1,1,6,7-Tetrafluoroindanes: improved liquid crystals for LCD-TV application†
Matthias
Bremer
* and
Lars
Lietzau
Merck KGaA – Division of Liquid Crystals, R&D Chemistry Section, Frankfurterstr. 250, D-64271, Darmstadt, Germany. E-mail: matthias.bremer@merck.de
First published on 8th December 2004
Abstract
New materials based on the 1,1,6,7-tetrafluoroindane skeleton were synthesized via ortho-metallation and intramolecular Heck cyclization followed by an oxidative fluorination procedure. The materials offer improved properties over liquid crystals currently employed in flat panel LCD-TVs.
A fast-growing flat panel display industry constantly requires improved materials for the manufacture of high resolution Active Matrix Liquid Crystal Displays (AM-LCDs), especially for flat panel large size TV applications. Research in the “device chemicals” area focuses in particular on fluorinated liquid crystals, which combine the necessary polarity with chemical stability and low polarizability.1
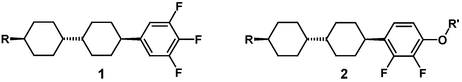
The presence of a permanent dipole moment is a prerequisite for the nematic liquid crystal being moved (switched) in an electric field. There are two basic types of LCs, which either have the dipole moment oriented parallel or perpendicular to the long molecular axis, examples being 1 and 2, respectively:
Compounds of type 2 (R = n-alkyl) have become popular in the mid 1990s when the so-called Multi-Domain Vertical Alignment technology (MVA)2 was introduced, which is now widely employed in desktop computer monitors. In the MVA mode, the molecules are oriented perpendicular to the glass plates in the off-state (dark) and are switched to a parallel orientation when the electric field is applied (bright). This mode offers intrinsically better contrast and faster switching than the conventional TN mode (where compounds of type 1 are used), and therefore is the technology of choice for television. LCD-TV sets with sizes of 37 inches and larger (screen diagonal) are now commercially available. For the two major technologies, “Advanced Super View” (ASV)3 and “Patterned Vertical Alignment” (PVA)4 liquid crystals of type 2 are required as well. Liquid crystals for display applications have to fulfil a complicated interdependent set of properties.1 First of all, there must be a broad nematic phase range from typically −30 °C to +80 °C. The absolute value for the dielectric anisotropy Δε should be large to decrease the operating voltage towards lower power consumption. The rotational viscosity γ1 should be as low as possible to allow fast switching, and the birefringence Δn has to be adjusted to fit the precise display configuration, in particular the cell gap. In addition, the elastic constants of the liquid crystal also influence the operating voltage. A suitable set of properties cannot be achieved with a single material, instead a mixture of up to 30 compounds has to be optimized to fulfil the LCD manufacturers’ request.
In the molecular design of new liquid crystals electrooptical properties can now be predicted quite precisely based on molecular modelling,1,5 and there are some qualitative empirical structure–property relationships regarding nematogenicity and viscosity. Nevertheless, full evaluation of a new structure’s value is still only possible after synthesis, especially because this evaluation has to be carried out in application-relevant LC mixtures.
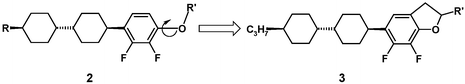
In the past years many attempts have been made to improve the properties of nematic liquid crystals based on the 2,3-difluorophenyl moiety present in 2.6–10 In addition to the fluorine atoms, polarity is provided by the alkoxy group. However, because of nearly free rotation around the carbon–oxygen bond, the average dipole moment and hence the absolute dielectric anisotropy in 2 are reduced. A straightforward approach is to fix the side chain to the aromatic ring leading to benzofurans or related dihydrobenzofurans 3.
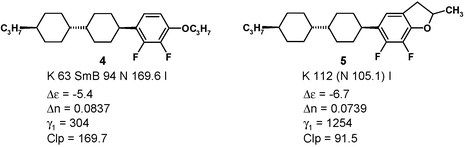
After materials of structure 3 were synthesized,11,12 the expected increase in polarity was observed but other properties deteriorated:
Not only is the rotational viscosity γ1 increased dramatically, the phase behavior is also poorer: 4 exhibits a broad nematic phase and a smaller smectic phase, 5 is only monotropically nematic with a temperature range of merely 7 K, and the extrapolated clearing point (Clp) is even 80 K lower.
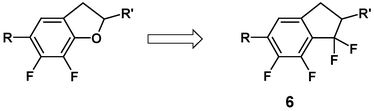
Keeping the idea of fixing the most polar conformation but replacing the oxygen by a difluoromethylene group led to new LCs based on the 1,1,6,7-tetrafluorindane skeleton 6:
The synthesis of novel materials of this type is shown in Scheme 1. The key steps are a directed ortho-metallation13 and functionalization with an appropriate α,β-unsaturated aldehyde followed by an intramolecular Heck cyclization.14 This approach offers complete control with regard to regiochemistry. Unfortunately, it was not possible to convert the intermediate 2-indanones 7 directly to the target molecules by using diethylaminosulfur trifluoride (DAST) or related reagents,15 although fluorination with sulfur tetrafluoride remains to be tested. Furthermore, the indirect approach16 was complicated by partial bromination in the oxidative fluorination procedure. The mixture of 8 and 9 was directly used for the final synthetic steps, which involve elimination of hydrogen bromide and finally catalytic hydrogenation.
![Synthetic route to tri- and tetrafluoroindanes (L = H, F). LDA = lithium diisopropylamide; R =
n-alkyl; DBH = 1,3-dibromo-5,5-dimethylhydantoin; DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; DAST = diethylaminosulfur trifluoride.](/image/article/2005/NJ/b414312d/b414312d-s1.gif) | ||
| Scheme 1 Synthetic route to tri- and tetrafluoroindanes (L = H, F). LDA = lithium diisopropylamide; R = n-alkyl; DBH = 1,3-dibromo-5,5-dimethylhydantoin; DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene; DAST = diethylaminosulfur trifluoride. | ||
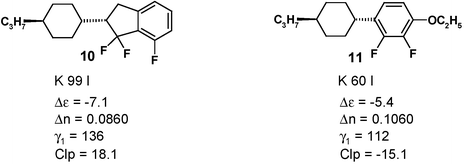
Trifluoroindanes (L = H) already offer interesting properties:
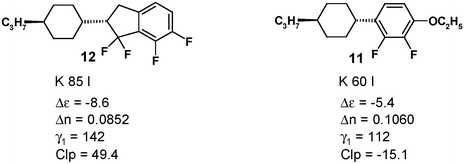
The material does not exhibit any mesophase in the pure state but the “virtual” clearing point of 10 measured in an application relevant LC mixture12 is more than 30 K higher than that of the reference compound 11. Although indane 10 does not contain any additional polar oxygen containing substituent, the dielectric anisotropy Δε is increased compared to 11.
The introduction of a fourth fluorine atