yasumoto, Tetrahedron, 2000, 56, 8995 CrossRef CAS.
- M. Inoue, M. Hirama, M. Satake, K. Sugiyama and T. Yasumoto, Toxicon, 2003, 41, 469 CrossRef CAS.
- Y.-Y. Lin, M. Risk, S. M. Ray, D. Van Engen, J. Clardy, J. Golik, J. C. James and K. Nakanishi, J. Am. Chem. Soc., 1981, 103, 6773 CrossRef CAS.
- T. K. Han, M. Derby, D. F. Martin, S. D. Wright and M. L. Dao, Int. J. Toxicol., 2003, 22, 73 CrossRef CAS.
- M. T. Hamann and P. J. Scheuer, J. Am. Chem. Soc., 1993, 115, 5825 CrossRef CAS.
- G. Goetz, W. Y. Yoshida and P. J. Scheuer, Tetrahedron, 1999, 55, 7739 CrossRef CAS.
- I. Bonnard, I. Manzanares and K. L. Rinehart, J. Nat. Prod., 2003, 66, 1466 CrossRef CAS.
- V. Smyrniotopoulos, D. Abatis, L.-A. Tziveleka, C. Tsitsimpikou, V. Roussis, A. Loukis and C. Vagias, J. Nat. Prod., 2003, 66, 21 CrossRef CAS.
- V. J. Paul and W. Fenical, Mar. Ecol. Prog. Ser., 1986, 34, 157 CrossRef CAS.
- L. Commeiras, R. Valls, M. Santelli and J.-L. Parrain, Synlett., 2003, 1716.
- K. M. Fisch, V. Böhm, A. D. Wright and G. M. König, J. Nat. Prod., 2003, 66, 968 CrossRef CAS.
- S.-E. N. Ayyad, O. B. Abdel-Halim, W. T. Shier and T. R. Hoye, Z. Naturforsch., C: Biosci., 2003, 58, 33 CAS.
- S. R. Gedara, O. B. Abdel-Halim, S. H. El-Sharkawy, O. M. Salama, T. W. Shier and A. F. Halim, Z. Naturforsch., C: Biosci., 2003, 58, 17 CAS.
- M. S. Ali and M. K. Pervez, Z. Naturforsch., B: Chem. Sci., 2003, 58, 438 CAS.
- M. S. Ali and M. K. Pervez, Nat. Prod. Res., 2003, 17, 281 CrossRef CAS.
- M. S. Ali, M. K. Pervez, M. Saleem and F. Ahmed, Nat. Prod. Res., 2003, 17, 301 CrossRef CAS.
- H. Soto, J. Rovirosa and A. San-Martín, Z. Naturforsch., B: Chem. Sci., 2003, 58, 795 CAS.
- K. Kousaka, N. Ogi, Y. Akazawa, M. Fujieda, Y. Yamamoto, Y. Takada and J. Kimura, J. Nat. Prod., 2003, 66, 1318 CrossRef CAS.
- S. Carmeli, R. E. Moore and G. M. L. Patterson, J. Nat. Prod., 1990, 53, 1533 CrossRef CAS.
- S. Carmely, M. Rotem and Y. Kashman, Magn. Reson. Chem., 1986, 24, 343 CAS.
- I. Kitagawa, M. Kobayashi, T. Katori, M. Yamashita, J. Tanaka, M. Doi and T. Ishida, J. Am. Chem. Soc., 1990, 112, 3710 CrossRef CAS.
- J. Kubanek, P. R. Jensen, P. A. Keifer, M. C. Sullards, D. O. Collins and W. Fenical, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6916 CrossRef CAS.
- S.-E. N. Ayyad, S. Z. A. Sowellim, M. S. El-Hosini and A. Abo-Atia, Z. Naturforsch., C: Biosci., 2003, 58, 333 CAS.
- H. Tang, Y. Yi, X. Yao, Q. Xu, S. Zhang and H. Lin, Zhongguo Haiyang Yaowu, 2003, 22, 28 Search PubMed.
- H. S. Kang, H. Y. Chung, J. H. Jung, B. W. Son and J. S. Choi, Chem. Pharm. Bull., 2003, 51, 1012 CrossRef CAS.
- C. Ireland and D. J. Faulkner, J. Org. Chem., 1977, 42, 3157 CrossRef CAS.
- J. P. Barbosa, V. L. Teixeira, R. Villaça, R. C. Pereira, J. L. Abrantes and I. C. P. da Paixão Frugulhetti, Biochem. Syst. Ecol., 2003, 31, 1451 CrossRef CAS.
- N. Takada, R. Watanabe, K. Suenaga, K. Yamada and D. Uemura, J. Nat. Prod., 2001, 64, 653 CrossRef CAS.
- Y. Li, B. Lu, C. Li and Y. Li, Synth. Commun., 2003, 33, 1417 CrossRef CAS.
- L. R. de Carvalho, M. T. Fujii, N. F. Roque, M. J. Kato and J. H. G. Lago, Tetrahedron Lett., 2003, 44, 2637 CrossRef CAS.
- G. Guella, D. Skropeta, I. Mancini and F. Pietra, Chem. Eur. J., 2003, 9, 5770 CrossRef CAS.
- G. Topcu, Z. Aydogmus, S. Imre, A. C. Gören, J. M. Pezzuto, J. A. Clement and D. G. I. Kingston, J. Nat. Prod., 2003, 66, 1505 CrossRef CAS.
- A. D. Wright, E. Goclik and G. M. König, J. Nat. Prod., 2003, 66, 435 CrossRef CAS.
- D. Iliopoulou, N. Mihopoulos, C. Vagias, P. Papazafiri and V. Roussis, J. Org. Chem., 2003, 68, 7667 CrossRef.
- N. Mihopoulos, C. Vagias, E. Mikros, M. Scoullos and V. Roussis, Tetrahedron Lett., 2001, 42, 3749 CrossRef CAS.
- D. Iliopoulou, N. Mihopoulos, V. Roussis and C. Vagias, J. Nat. Prod., 2003, 66, 1225 CrossRef CAS.
- X. Fan, N.-J. Xu and J.-G. Shi, J. Nat. Prod., 2003, 66, 455 CrossRef CAS.
- X. Fan, N. J. Xu and J. G. Shi, Chin. Chem. Lett., 2003, 14, 1045 CAS.
- J. A. Shepherd, W. W. Poon, D. C. Myles and C. F. Clarke, Tetrahedron Lett., 1996, 37, 2395 CrossRef CAS.
- X. Fan, N. J. Xu and J. G. Shi, Chinese Chemical Letters, 2003, 14, 939 Search PubMed.
- N. Xu, X. Fan, X. Yan, X. Li, R. Niu and C. K. Tseng, Phytochemistry, 2003, 62, 1221 CrossRef CAS.
- M. Kuniyoshi, N. Oshiro, T. Miono and T. Higa, J. Chin. Chem. Soc., 2003, 50, 167 CAS.
- M. Norte, J. J. Fernández, M. L. Souto, J. A. Gavín and M. D. Garcia-Grávalos, Tetrahedron, 1997, 53, 3173 CrossRef CAS.
- M. K. Pec, A. Aguirre, K. Moser-Thier, J. J. Fernandez, M. L. Souto, J. Dorta, F. Diaz-Gonzalez and J. Villar, Biochem. Pharmacol., 2003, 65, 1451 CrossRef CAS.
- J. J. Sims, G. H. Y. Lin and R. M. Wing, Tetrahedron Lett., 1974, 39, 3487 CrossRef.
- C. S. Vairappan, Biomol. Eng., 2003, 20, 255 Search PubMed.
- A. G. González, J. Darias, A. Díaz, J. D. Fourneron, J. D. Martín and C. Pérez, Tetrahedron Lett., 1976, 35, 3051 CrossRef.
- A. G. González, M. J. Delgado, V. S. Martín, M. Martínez-Ripoll and J. Fayos, Tetrahedron Lett., 1979, 29, 2717 CrossRef.
- M. Pedersen, P. Saenger and L. Fries, Phytochemistry, 1974, 13, 2273 CrossRef CAS.
- J. M. Kuhajek and D. Schlenk, Comp. Biochem. Physiol., 2003, 134C, 473 Search PubMed.
- A. G. González, J. D. Martín, V. S. Martín, M. Norte, R. Pérez and J. Ruano, Tetrahedron, 1982, 38, 1009 CrossRef CAS.
- M. Norte, A. G. González, F. Cataldo, M. L. Rodriguez and I. Brito, Tetrahedron, 1991, 47, 9411 CrossRef CAS.
- H. Kim, W. J. Choi, J. Jung, S. Kim and D. Kim, J. Am. Chem. Soc., 2003, 125, 10238 CrossRef CAS.
- M. Suzuki, Y. Misano, Y. Matsuo and M. Masuda, Phytochemistry, 1996, 43, 121 CrossRef CAS.
- H. Lee, H. Kim, S. Baek, S. Kim and D. Kim, Tetrahedron Lett., 2003, 44, 6609 CrossRef CAS.
- L. Zaman, O. Arakawa, A. Shimosu, Y. Onoue, S. Nishio, Y. Shida and T. Noguchi, Toxicon, 1997, 35, 205 CrossRef CAS.
- Y. Ni, K. K. D. Amarasinghe, B. Ksebati and J. Montgomery, Org. Lett., 2003, 5, 3771 CrossRef CAS.
- V. Costantino, E. Fattorusso, C. Imperatore and A. Mangoni, Eur. J. Org. Chem., 2003, 1433 CrossRef CAS.
- V. Ledroit, C. Debitus, C. Lavaud and G. Massiot, Tetrahedron Lett., 2003, 44, 225 CrossRef CAS.
- W. Wang, Y. M. Lee, J. Hong, C.-O. Lee, J. H. Park and J. H. Jung, Nat. Prod. Sci., 2003, 9, 241 CAS.
- S.-Y. Lee, Q. Zhao, K. Choi, J. Hong, D. S. Lee, C.-O. Lee and J. H. Jung, Nat. Prod. Sci., 2003, 9, 232 CAS.
- Q. Zhao, Y. Liu, J. Hong, C.-O. Lee, J. H. Park, D. S. Lee and J. H. Jung, Nat. Prod. Sci., 2003, 9, 18 CAS.
- M. Tsuda, T. Endo, M. Perpelescu, S. Yoshida, K. Watanabe, J. Fromont, Y. Mikami and J. Kobayashi, Tetrahedron, 2003, 59, 1137 CrossRef CAS.
- T. Řezanka and V. M. Dembitsky, Eur. J. Org. Chem., 2003, 2144 CrossRef CAS.
- M. Fujita, Y. Nakao, S. Matsunaga, R. W. M. van Soest, Y. Itoh, M. Seiki and N. Fusetani, J. Nat. Prod., 2003, 66, 569 CrossRef CAS.
- Q. Zhao, S.-Y. Lee, J. Hong, C.-O. Lee, K. S. Im, C. J. Sim, D. S. Lee and J. H. Jung, J. Nat. Prod., 2003, 66, 408 CrossRef CAS.
- Q. Zhao, T. A. Mansoor, J. Hong, C.-O. Lee, K. S. Im, D. S. Lee and J. H. Jung, J. Nat. Prod., 2003, 66, 725 CrossRef CAS.
- H.-S. Lee, J.-R. Rho, C. J. Sim and J. Shin, J. Nat. Prod., 2003, 66, 566 CrossRef CAS.
- J. C. Braekman, D. Daloze, C. Devijver, D. Dubut and R. W. M. van Soest, J. Nat. Prod., 2003, 66, 871 CrossRef CAS.
- M. L. Lerch, M. K. Harper and D. J. Faulkner, J. Nat. Prod., 2003, 66, 667 CrossRef CAS.
- R. P. de Jesus and D. J. Faulkner, J. Nat. Prod., 2003, 66, 671 CrossRef CAS.
- R. P. Walker and D. J. Faulkner, J. Org. Chem., 1981, 46, 1475 CrossRef CAS.
- D. T. A. Youssef, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2003, 66, 861 CrossRef CAS.
- S. Ohta, H. Okada, H. Kobayashi, J. M. Oclarit and S. Ikegami, Tetrahedron Lett., 1993, 34, 5935 CrossRef CAS.
- M. Ojika, Y. Itou and Y. Sakagami, Biosci. Biotechnol. Biochem., 2003, 67, 1568 CrossRef CAS.
- C. Jiménez and P. Crews, J. Nat. Prod., 1990, 53, 978 CrossRef.
- M. Ichihashi and K. Mori, Biosci. Biotechnol. Biochem., 2003, 67, 329 CrossRef CAS.
- N. M. Carballeria and J. Alicea, Lipids, 2001, 36, 83.
- N. M. Carballeira, H. Cruz, E. A. Orellano and F. A. González, Chem. Phys. Lipids, 2003, 126, 149 CrossRef CAS.
- K. Watanabe, Y. Tsuda, Y. Yamane, H. Takahashi, K. Higuchi, H. Naoki, T. Fujita and R. M. W. van Soest, Tetrahedron Lett., 2000, 41, 9271 CrossRef CAS.
- S. Reber, T. F. Knöpfel and E. M. Carreira, Tetrahedron, 2003, 59, 6813 CrossRef CAS.
- S. Tsukamoto, H. Kato, H. Hirota and N. Fusetani, J. Nat. Prod., 1997, 60, 126 CrossRef CAS.
- A. Umeyama, C. Nagano and S. Arihara, J. Nat. Prod., 1997, 60, 131 CrossRef CAS.
- S. López, F. Fernández-Trillo, L. Castedo and C. Saá, Org. Lett., 2003, 5, 3725 CrossRef CAS.
- I. van Altena, R. van Soest, M. Roberge and R. J. Andersen, J. Nat. Prod., 2003, 66, 561 CrossRef CAS.
- A. Rudi, R. Afanii, L. G. Gravalos, M. Aknin, E. Gaydou, J. Vacelet and Y. Kashman, J. Nat. Prod., 2003, 66, 682 CrossRef CAS.
- C.-Y. Wang, B.-G. Wang, S. Wiryowidagdo, V. Wray, R. van Soest, K. G. Steube, H.-S. Guan, P. Proksch and R. Ebel, J. Nat. Prod., 2003, 66, 51 CrossRef CAS.
- M. del-S. Jiménez, S. P. Garzón and A. D. Rodríguez, J. Nat. Prod., 2003, 66, 655 CrossRef.
- M. Yanai, S. Ohta, E. Ohta, T. Hirata and S. Ikegami, Bioorg. Med. Chem., 2003, 11, 1715 CrossRef CAS.
- K. L. Erikson, J. A. Beutler, J. H. Cardellina and M. R. Boyd, Tetrahedron, 1995, 51, 11953 CrossRef CAS.
- C. M. Cerda-García-Rojas and D. J. Faulkner, Tetrahedron, 1995, 51, 1087 CrossRef CAS.
- I. R. Czuba, S. Zammit and M. A. Rizzacasa, Org. Biomol. Chem., 2003, 1, 2044 RSC.
- W. M. Bandaranayake, G. Pattenden and W. A. Wickramasinghe, Trends Comp. Biochem. Physiol., 2002, 9, 205 Search PubMed.
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2004, 21, 1 RSC.
- K. Tachibana, P. J. Scheuer, Y. Tsukitani, H. Kikuchi, D. van Engen, J. Clardy, Y. Gopichand and F. J. Schmitz, J. Am. Chem. Soc., 1981, 103, 2469 CrossRef CAS.
- T. Yasumoto, M. Murata, Y. Oshima, M. Sano, G. K. Matsumoto and J. Clardy, Tetrahedron, 1985, 41, 1019 CrossRef CAS.
- Y. Murakami, Y. Oshima and T. Yasumoto, Bull. Jap. Soc. Sci. Fish., 1982, 48, 69 Search PubMed.
- M. Wiens, B. Luckas, F. Brümmer, M. Shokry, A. Ammar, R. Steffen, R. Batel, B. Diehl-Seifert, H. C. Schröder and W. E. G. Müller, Mar. Biol., 2003, 142, 213 Search PubMed.
- R. Britton, M. Roberge, C. Brown, R. van Soest and R. J. Andersen, J. Nat. Prod., 2003, 66, 838 CrossRef CAS.
- R. Talpir, Y. Benayahu, Y. Kashman, L. Pannell and M. Schleyer, Tetrahedron Lett., 1994, 35, 4453 CrossRef CAS.
- P. Crews, J. J. Farias, R. Emrich and P. A. Keifer, J. Org. Chem., 1994, 59, 2932 CrossRef CAS.
- C. Chevallier, A. D. Richardson, M. C. Edler, E. Hamel, M. K. Harper and C. M. Ireland, Org. Lett., 2003, 5, 3737 CrossRef CAS.
- Y. Nakao, J. Kuo, W. Y. Yoshida, M. Kelly and P. J. Scheuer, Org. Lett., 2003, 5, 1387 CrossRef CAS.
- Y. Sera, K. Adachi, K. Fujii and Y. Shizuri, J. Nat. Prod., 2003, 66, 719 CrossRef CAS.
- K. L. Erickson, K. R. Gustafson, D. J. Milanowski, L. K. Pannell, J. R. Klose and M. R. Boyd, Tetrahedron, 2003, 59, 10231 CrossRef CAS.
- K. L. Erickson, K. R. Gustafson, L. K. Pannell, J. A. Beutler and M. R. Boyd, J. Nat. Prod., 2002, 65, 1303 CrossRef CAS.
- S. Kehraus, G. M. König, A. D. Wright and G. Woerheide, J. Org. Chem., 2002, 67, 4989 CrossRef CAS.
- W. Wang and F. Nan, J. Org. Chem., 2003, 68, 1636 CrossRef CAS.
- L. T. Tan, R. T. Williamson, W. H. Gerwick, K. S. Watts, K. McGough and R. Jacobs, J. Org. Chem., 2000, 65, 419 CrossRef CAS.
- S. Deng and J. Taunton, J. Am. Chem. Soc., 2002, 124, 916 CrossRef CAS.
- F. Yokokawa, T. Shiori, Y. In, K. Minoura and T. Ishida, Pept. Sci., 2002, 39, 41 Search PubMed.
- G. R. Pettit, Z. Cichacz, J. Barkoczy, A. C. Dorsaz, D. L. Herald, M. D. Williams, D. L. Doubek, J. M. Schmidt, L. P. Tackett, D. C. Brune, R. L. Cerny, J. N. A. Hooper and G. J. Bakus, J. Nat. Prod., 1993, 56, 260 CrossRef CAS.
- G. R. Pettit, R. Tan, Y. Ichihara, M. D. Williams, D. L. Doubek, L. P. Tackett, J. M. Schmidt, R. L. Cerny, M. R. Boyd and J. N. A. Hooper, J. Nat. Prod., 1995, 58, 961 CrossRef CAS.
- A. Napolitano, M. Rodriquez, I. Bruno, S. Marzocco, G. Autore, R. Riccio and L. Gomez-Paloma, Tetrahedron, 2003, 59, 10203 CrossRef CAS.
- G. R. Pettit and R. Tan, Bioorg. Med. Chem. Lett., 2003, 13, 685 CrossRef CAS.
- W.-L. Li, Y.-H. Yi, H.-M. Wu, Q.-Z. Xu, H.-F. Tang, D.-Z. Zhou, H.-W. Lin and Z.-H. Wang, J. Nat. Prod., 2003, 66, 146 CrossRef CAS.
- D. E. Williams, M. Roberge, R. van Soest and R. J. Andersen, J. Am. Chem. Soc., 2003, 125, 5296 CrossRef CAS.
- L. M. West, P. T. Northcote and C. N. Battershill, J. Org. Chem., 2000, 65, 445 CrossRef CAS.
- X. Liao, Y. Wu and J. K. de Brabander, Angew. Chem., Int. Ed., 2003, 42, 1648 CrossRef CAS.
- M. V. D'Auria, L. Gomez-Paloma, L. Minale, A. Zampella, J. F. Verbist, C. Roussakis, C. Debitus and J. Patissou, Tetrahedron, 1994, 50, 4829 CrossRef CAS.
- A. Zampella, V. Sepe, R. D'Orsi, G. Bifulco, C. Bassarello and M. V. D'Auria, Tetrahedron: Asymmetry, 2003, 14, 1787 CrossRef CAS.
- N. Fusetani, T. Sugawara, S. Matsunaga and H. Hirota, J. Org. Chem., 1991, 56, 4971 CrossRef CAS.
- A. B. Smith III, C. M. Adams, S. A. Barbosa and A. P. Degnan, J. Am. Chem. Soc., 2003, 125, 350 CrossRef.
- P. A. Horton, F. E. Koehn, R. E. Longley and O. J. McConnell, J. Am. Chem. Soc., 1994, 116, 6015 CrossRef CAS.
- H. Y. Song, J. M. Joo, J. W. Kang, D.-S. Kim, C.-K. Jung, H. S. Kwak, J. H. Park, E. Lee, C. Y. Hong, S. Jeong, K. Jeon and J. H. Park, J. Org. Chem., 2003, 68, 8080 CrossRef.
- R. S. Norton, K. D. Croft and R. J. Wells, Tetrahedron, 1981, 37, 2341 CrossRef CAS.
- J. Á. de la Fuente, S. Manzanaro, M. J. Martín, T. G. de Quesada, I. Reymundo, S. M. Luengo and F. Gago, J. Med. Chem., 2003, 46, 5208 CrossRef CAS.
- R. Sakai, H. Matsubara, K. Shimamoto, M. Jimbo, H. Kamiya and M. Namikoshi, J. Nat. Prod., 2003, 66, 784 CrossRef CAS.
- N. Lysek, R. Kinscherf, R. Claus and T. Lindel, Z. Naturforsch., C: Biosci., 2003, 58, 568 CAS.
- C. Campagnuolo, C. Fattorusso, E. Fattorusso, A. Ianaro, B. Pisano and O. Taglialatela-Scafati, Org. Lett., 2003, 5, 673 CrossRef CAS.
- C. A. Volk and M. Köck, Org. Lett., 2003, 5, 3567 CrossRef CAS.
- Y. Kashman, G. Koren-Goldshlager, M. D. Garcia Gravalos and M. Schleyer, Tetrahedron Lett., 1999, 40, 997 CrossRef CAS.
- M. R. Heinrich, W. Steglich, M. G. Banwell and Y. Kashman, Tetrahedron, 2003, 59, 9239 CrossRef CAS.
- S. Sperry and P. Crews, Tetrahedron Lett., 1996, 37, 2389 CrossRef CAS.
- J. C. Daab and F. Bracher, Monatsh. Chem., 2003, 134, 573 CrossRef CAS.
- S. Tsukamoto, M. Takahashi, S. Matsunaga, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 2000, 63, 682 CrossRef CAS.
- W. R. F. Goundry, J. E. Baldwin and V. Lee, Tetrahedron, 2003, 59, 1719 CrossRef CAS.
- M. Tsuda, K. Hirano, T. Kubota and J. Kobayashi, Tetrahedron Lett., 1999, 40, 4819 CrossRef CAS.
- B. B. Snider and B. Shi, Tetrahedron Lett., 2001, 42, 1639 CrossRef CAS.
- S. P. Romeril, V. Lee, T. D. W. Claridge and J. E. Baldwin, Tetrahedron Lett., 2002, 43, 327 CrossRef CAS.
- Y. Morimoto, S. Kitao, T. Okita and T. Shoji, Org. Lett., 2003, 5, 2611 CrossRef CAS.
- S. P. Romeril, V. Lee, J. E. Baldwin, T. D. W. Claridge and B. Odell, Tetrahedron Lett., 2003, 44, 7757 CrossRef CAS.
- J. C. Braekman, D. Daloze, P. Macedo de Abreu, C. Piccinni-Leopardi, G. Germain and M. van Meerssche, Tetrahedron Lett., 1982, 23, 4277 CrossRef CAS.
- J. C. Braekman, D. Daloze, N. Defay and D. Zimmermann, Bull. Soc. Chim. Belg., 1984, 93, 941 CAS.
- T. V. Goud, N. S. Reddy, N. R. Swamy, T. S. Ram and Y. Venkateswarlu, Biol. Pharm. Bull., 2003, 26, 1498 CrossRef CAS.
- J. Kobayashi, D. Watanabe, N. Kawasaki and M. Tsuda, J. Org. Chem., 1997, 62, 9236 CrossRef CAS.
- T. Nagata, M. Nakagawa and A. Nishida, J. Am. Chem. Soc., 2003, 125, 7484 CrossRef CAS.
- K. V. Rao, B. D. Santarsiero, A. D. Mesecar, R. F. Schinazi, B. L. Tekwani and M. T. Hamann, J. Nat. Prod., 2003, 66, 823 CrossRef CAS.
- N. L. Segraves, S. Lopez, T. A. Johnson, S. A. Said, X. Fu, F. J. Schmitz, H. Pietraszkiewicz, F. A. Valeriote and P. Crews, Tetrahedron Lett., 2003, 44, 3471 CrossRef CAS.
- C. Campagnuolo, E. Fattorusso and O. Taglialatela-Scafati, Eur. J. Org. Chem., 2003, 284 CrossRef CAS.
- D. B. Stierle and D. J. Faulkner, J. Nat. Prod., 1991, 54, 1131 CrossRef CAS.
- N. K. Utkina, A. V. Gerasimenko and D. Y. Popov, Russ. Chem. Bull., 2003, 52, 258 CrossRef CAS.
- C. M. Zeng, M. Ishibashi, K. Matsumoto, S. Nakaike and J. Kobayashi, Tetrahedron, 1993, 49, 8337 CrossRef CAS.
- S. Aoki, H. Wei, K. Matsui, R. Rachmat and M. Kobayashi, Bioorg. Med. Chem., 2003, 11, 1969 CrossRef CAS.
- L. Calcul, A. Longeon, A. Al-Mourabit, M. Guyot and M. Bourguet-Kondracki, Tetrahedron, 2003, 59, 6539 CrossRef CAS.
- N. Oku, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2003, 66, 1136 CrossRef CAS.
- G. R. Pettit, J. C. Collins, J. C. Knight, D. L. Herald, R. A. Nieman, M. D. Williams and R. K. Pettit, J. Nat. Prod., 2003, 66, 544 CrossRef CAS.
- K. Warabi, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Org. Chem., 2003, 68, 2765 CrossRef CAS.
- E. Kourany-Lefoll, O. Laprévote, T. Sévenet, A. Montagnac, M. Païs and C. Debitus, Tetrahedron, 1994, 50, 3415 CrossRef CAS.
- B. J. Neubert and B. B. Snider, Org. Lett., 2003, 5, 765 CrossRef CAS.
- P. Crews, D. P. Clark and K. Tenney, J. Nat. Prod., 2003, 66, 177 CrossRef CAS.
- R. A. Edrada, C. C. Stessman and P. Crews, J. Nat. Prod., 2003, 66, 939 CrossRef CAS.
- K. A. Alvi, B. M. Peters, L. M. Hunter and P. Crews, Tetrahedron, 1993, 49, 329 CrossRef.
- S. Nakamura, I. Kawasaki, M. Yamashita and S. Ohta, Heterocycles, 2003, 60, 583 CrossRef CAS.
- M. Assmann, S. Zea and M. Köck, J. Nat. Prod., 2001, 64, 1593 CrossRef CAS.
- G. Breckle, K. Polborn and T. Lindel, Z. Naturforsch., B: Chem. Sci., 2003, 58, 451 CAS.
- M. Fujita, Y. Nakao, S. Matsunaga, M. Seiki, Y. Itoh, J. Yamashita, R. W. M. van Soest and N. Fusetani, J. Am. Chem. Soc., 2003, 125, 15700 CrossRef CAS.
- R. G. Linington, D. E. Williams, A. Tahir, R. van Soest and R. J. Andersen, Org. Lett., 2003, 5, 2735 CrossRef CAS.
- S. Nishimura, S. Matsunaga, M. Shibazaki, K. Suzuki, K. Furihata, R. W. M. van Soest and N. Fusetani, Org. Lett., 2003, 5, 2255 CrossRef CAS.
- E. A. Jares-Erijman, R. Sakai and K. L. Rinehart, J. Org. Chem., 1991, 56, 5712 CrossRef CAS.
- E. Palagiano, S. De Marino, L. Minale, R. Riccio, F. Zollo, M. Iorizzi, J. B. Carré, C. Debitus, L. Lucarain and J. Provost, Tetrahedron, 1995, 51, 3675 CrossRef CAS.
- L. Chang, N. F. Whittaker and C. A. Bewley, J. Nat. Prod., 2003, 66, 1490 CrossRef CAS.
- S.-W. Yang, T.-M. Chang, S. A. Pomponi, G. Chen, A. E. Wright, M. Patel, V. Gullo, B. Pramanik and M. Chu, J. Antibiot., 2003, 56, 970 CAS.
- E. Manzo, R. van Soest, L. Matainaho, M. Roberge and R. J. Andersen, Org. Lett., 2003, 5, 4591 CrossRef CAS.
- T. V. Goud, M. Srinivasulu, V. L. N. Reddy, A. V. Reddy, T. P. Rao, D. S. Kumar, U. S. Murty and Y. Venkateswarlu, Chem. Pharm. Bull., 2003, 51, 990 CrossRef.
- K. Moody, R. H. Thomson, E. Fattorusso, L. Minale and G. Sodano, J. Chem. Soc., Perkins Trans. 1, 1972, 18 RSC.
- R. Encarnación-Dimayuga, M. R. Ramírez and J. Luna-Herrera, Pharm. Biol., 2003, 41, 384 CrossRef CAS.
- X.-H. Xu, G.-M. Yao, Y.-M. Li, J.-H. Lu, C. Lin, X. Wang and C.-H. Kong, J. Nat. Prod., 2003, 66, 285 CrossRef CAS.
- H.-D. Yoo, D. Leung, J. Sanghara, D. Daley, R. van Soest and R. J. Andersen, Pharm. Biol., 2003, 41, 223 CrossRef CAS.
- R. Kazlauskas, P. T. Murphy, R. G. Warren, R. J. Wells and J. F. Blount, Aust. J. Chem., 1978, 31, 2685 CAS.
- A. Bernet, J. Schröder and K. Seifert, Helv. Chim. Acta, 2003, 86, 2009 CrossRef CAS.
- V. J. R. V. Mukku, R. A. Edrada, F. J. Schmitz, M. K. Shanks, B. Chaudhuri and D. Fabbro, J. Nat. Prod., 2003, 66, 686 CrossRef CAS.
- B. Sullivan, P. Djura, D. E. McIntyre and D. J. Faulkner, Tetrahedron, 1981, 37, 979 CrossRef CAS.
- H. Mitome, T. Nagasawa, H. Miyaoka, Y. Yamada and R. W. M. van Soest, J. Nat. Prod., 2003, 66, 46 CrossRef CAS.
- N. K. Utkina, V. A. Denisenko, O. V. Scholokova, M. V. Virovaya and N. G. Prokof'eva, Tetrahedron Lett., 2003, 44, 101 CrossRef CAS.
- I. C. Piña, M. L. Sanders and P. Crews, J. Nat. Prod., 2003, 66, 2 CrossRef CAS.
- J. S. Simpson, P. Raniga and M. J. Garson, Tetrahedron Lett., 1997, 38, 7947 CrossRef CAS.
- A. Brust and M. J. Garson, Tetrahedron Lett., 2003, 44, 327 CrossRef CAS.
- Y. Nogata, E. Yoshimura, K. Shinshima, Y. Kitano and I. Sakaguchi, Biofouling, 2003, 19, 193 Search PubMed.
- Yasman, R. A. Edrada, V. Wray and P. Proksch, J. Nat. Prod., 2003, 66, 1512 CrossRef.
- A. T. Pham, T. Ichiba, W. Y. Yoshida, P. J. Scheuer, T. Uchida, J.-I. Tanaka and T. Higa, Tetrahedron Lett., 1991, 32, 4843 CrossRef CAS.
- H.-Y. He, J. Salva, R. F. Catalos and D. J. Faulkner, J. Org. Chem., 1992, 57, 3191 CrossRef CAS.
- A. Srikishna and S. Gharpure, J. Chem. Soc., Perkin Trans. 1, 2000, 3191 RSC.
- A. Srikrishna, S. J. Gharpure and P. Venugopalan, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2003, 42, 129.
- K. Kodama, R. Higuchi, T. Miyamoto and R. W. M. van Soest, Org. Lett., 2003, 5, 169 CrossRef CAS.
- N. Soji, A. Umeyama, M. Teranaka and S. Arihara, J. Nat. Prod., 1996, 59, 448 CrossRef CAS.
- S. Díaz, J. Cuesta, A. González and J. Bonjoch, J. Org. Chem., 2003, 68, 7400 CrossRef CAS.
- W. C. Taylor and S. Toth, Aust. J. Chem., 1997, 50, 895 CAS.
- M. Arnó, M. A. González and R. J. Zaragozá, J. Org. Chem., 2003, 68, 1242 CrossRef CAS.
- R. Lucas, A. Casapullo, L. Ciasullo, L. Gomez-Paloma and M. Payá, Life Sci., 2003, 72, 2543 CrossRef CAS.
- L. Ciasullo, A. Cutignano, A. Casapullo, R. Puliti, C. A. Mattia, C. Debitus, R. Riccio and L. Gomez-Paloma, J. Nat. Prod., 2002, 65, 1210 CrossRef CAS.
- P. Phuwapraisirisan, S. Matsunaga, N. Fusetani, N. Chaitanawisuti, S. Kritsanapuntu and P. Menasveta, J. Nat. Prod., 2003, 66, 289 CrossRef CAS.
- Y. Liu, T. A. Mansoor, J. Hong, C.-O. Lee, C. J. Sim, K. S. Im, N. D. Kim and J. H. Jung, J. Nat. Prod., 2003, 66, 1451 CrossRef CAS.
- Y. Liu, J. Hong, C.-O. Lee, K. S. Im, N. D. Kim, J. S. Choi and J. H. Jung, J. Nat. Prod., 2002, 65, 1307 CrossRef CAS.
- H. H. Issa, J. Tanaka and T. Higa, J. Nat. Prod., 2003, 66, 251 CrossRef CAS.
- T. N. Makarieva, J.-R. Rho, H.-S. Lee, E. A. Santalova, V. Stonik and J. Shin, J. Nat. Prod., 2003, 66, 1010 CrossRef CAS.
- S.-W. Yang, T.-M. Chan, S. A. Pomponi, W. Gonsiorek, G. Chen, A. E. Wright, W. Hipkin, M. Patel, V. Gullo, B. Pramanik, P. Zavodny and M. Chu, J. Antibiot., 2003, 56, 783 CAS.
- A. R. Díaz-Marrero, I. Brito, E. Dorta, M. Cueto, A. San-Martín and J. Darias, Tetrahedron Lett., 2003, 44, 5939 CrossRef CAS.
- S. De Rosa, A. Crispino, A. De Giulio, C. Iodice, P. Amodeo and T. Tancredi, J. Nat. Prod., 1999, 62, 1316 CrossRef.
- D. Demeke and C. J. Forsyth, Org. Lett., 2003, 5, 991 CrossRef CAS.
- M. Tsoukatou, H. Siapi, C. Vagias and V. Roussis, J. Nat. Prod., 2003, 66, 444 CrossRef.
- S. Tsukamoto, S. Miura, R. W. M. van Soest and T. Ohta, J. Nat. Prod., 2003, 66, 438 CrossRef CAS.
- S. Tsukamoto, M. Tatsuno, R. W. M. van Soest, H. Yokosawa and T. Ohta, J. Nat. Prod., 2003, 66, 1181 CrossRef CAS.
- R. A. Keyzers, P. T. Northcote and M. V. Berridge, Aust. J. Chem., 2003, 56, 279 CrossRef CAS.
- N. Fusetani, S. Matsunaga and S. Konosu, Tetrahedron Lett., 1981, 22, 1985 CrossRef CAS.
- S.-W. Yang, A. Buivich, T.-M. Chan, M. Smith, J. Lachowicz, S. A. Pomponi, A. E. Wright, R. Mierzwa, M. Patel, V. Gullo and M. Chu, Bioorg. Med. Chem. Lett., 2003, 13, 1791 CrossRef CAS.
- S.-W. Yang, T.-M. Chan, S. A. Pomponi, G. Chen, D. Loebenberg, A. Wright, M. Patel, V. Gullo, B. Pramanik and M. Chu, J. Antibiot., 2003, 56, 186 CAS.
- A. S. Antonov, S. S. Afiyatullov, A. I. Kalinovsky, L. P. Ponomarenko, P. S. Dmitrenok, D. L. Aminin, I. G. Agafonova and V. A. Stonik, J. Nat. Prod., 2003, 66, 1082 CrossRef CAS.
- C. P. Ridley and D. J. Faulkner, J. Nat. Prod., 2003, 66, 1536 CrossRef CAS.
- T. Teruya, S. Nakagawa, T. Koyama, K. Suenaga, M. Kita and D. Uemura, Tetrahedron Lett., 2003, 44, 5171 CrossRef CAS.
- S. Ohta, M. Uno, M. Tokumasu, Y. Hiraga and S. Ikegami, Tetrahedron Lett., 1996, 37, 7765 CrossRef CAS.
- Y. Mizushina, C. Murakami, H. Takikawa, N. Kasai, X. Xu, K. Mori, M. Oshige, T. Yamaguchi, M. Saneyoshi, N. Shimazaki, O. Koiwai, H. Yoshida, F. Sugawara and K. Sakaguchi, J. Biochem., 2003, 133, 541 CrossRef CAS.
- I. Carletti, C. Long, C. Funel and P. Amade, J. Nat. Prod., 2003, 66, 25 CrossRef CAS.
- C. B. Rao, V. C. Sekhar, D. V. Rao, B. Sarvani and D. K. M. Lakshmi, Asian J. Chem., 2003, 15, 1161 Search PubMed.
- A. S. Dmitrenok, P. Radhika, V. Anjaneyulu, S. Subrahmanyam, P. V. Subba Rao, P. S. Dmitrenok and V. M. Boguslavsky, Russ. Chem. Bull., 2003, 52, 1868 CrossRef CAS.
- A. Patra and A. Majumdar, ARKIVOC, 2003, 133 Search PubMed.
- M. Ojika, M. K. Islam, T. Shintani, Y. Zhang, T. Okamoto and Y. Sakagami, Biosci. Biotechnol. Biochem., 2003, 67, 1410 CrossRef CAS.
- M. Hirono, M. Ojika, H. Mimura, Y. Nakanishi and M. Maeshima, J. Biochem., 2003, 133, 811 CrossRef CAS.
- Y. T. Chang, C. L. Lin, A. T. Khalil and Y. C. Shen, Chin. Pharm. J., 2003, 55, 129 Search PubMed.
- T. Iwagawa, M. Miyazaki, H. Okamura, M. Nakatani, M. Doe and K. Takemura, Tetrahedron Lett., 2003, 44, 2533 CrossRef CAS.
- T. Řezanka and V. M. Dembitsky, Eur. J. Org. Chem., 2003, 309 CrossRef.
- K. Watanabe, M. Sekine and K. Iguchi, J. Nat. Prod., 2003, 66, 1434 CrossRef CAS.
- K. Watanabe, M. Sekine and K. Iguchi, Chem. Pharm. Bull., 2003, 51, 909 CrossRef CAS.
- N. Hashimoto, S. Fujiwara, K. Watanabe, K. Iguchi and M. Tsuzuki, Lipids, 2003, 38, 991 CAS.
- K. B. Iken and B. J. Baker, J. Nat. Prod., 2003, 66, 888 CrossRef CAS.
- M. Gavagnin, E. Mollo, F. Castelluccio, A. Crispino and G. Cimino, J. Nat. Prod., 2003, 66, 1517 CrossRef CAS.
- K. Iguchi, K. Mori, M. Suzuki, H. Takahashi and Y. Yamada, Chem. Lett., 1986, 1789 CAS.
- E. P. Kündig, R. Cannas, M. Laxmisha, L. Ronggang and S. Tchertchian, J. Am. Chem. Soc., 2003, 125, 5642 CrossRef.
- J. Tanaka, H. Miki and T. Higa, J. Nat. Prod., 1992, 55, 1522 CrossRef CAS.
- H. K. Yim, Y. Liao and H. N. C. Wong, Tetrahedron, 2003, 59, 1877 CrossRef CAS.
- J. Y. Su, Y. Y. Kuang and L. M. Zeng, Huaxue Xuebao, 2003, 61, 1097 CAS.
- A. Ata, J. Ackerman and P. Radhika, Tetrahedron Lett., 2003, 44, 6951 CrossRef CAS.
- K. Mori, K. Iguchi, N. Yamada, Y. Yamada and Y. Inouye, Chem. Pharm. Bull., 1988, 36, 2840 CAS.
- J. Shin and W. Fenical, J. Org. Chem., 1991, 56, 3392 CrossRef CAS.
- S. A. Look and W. Fenical, J. Org. Chem., 1982, 47, 4129 CrossRef CAS.
- H. Miyaoka, Y. Isaji, H. Mitome and Y. Yamada, Tetrahedron, 2003, 59, 61 CrossRef CAS.
- B. F. Bowden, J. C. Coll, J. M. Gulbis, M. F. Mackay and R. H. Willis, Aust. J. Chem., 1986, 39, 803 CAS.
- D. R. Williams and R. W. Heidebrecht, J. Am. Chem. Soc., 2003, 125, 1843 CrossRef CAS.
- J. Marrero, A. D. Rodríguez, P. Baran and R. G. Raptis, J. Org. Chem., 2003, 68, 4977 CrossRef CAS.
- A. D. Rodríguez, E. González and S. D. Huang, J. Org. Chem., 1998, 63, 7083 CrossRef CAS.
- T. J. Heckrodt and J. Mulzer, J. Am. Chem. Soc., 2003, 125, 4680 CrossRef CAS.
- A. D. Rodríguez, C. Ramirez, I. I. Rodríguez and C. L. Barnes, J. Org. Chem., 2000, 65, 1390 CrossRef CAS.
- A. D. Rodríguez and C. Ramirez, Org. Lett., 2000, 2, 507 CrossRef CAS.
- A. I. Kim and S. D. Rychnovsky, Angew. Chem., Int. Ed., 2003, 42, 1267 CrossRef CAS.
- Y. P. Shi, I. I. Rodríguez and A. D. Rodríguez, Tetrahedron Lett., 2003, 44, 3249 CrossRef CAS.
- A. Ata, R. G. Kerr, C. E. Moya and R. S. Jacobs, Tetrahedron, 2003, 59, 4215 CrossRef CAS.
- A. C. Kohl, A. Ata and R. G. Kerr, J. Ind. Microbiol. Biotechnol., 2003, 30, 495 CrossRef CAS.
- L. D. Mydlarz, R. S. Jacobs, J. Boehnlein and R. G. Kerr, Chem. Biol., 2003, 10, 1051 CrossRef CAS.
- A. D. Rodríguez, C. Ramirez, I. I. Rodríguez and E. González, Org. Lett., 1999, 1, 527 CrossRef CAS.
- J. P. Davidson and E. J. Corey, J. Am. Chem. Soc., 2003, 125, 13486 CrossRef CAS.
- I. I. Rodríguez and A. D. Rodríguez, J. Nat. Prod., 2003, 66, 855 CrossRef CAS.
- A. D. Rodríguez, C. Ramirez and I. I. Rodríguez, J. Nat. Prod., 1999, 62, 997 CrossRef CAS.
- A. D. Rodríguez and Y. P. Shi, Tetrahedron, 2000, 56, 9015 CrossRef CAS.
- P. Baran, R. G. Raptis, A. D. Rodríguez, I. I. Rodríguez and Y. P. Shi, J. Chem. Crystallogr., 2003, 33, 711 CrossRef CAS.
- H. Gross, S. Kehraus, M. Nett, G. M. König, W. Beil and A. D. Wright, Org. Biomol. Chem., 2003, 1, 944 RSC.
- S. Carmely, A. Groweiss and Y. Kashman, J. Org. Chem., 1981, 46, 4279 CrossRef CAS.
- X. H. Xu, C. H. Kong, C. J. Lin, X. Wang and J. H. Lu, Gaodeng Xuexiao Huaxue Xuebao, 2003, 24, 1023 CAS.
- X. H. Xu, C. H. Kong, C. J. Lin, X. Wang, Y. D. Zhu and H. S. Yang, Chin. J. Chem., 2003, 21, 1506 CAS.
- P. W. Hsieh, F. R. Chang, A. T. McPhail, K. H. Lee and Y. C. Wu, Nat. Prod. Res., 2003, 17, 409 CrossRef CAS.
- Y. Kashman, M. Bodner, Y. Loya and Y. Benayahu, Isr. J. Chem., 1977, 16, 1 CAS.
- A. F. Ahmed, R. T. Shiue, G. H. Wang, C. F. Dai, Y. H. Kuo and J. H. Sheu, Tetrahedron, 2003, 59, 7337 CrossRef CAS.
- K. I. Marville, S. McLean, W. F. Reynolds and W. F. Tinto, J. Nat. Prod., 2003, 66, 1284 CrossRef.
- J. Marrero, A. D. Rodríguez, P. Baran and R. G. Raptis, Org. Lett., 2003, 5, 2551 CrossRef CAS.
- Y. C. Shen, Y. L. Pan, C. L. Ko, Y. H. Kuo and C. Y. Chen, J. Chin. Chem. Soc., 2003, 50, 471 CAS.
- J. H. Sheu, G. H. Wang, C. Y. Duh and K. Soong, J. Nat. Prod., 2003, 66, 662 CrossRef CAS.
- Y. Nakao, S. Yoshida, S. Matsunaga and N. Fusetani, J. Nat. Prod., 2003, 66, 524 CrossRef CAS.
- T. Kusumi, H. Uchida, M. O. Ishitsuka, H. Yamamoto and H. Kakisawa, Chem. Lett., 1988, 1077 CAS.
- O. Corminboeuf, L. E. Overman and L. D. Pennington, Org. Lett., 2003, 5, 1543 CrossRef CAS.
- A. D. Rodríguez and O. M. Cóbar, Chem. Pharm. Bull., 1995, 43, 1853 CAS.
- O. Corminboeuf, L. E. Overman and L. D. Pennington, J. Am. Chem. Soc., 2003, 125, 6650 CrossRef.
- C. A. Ospina, A. D. Rodríguez, E. Ortega-Barria and T. L. Capson, J. Nat. Prod., 2003, 66, 357 CrossRef CAS.
- A. D. Rodríguez and O. M. Cóbar, Tetrahedron, 1995, 51, 6869 CrossRef CAS.
- B. F. Bowden, J. C. Coll and I. M. Vasilescu, Aust. J. Chem., 1989, 42, 1705 CAS.
- A. S. R. Anjaneyulu, V. L. Rao, V. G. Sastry, M. J. R. V. Venugopal and F. J. Schmitz, J. Nat. Prod., 2003, 66, 507 CrossRef CAS.
- P. J. Sung, T. Y. Fan, L. S. Fang, J. H. Sheu, S. L. Wu, G. H. Wang and M. R. Lin, Heterocycles, 2003, 61, 587 CrossRef CAS.
- Y. C. Shen, Y. C. Lin, C. L. Ko and L. T. Wang, J. Nat. Prod., 2003, 66, 302 CrossRef CAS.
- Y. C. Shen, Y. C. Lin and Y. L. Huang, J. Chin. Chem. Soc., 2003, 50, 1267 CAS.
- N. Krishna, P. Muralidhar, M. M. K. Kumar, D. V. Rao and C. B. Rao, Asian J. Chem., 2003, 15, 344 Search PubMed.
- P. J. Sung and T. Y. Fan, Heterocycles, 2003, 60, 1199 CrossRef CAS.
- S. L. Wu, P. J. Sung, J. H. Su and J. H. Sheu, J. Nat. Prod., 2003, 66, 1252 CrossRef CAS.
- P. J. Sung, T. Y. Fan, L. S. Fang, S. L. Wu, J. J. Li, M. C. Chen, Y. M. Cheng and G. H. Wang, Chem. Pharm. Bull., 2003, 51, 1429 CrossRef CAS.
- T. Iwagawa, N. Nishitani, S. Kurosaki, H. Okamura, M. Nakatani, M. Doe and K. Takemura, J. Nat. Prod., 2003, 66, 1412 CrossRef CAS.
- O. Taglialatela-Scafati, K. S. Craig, D. Rebérioux, M. Roberge and R. J. Andersen, Eur. J. Org. Chem., 2003, 3515 CrossRef CAS.
- O. Taglialatela-Scafati, U. Deo-Jangra, M. Campbell, M. Roberge and R. J. Andersen, Org. Lett., 2002, 4, 4085 CrossRef.
- D. Banjoo, B. S. Mootoo, R. S. Ramsewak, R. Sharma, A. J. Lough, S. McLean and W. F. Reynolds, J. Nat. Prod., 2002, 65, 314 CrossRef CAS.
- R. Dookran, D. Maharaj, B. S. Mootoo, R. Ramsewak, S. McLean, W. F. Reynolds and W. F. Tinto, J. Nat. Prod., 1993, 56, 1051 CrossRef CAS.
- Y. Kashman and A. Groweiss, Tetrahedron Lett., 1978, 4833 CrossRef CAS.
- H. Miyaoka, M. Nakano, K. Iguchi and Y. Yamada, Heterocycles, 2003, 61, 189 CrossRef CAS.
- H. Miyaoka, H. Mitome, H. M. Nakano and Y. Yamada, Tetrahedron, 2000, 56, 7737 CrossRef CAS.
- B. F. Bowden, B. J. Cusack and A. Dangel, Mar. Drugs, 2003, 18 Search PubMed.
- J. C. Braekman, D. Daloze, B. Tursch, J. P. Declercq, G. Germain and M. van Meerssche, Bull. Soc. Chim. Belg., 1979, 88, 71 CAS.
- A. Fontana, M. L. Ciavatta and G. Cimino, J. Org. Chem., 1998, 63, 2845 CrossRef CAS.
- H. Miyaoka, M. Yamanishi, Y. Kajiwara and Y. Yamada, J. Org. Chem., 2003, 68, 3476 CrossRef CAS.
- I. S. Marcos, A. B. Pedrero, M. J. Sexmero, D. Diez, P. Basabe, N. García, R. F. Moro, H. B. Broughton, F. Mollinedo and J. G. Urones, J. Org. Chem., 2003, 68, 7496 CrossRef CAS.
- C. Subrahmanyam, S. R. Kumar and G. D. Reddy, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2003, 42, 219.
- J. H. Sheu, L. F. Huang, S. P. Chen, Y. L. Yang, P. J. Sung, G. H. Wang, J. H. Su, C. H. Chao, W. P. Hu and J. J. Wang, J. Nat. Prod., 2003, 66, 917 CrossRef CAS.
- A. D. Wright, E. Goclik and G. M. König, J. Nat. Prod., 2003, 66, 157 CrossRef CAS.
- A. S. R. Anjaneyulu, V. L. Rao and V. G. Sastry, Nat. Prod. Res., 2003, 17, 149 CrossRef CAS.
- A. F. Ahmed, C. F. Dai, Y. H. Kuo and J. H. Sheu, Steroids, 2003, 68, 377 CrossRef CAS.
- W. H. Zhang, W. K. Liu and C. T. Che, Chem. Pharm. Bull., 2003, 51, 1009 CrossRef CAS.
- W. J. Lan, C. W. Lin, J. Y. Su and L. M. Zeng, Gaodeng Xuexiao Huaxue Xuebao, 2003, 24, 2019 CAS.
- S. Diochot, E. Loret, T. Bruhn, L. Béress and M. Lazdunski, Mol. Pharmacol., 2003, 64, 59 CrossRef CAS.
- P. Maček and D. Lebez, Toxicon, 1988, 26, 441 CrossRef CAS.
- B. B. Bonev, Y. H. Lam, G. Anderluh, A. Watts, R. S. Norton and F. Separovic, Biophys. J., 2003, 84, 2382 CrossRef CAS.
- G. Anderluh, M. D. Serra, G. Viero, G. Guella, P. Maček and G. Menestrina, J. Biol. Chem., 2003, 278, 45216 CrossRef CAS.
- P. Malovrh, G. Viero, M. D. Serra, Z. Podlesek, J. H. Lakey, P. Maček, G. Menestrina and G. Anderluh, J. Biol. Chem., 2003, 278, 22678 CrossRef CAS.
- G. Anderluh, P. Maček and J. H. Lakey, Toxicon, 2003, 42, 225 CrossRef CAS.
- B. Yao, M. R. Prinsep, B. K. Nicholson and D. P. Gordon, J. Nat. Prod., 2003, 66, 1074 CrossRef CAS.
- D. T. Harwood, S. Urban, J. W. Blunt and M. H. G. Munro, Nat. Prod. Res., 2003, 17, 15 CrossRef CAS.
- L. Peters, G. M. König, H. Terlau and A. D. Wright, J. Nat. Prod., 2002, 65, 1633 CrossRef CAS.
- L. Peters, G. M. König, A. D. Wright, R. Pukall, E. Stackebrandt, L. Eberl and K. Riedel, Appl. Environ. Microbiol., 2003, 69, 3469 CrossRef CAS.
- P. Wulff, J. S. Carle and C. Christophersen, J. Chem. Soc., Perkin Trans. 1, 1981, 2895 RSC.
- J. S. Carle and C. Christophersen, J. Org. Chem., 1981, 46, 3440 CrossRef CAS.
- M. V. Laycock, J. L. C. Wright, J. A. Findlay and A. D. Patil, Can. J. Chem., 1986, 64, 1312 CAS.
- J. L. C. Wright, J. Nat. Prod., 1984, 47, 893 CrossRef CAS.
- Y. H. Choi, A. Park, F. J. Schmitz and I. van Altena, J. Nat. Prod., 1993, 56, 1431 CrossRef CAS.
- V. F. Ferreira, A. Park, F. J. Schmitz and F. A. Valeriote, Tetrahedron, 2003, 59, 1349 CrossRef CAS.
- A. Cutignano, A. Fontana, L. Renzulli and G. Cimino, J. Nat. Prod., 2003, 66, 1399 CrossRef CAS.
- R. R. Vardaro, V. Di Marzo and G. Cimino, Tetrahedron Lett., 1992, 33, 2875 CrossRef CAS.
- C. Ireland and P. J. Scheuer, Science, 1979, 205, 922 CrossRef CAS.
- M. Gavagnin, A. Spinella, F. Castelluccio, G. Cimino and A. Marin, J. Nat. Prod., 1994, 57, 298 CrossRef CAS.
- A. K. Miller and D. Trauner, Angew. Chem., Int. Ed., 2003, 42, 549 CrossRef CAS.
- K. J. James, M. D. Sierra, M. Lehane, A. B. Magdalena and A. Furey, Toxicon, 2003, 41, 277 CrossRef CAS.
- M. Satake, K. Ofuji, H. Naoki, K. J. James, A. Furey, T. McMahon, J. Silke and T. Yasumoto, J. Am. Chem. Soc., 1998, 120, 9967 CrossRef CAS.
- K. C. Nicolaou, Y. Li, N. Uesaka, T. V. Koftis, S. Vyskocil, T. Ling, M. Govindasamy, W. Qian, F. Bernal and D. Y. K. Chen, Angew. Chem., Int. Ed., 2003, 42, 3643 CrossRef CAS.
- K. C. Nicolaou, D. Y. K. Chen, Y. Li, W. Qian, T. Ling, S. Vyskocil, T. V. Koftis, M. Govindasamy and N. Uesaka, Angew. Chem., Int. Ed., 2003, 42, 3649 CrossRef CAS.
- A. Tarui, K. Shibata, S. Takahashi, Y. Kera, T. Munegumi and R. H. Yamada, Comp. Biochem. Physiol., 2003, 134B, 79 Search PubMed.
- W. P. Kelley, A. M. Wolters, J. T. Sack, R. A. Jockusch, J. C. Jurchen, E. R. Williams, J. V. Sweedler and W. F. Gilly, J. Biol. Chem., 2003, 278, 34934 CrossRef CAS.
- L. Meijer, A. L. Skaltsounis, P. Magiatis, P. Polychronopoulos, M. Knockaert, M. Leost, X. P. Ryan, C. A. Vonica, A. Brivanlou, R. Dajani, C. Crovace, C. Tarricone, A. Musacchio, S. M. Roe, L. Pearl and P. Greengard, Chem. Biol., 2003, 10, 1255 CrossRef CAS.
- A. Cutignano, A. Tramice, S. De Caro, G. Villani, G. Cimino and A. Fontana, Angew. Chem., Int. Ed., 2003, 42, 2633 CrossRef CAS.
- A. Spinella, L. A. Alvarez, A. Passeggio and G. Cimino, Tetrahedron, 1993, 49, 1307 CrossRef CAS.
- O. Grovel, Y. F. Pouchus and J. F. Verbist, Toxicon, 2003, 42, 297 CrossRef CAS.
- R. J. Andersen, D. J. Faulkner, H. C. Heng, G. D. van Duyne and J. Clardy, J. Am. Chem. Soc., 1985, 107, 5492 CrossRef CAS.
- M. Facompré, C. Tardy, C. Bal-Mahieu, P. Colson, C. Perez, I. Manzanares, C. Cuevas and C. Bailly, Cancer Res., 2003, 63, 7392 CAS.
- Y. Furukawa, K. Nakamaru, K. Sasaki, Y. Fujisawa, H. Minakata, S. Ohta, F. Morishita, O. Matsushima, L. Li, V. Alexeeva, T. A. Ellis, N. C. Dembrow, J. Jing, J. V. Sweedler, K. R. Weiss and F. S. Vilim, J. Neurophysiol., 2003, 89, 3114 Search PubMed.
- E. C. Jimenez, R. P. Shetty, M. Lirazan, J. Rivier, C. Walker, F. C. Abogadie, D. Yoshikami, L. J. Cruz and B. M. Olivera, J. Neurochem., 2003, 85, 610 CAS.
- R. Jacobsen, D. Yoshikami, M. Ellison, J. Martinez, W. R. Gray, G. E. Cartier, K. J. Shon, D. R. Groebe, S. N. Abramson, B. M. Olivera and J. M. McIntosh, J. Biol. Chem., 1997, 272, 22531 CrossRef CAS.
- S. W. Chi, K. H. Park, J. E. Suk, B. M. Olivera, J. M. McIntosh and K. H. Han, J. Biol. Chem., 2003, 278, 42208 CrossRef CAS.
- C. X. Fan, X. K. Chen, C. Zhang, L. X. Wang, K. L. Duan, L. L. He, Y. Cao, S. Y. Liu, M. N. Zhong, C. Ulens, J. Tytgat, J. S. Chen, C. W. Chi and Z. Zhou, J. Biol. Chem., 2003, 278, 12624 CrossRef CAS.
- A. López-Macià, J. C. Jiménez, M. Royo, E. Giralt and F. Albericio, J. Am. Chem. Soc., 2001, 123, 11398 CrossRef CAS.
- Y. Suarez, L. Gonzalez, A. Cuadrado, M. Berciano, M. Lafarga and A. Munoz, Mol. Cancer Ther., 2003, 2, 863 Search PubMed.
- G. R. Pettit, Y. Kamano, H. Kizu, C. Dufresne, C. L. Herald, R. J. Bontems, J. M. Schmidt, F. E. Boettner and R. A. Nieman, Heterocycles, 1989, 28, 553 CrossRef CAS.
- T. Oda, Z. D. Crane, C. W. Dicus, B. A. Sufi and R. B. Bates, J. Mol. Biol., 2003, 328, 319 CrossRef CAS.
- R. Iijima, J. Kisugi and M. Yamazaki, Dev. Comp. Immunol., 2003, 27, 305 Search PubMed.
- R. Garimella, Y. Xu, C. H. Schein, K. Rajarathnam, G. T. Nagle, S. D. Painter and W. Braun, Biochemistry, 2003, 42, 9970 CrossRef CAS.
- M. Gavagnin, M. Carbone, E. Mollo and G. Cimino, Tetrahedron Lett., 2003, 44, 1495 CrossRef CAS.
- A. Spinella, L. A. Alvarez, C. Avila and G. Cimino, Tetrahedron Lett., 1994, 35, 8665 CrossRef CAS.
- A. Fontana, A. Tramice, A. Cutignano, G. d'Ippolito, M. Gavagnin and G. Cimino, J. Org. Chem., 2003, 68, 2405 CrossRef CAS.
- M. Gavagnin, M. Carbone, E. Mollo and G. Cimino, Tetrahedron, 2003, 59, 5579 CrossRef CAS.
- M. T. Davies-Coleman and D. J. Faulkner, Tetrahedron, 1991, 47, 9743 CrossRef CAS.
- M. Gavagnin, A. de Napoli, G. Cimino, K. Iken, C. Avila and F. J. Garcia, Tetrahedron: Asymmetry, 1999, 10, 2647 CrossRef CAS.
- G. Cimino, M. Gavagnin, G. Sodano, R. Puliti, C. A. Mattia and L. Mazzarella, Tetrahedron, 1988, 44, 2301 CrossRef CAS.
- M. Gavagnin, A. Spinella, G. Cimino and G. Sodano, Tetrahedron Lett., 1990, 31, 6093 CrossRef CAS.
- A. Fontana, A. Tramice, A. Cutignano, G. d'Ippolito, L. Renzulli and G. Cimino, Eur. J. Org. Chem., 2003, 3104 CrossRef CAS.
- A. R. Díaz-Marrero, E. Dorta, M. Cueto, J. Rovirosa, A. San-Martín, A. Loyola and J. Darias, Tetrahedron, 2003, 59, 4805 CrossRef CAS.
- Y. Okamoto, N. Nitanda, M. Ojika and Y. Sakagami, Biosci. Biotechnol. Biochem., 2001, 65, 474 CrossRef CAS.
- Y. Okamoto, N. Nitanda, M. Ojika and Y. Sakagami, Biosci. Biotechnol. Biochem., 2003, 67, 460 CAS.
- M. Suzuki and E. Kurosawa, Phytochemistry, 1985, 24, 1999 CrossRef CAS.
- M. B. Ksebati and F. J. Schmitz, J. Org. Chem., 1987, 52, 3766 CrossRef CAS.
- E. J. Dumdei, J. Kubanek, J. E. Coleman, J. Pika, R. J. Andersen, J. R. Steiner and J. Clardy, Can. J. Chem., 1997, 75, 773 CAS.
- A. R. Díaz-Marrero, E. Dorta, M. Cueto, J. Rovirosa, A. San-Martín, A. Loyola and J. Darias, ARKIVOC, 2003, 107 Search PubMed.
- A. Aiello, E. Fattorusso, A. Mangoni and M. Menna, Eur. J. Org. Chem., 2003, 734 CrossRef CAS.
- T. C. McKee, D. L. Galinis, L. K. Pannell, J. H. Cardellina, J. Laakso, C. M. Ireland, L. Murray, R. J. Capon and M. R. Boyd, J. Org. Chem., 1998, 63, 7805 CrossRef CAS.
- R. Shen, C. T. Lin, E. J. Bowman, B. J. Bowman and J. A. Porco, J. Am. Chem. Soc., 2003, 125, 7889 CrossRef CAS.
- J. Kobayashi, J. F. Cheng, T. Ohta, H. Nakamura, S. Nozoe, Y. Hirata, Y. Ohizumi and T. Sasaki, J. Org. Chem., 1988, 53, 6147 CrossRef CAS.
- M. Tsuda, K. Nozawa, K. Shimbo, H. Ishiyama, E. Fukushi, J. Kawabata and J. Kobayashi, Tetrahedron Lett., 2003, 44, 1395 CrossRef CAS.
- H. H. Issa, J. Tanaka, R. Rachmat and T. Higa, Tetrahedron Lett., 2003, 44, 1243 CrossRef CAS.
- L. Garrido, E. Zubía, M. J. Ortega and J. Salvá, J. Org. Chem., 2003, 68, 293 CrossRef CAS.
- A. Rudi, L. Chill, M. Aknin and Y. Kashman, J. Nat. Prod., 2003, 66, 575 CrossRef CAS.
- L. J. Perez and D. J. Faulkner, J. Nat. Prod., 2003, 66, 247 CrossRef CAS.
- A. R. Carroll, B. F. Bowden, J. C. Coll, D. C. R. Hockless, B. W. Skelton and A. H. White, Aust. J. Chem., 1994, 47, 61 CAS.
- B. McKeever and G. Pattenden, Tetrahedron, 2003, 59, 2701 CrossRef CAS.
- A. R. Carroll, J. C. Coll, D. J. Bourne, J. K. MacLeod, M. T. Zabriskie, C. M. Ireland and B. F. Bowden, Aust. J. Chem., 1996, 49, 659.
- P. Wipf and Y. Uto, J. Org. Chem., 2000, 65, 1037 CrossRef CAS.
- X. Salvatella, J. M. Caba, F. Albericio and E. Giralt, J. Org. Chem., 2003, 68, 211 CrossRef CAS.
- M. M. Joullié, M. S. Leonard, P. Portonovo, B. Liang, X. Ding and J. J. La Clair, Bioconjugate Chem., 2003, 14, 30 CrossRef CAS.
- J. A. Tincu, A. G. Craig and S. W. Taylor, Biochem. Biophys. Res. Commun., 2000, 270, 421 CrossRef CAS.
- J. A. Tincu, L. P. Menzel, R. Azimov, J. Sands, T. Hong, A. J. Waring, S. W. Taylor and R. I. Lehrer, J. Biol. Chem., 2003, 278, 13546 CrossRef CAS.
- W. S. Jang, K. N. Kim, Y. S. Lee, M. H. Nam and I. H. Lee, FEBS Lett., 2002, 521, 81 CrossRef CAS.
- W. S. Jang, C. H. Kim, K. N. Kim, S. Y. Park, J. H. Lee, S. M. Son and I. H. Lee, Antimicrob. Agents Chemother., 2003, 47, 2481 CrossRef CAS.
- J. L. Urdiales, P. Morata, I. Nunez de Castro and F. Sanchez-Jimenez, Cancer Lett. (Shannon, Irel.), 1996, 102, 31 Search PubMed.
- M. Broggini, S. V. Marchini, E. Galliera, P. Borsotti, G. Taraboletti, E. Erba, M. Sironi, J. Jimeno, G. T. Faircloth, R. Giavazzi and M. D'Incalci, Leukemia, 2003, 17, 52 CrossRef CAS.
- M. Pérez, M. Sadqi, V. Muñoz and J. Ávila, Biochim. Biophys. Acta, 2003, 1639, 133 CAS.
- K. Fukui, T. Ueki, H. Ohya and H. Michibata, J. Am. Chem. Soc., 2003, 125, 6352 CrossRef CAS.
- S. Hirsch, A. Miroz, P. McCarthy and Y. Kashman, Tetrahedron Lett., 1989, 30, 4291 CrossRef CAS.
- E. Vaz, M. Fernandez-Suarez and L. Muñoz, Tetrahedron: Asymmetry, 2003, 14, 1935 CrossRef CAS.
- M. F. Raub, J. H. Cardellina, M. I. Choudhary, C. Z. Ni, J. Clardy and M. C. Alley, J. Am. Chem. Soc., 1991, 113, 3178 CrossRef CAS.
- C. Agami, F. Couty, G. Evano, F. Darro and R. Kiss, Eur. J. Org. Chem., 2003, 2062 CrossRef CAS.
- J. F. Biard, S. Guyot, C. Roussakis, J. F. Verbist, J. Vercauteren, J. F. Weber and K. Boukef, Tetrahedron Lett., 1994, 35, 2691 CrossRef CAS.
- M. Jugé, N. Grimaud, J. F. Biard, M. P. Sauviat, M. Nabil, J. F. Verbist and J. Y. Petit, Toxicon, 2001, 39, 1231 CrossRef CAS.
- S. M. Weinreb, Acc. Chem. Res., 2003, 36, 59 CrossRef CAS.
- C. Kibayashi, S. Aoyagi and H. Abe, Bull. Chem. Soc. Jpn., 2003, 76, 2059 CrossRef CAS.
- C. Li and A. J. Blackman, Aust. J. Chem., 1994, 47, 1355 CAS.
- B. M. Trost and M. T. Rudd, Org. Lett., 2003, 5, 4599 CrossRef CAS.
- A. Aiello, F. Borrelli, R. Capasso, E. Fattorusso, P. Luciano and M. Menna, Bioorg. Med. Chem. Lett., 2003, 13, 4481 CrossRef CAS.
- J. Bergman, Acta Chem. Scand., 1971, 25, 2865 CrossRef CAS.
- A. Abourriche, Y. Abboud, S. Maoufoud, H. Mohou, T. Seffaj, M. Charrouf, N. Chaib, A. Bennamara, N. Bontemps and C. Francisco, Il Farmaco, 2003, 58, 1351 Search PubMed.
- L. Chill, M. Aknin and Y. Kashman, Org. Lett., 2003, 5, 2433 CrossRef CAS.
- A. Aiello, E. Fattorusso, P. Luciano, M. Menna, G. Esposito, T. Iuvone and D. Pala, Eur. J. Org. Chem., 2003, 898 CrossRef CAS.
- D. R. Appleton and B. R. Copp, Tetrahedron Lett., 2003, 44, 8963 CrossRef CAS.
- B. S. Davidson, T. F. Molinski, L. R. Barrows and C. M. Ireland, J. Am. Chem. Soc., 1991, 113, 4709 CrossRef CAS.
- M. Litaudon and M. Guyot, Tetrahedron Lett., 1991, 32, 911 CrossRef CAS.
- M. Litaudon, F. Trigalo, M. T. Martin, F. Frappier and M. Guyot, Tetrahedron, 1994, 50, 5323 CrossRef CAS.
- T. Kimura, M. Hanzawa, S. Ogawa, R. Sato, T. Fujii and Y. Kawai, Heteroat. Chem., 2003, 14, 88 CrossRef CAS.
- R. A. Davis, I. T. Sandoval, G. P. Concepcion, R. M. da Rocha and C. M. Ireland, Tetrahedron, 2003, 59, 2855 CrossRef CAS.
- M. Tsuda, K. Nozawa, K. Shimbo and J. Kobayashi, J. Nat. Prod., 2003, 66, 292 CrossRef CAS.
- R. A. Davis, L. V. Christensen, A. D. Richardson, R. M. da Rocha and C. M. Ireland, Mar. Drugs, 2003, 27 Search PubMed.
- J. Kobayashi, J. F. Cheng, Y. Kikuchi, M. Ishibashi, S. Yamamura, Y. Ohizumi, T. Ohta and S. Nozoe, Tetrahedron Lett., 1990, 31, 4617 CrossRef CAS.
- P. Schupp, T. Poehner, R. A. Edrada, R. Ebel, A. Berg, V. Wray and P. Proksch, J. Nat. Prod., 2003, 66, 272 CrossRef CAS.
- N. Oku, S. Matsunaga and N. Fusetani, J. Am. Chem. Soc., 2003, 125, 2044 CrossRef CAS.
- A. N. Pearce, D. R. Appleton, R. C. Babcock and B. R. Copp, Tetrahedron Lett., 2003, 44, 3897 CrossRef CAS.
- B. R. Copp, J. Jompa, A. Tahir and C. M. Ireland, J. Org. Chem., 1998, 63, 8024 CrossRef CAS.
- S. Nakahara and A. Kubo, Heterocycles, 2003, 60, 2017 CrossRef CAS.
- Y. R. Torres, T. S. Bugni, R. G. S. Berlinck, C. M. Ireland, A. Magalhães, A. G. Ferreira and R. M. da Rocha, J. Org. Chem., 2002, 67, 5429 CrossRef CAS.
- L. Legentil, J. Bastide and E. Delfourne, Tetrahedron Lett., 2003, 44, 2473 CrossRef CAS.
- J. Kobayashi, J. F. Cheng, H. Nakamura, Y. Ohizumi, Y. Hirata, T. Sasaki, T. Ohta and S. Nozoe, Tetrahedron Lett., 1988, 29, 1177 CrossRef CAS.
- B. R. Copp, O. Kayser, R. Brun and A. F. Kiderlen, Planta Med., 2003, 69, 527 CrossRef CAS.
- B. Debnath, S. Gayen, S. Bhattacharya, S. Samanta and T. Jha, Bioorg. Med. Chem., 2003, 11, 5493 CrossRef CAS.
- E. Delfourne, F. Darro, P. Portefaix, C. Galaup, S. Bayssade, A. Bouteillé, L. Le Corre, J. Bastide, F. Collignon, B. Lesur, A. Frydman and R. Kiss, J. Med. Chem., 2002, 45, 3765 CrossRef CAS.
- S. S. Matsumoto, J. Biggs, B. R. Copp, J. A. Holden and L. R. Barrows, Chem. Res. Toxicol., 2003, 16, 113 CrossRef CAS.
- T. A. Foderaro, L. R. Barrows, P. Lassota and C. M. Ireland, J. Org. Chem., 1997, 62, 6064 CrossRef.
- A. Pouilhès, Y. Langlois and A. Chiaroni, Synlett, 2003, 10, 1488.
- A. R. Carroll, B. F. Bowden and J. C. Coll, Aust. J. Chem., 1993, 46, 489 CAS.
- M. V. R. Reddy and D. J. Faulkner, Tetrahedron, 1997, 53, 3457 CrossRef CAS.
- P. Cironi, I. Manzanares, F. Albericio and M. Álvarez, Org. Lett., 2003, 5, 2959 CrossRef CAS.
- K. C. Nicolaou, P. B. Rao, J. Hao, M. V. Reddy, G. Rassias, X. Huang, D. Y. K. Chen and S. A. Snyder, Angew. Chem., Int. Ed., 2003, 42, 1753 CrossRef CAS.
- A. W. G. Burgett, Q. Li, Q. Wei and P. G. Harran, Angew. Chem., Int. Ed., 2003, 42, 4961 CrossRef CAS.
- Z. Cruz-Monserrate, H. C. Vervoort, R. Bai, D. J. Newman, S. B. Howell, G. Los, J. T. Mullaney, M. D. Williams, G. R. Pettit, W. Fenical and E. Hamel, Mol. Pharmacol., 2003, 63, 1273 CrossRef CAS.
- K. L. Rinehart, T. G. Holt, N. L. Fregeau, J. G. Stroh, P. A. Keifer, F. Sun, L. H. Li and D. G. Martin, J. Org. Chem., 1990, 55, 4512 CrossRef CAS.
- A. E. Wright, D. A. Forleo, G. P. Gunawardana, S. P. Gunasekera, F. E. Koehn and O. J. McConnell, J. Org. Chem., 1990, 55, 4508 CrossRef CAS.
- K. L. Rinehart, T. G. Holt, N. L. Fregeau, J. G. Stroh, P. A. Keifer, F. Sun, L. H. Li and D. G. Martin, J. Org. Chem., 1991, 56, 1676 CrossRef CAS.
- R. Sakai, K. L. Rinehart, Y. Guan and A. H. J. Wang, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 11456 CAS.
- R. Sakai, E. A. Jares-Erijman, I. Manzanares, M. V. S. Elipe and K. L. Rinehart, J. Am. Chem. Soc., 1996, 118, 9017 CrossRef CAS.
- R. Menchaca, V. Martínez, A. Rodríguez, N. Rodríguez, M. Flores, P. Gallego, I. Manzanares and C. Cuevas, J. Org. Chem., 2003, 68, 8859 CrossRef CAS.
- M. D'Incalci and J. Jimeno, Expert Opin. Invest. drugs, 2003, 12, 1843 Search PubMed.
- C. Laverdiere, E. A. Kolb, J. G. Supko, R. Gorlick, P. A. Meyers, R. G. Maki, L. Wexler, G. D. Demetri, J. H. Healey, A. G. Huvos, A. M. Goorin, R. Bagatell, A. Ruiz-Casado, C. Guzman, J. Jimeno and D. Harmon, Cancer, 2003, 98, 832 CrossRef CAS.
- Ch. Van Kesteren, M. M. M. de Vooght, L. López-Lázaro, R. A. A. Mathôt, J. H. M. Schellens, J. M. Jimeno and J. H. Beijnen, Anti-Cancer Drugs, 2003, 14, 487 CrossRef CAS.
- S. Fukuzawa, S. Matsunaga and N. Fusetani, J. Org. Chem., 1995, 60, 608 CrossRef CAS.
- T. Komiya, N. Fusetani, S. Matsunaga, A. Kubo, F. J. Kaye, M. J. Kelley, K. Tamura, M. Yoshida, M. Fukuoka and K. Nakagawa, Cancer Chemother. Pharmacol., 2003, 51, 202 CAS.
- A. Aiello, G. Esposito, E. Fattorusso, T. Iuvone, P. Luciano and M. Menna, Steroids, 2003, 68, 719 CrossRef CAS.
- M. Yoshida, M. Murata, K. Inaba and M. Morisawa, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 14831 CrossRef CAS.
- T. Oishi, H. Tsuchikawa, M. Murata, M. Yoshida and M. Morisawa, Tetrahedron Lett., 2003, 44, 6387 CrossRef CAS.
- H. D. Chludil, A. M. Seldes and M. S. Maier, Z. Naturforsch., C: Biosci., 2003, 58, 433 CAS.
- M. E. Díaz de Vivar, A. M. Seldes and M. S. Maier, Lipids, 2002, 37, 597 CAS.
- M. S. Maier, A. Kuriss and A. M. Seldes, Lipids, 1998, 33, 825 CAS.
- M. Inagaki, K. Nakamura, S. Kawatake and R. Higuchi, Eur. J. Org. Chem., 2003, 325 CrossRef CAS.
- K. Yamada, A. Hamada, F. Kisa, T. Miyamoto and R. Higuchi, Chem. Pharm. Bull., 2003, 51, 46 CrossRef CAS.
- M. Kaneko, F. Kisa, K. Yamada, T. Miyamoto and R. Higuchi, Eur. J. Org. Chem., 2003, 1004 CrossRef CAS.
- W. Wang, F. Li, Y. Park, J. Hong, C. O. Lee, J. Y. Kong, S. Shin, K. S. Im and J. H. Jung, J. Nat. Prod., 2003, 66, 384 CrossRef CAS.
- R. Riccio, M. V. D'Auria, M. Iorizzi, L. Minale, D. Laurent and D. Duhet, Gazz. Chim. Ital., 1985, 115, 405 CAS.
- N. V. Ivanchina, A. A. Kicha, A. I. Kalinovsky, P. S. Dmitrenok and V. A. Stonik, J. Nat. Prod., 2003, 66, 298 CrossRef CAS.
- A. A. Kicha, N. V. Ivanchina, A. I. Kalinovsky, P. S. Dmitrenok and V. A. Stonik, Tetrahedron Lett., 2003, 44, 1935 CrossRef CAS.
- H. D. Chludil, A. P. Murray, A. M. Seldes and M. S. Maier, Stud. Nat. Prod. Chem., 2003, 28, 587 Search PubMed.
- N. G. Prokof'eva, E. L. Chaikina, A. A. Kicha and N. V. Ivanchina, Comp. Biochem. Physiol., 2003, 134B, 695 Search PubMed.
- W. H. Wang, F. M. Li, J. K. Hong, C. O. Lee, H. Y. Cho, K. S. Im and J. H. Jung, Chem. Pharm. Bull., 2003, 51, 435 CrossRef CAS.
- E. V. Levina, A. I. Kalinovskii, V. A. Stonik and P. S. Dmitrenok, Russ. Chem. Bull., 2003, 52, 1623 CrossRef CAS.
- Z. R. Zou, Y. H. Yi, H. M. Wu, J. H. Wu, C. C. Liaw and K. H. Lee, J. Nat. Prod., 2003, 66, 1055 CrossRef CAS.
- S. A. Avilov, A. S. Antonov, A. S. Silchenko, V. I. Kalinin, A. I. Kalinovsky, P. S. Dmitrenok, V. A. Stonik, R. Riguera and C. Jimenez, J. Nat. Prod., 2003, 66, 910 CrossRef CAS.
- M. Sandvoss, A. Preiss, K. Levsen, R. Weisemann and M. Spraul, Magn. Reson. Chem., 2003, 41, 949 CrossRef CAS.
- S. de Marino, N. Borbone, M. Iorizzi, G. Esposito, J. B. McClintock and F. Zollo, J. Nat. Prod., 2003, 66, 515 CrossRef CAS.
- H. Nakagawa, T. Tanigawa, K. Tomita, Y. Tomihara, Y. Araki and E. Tachikawa, J. Toxicol. Toxin Rev., 2003, 22, 633 Search PubMed.
- T. Barsby, C. E. Kicklighter, M. E. Hay, M. C. Sullards and J. Kubanek, J. Nat. Prod., 2003, 66, 1110 CrossRef CAS.
- T. Goto, Y. Kishi, S. Takahashi and Y. Hirata, Tetrahedron, 1965, 21, 2059 CrossRef CAS.
- K. Tsuda, S. Ikuma, M. Kawamura, R. Tachikawa, K. Sakai, C. Tamura and O. Amakasu, Chem. Pharm. Bull., 1964, 12, 1357 CAS.
- R. B. Woodward, Pure Appl. Chem., 1964, 9, 49 CrossRef CAS.
- N. Ohyabu, T. Nishikawa and M. Isobe, J. Am. Chem. Soc., 2003, 125, 8798 CrossRef.
- A. Hinman and J. Du Bois, J. Am. Chem. Soc., 2003, 125, 11510 CrossRef CAS.
- D. J. Faulkner, Tetrahedron, 1977, 33, 1421 CrossRef CAS.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2004, 67, 11216.
- W. Bergmann and R. J. Feeney, J. Am. Chem. Soc., 1950, 72, 2809 CrossRef CAS.
- W. Bergmann and R. J. Feeney, J. Org. Chem., 1951, 16, 981 CrossRef CAS.
- W. Bergmann and R. J. Feeney, J. Org. Chem., 1955, 20, 1501 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |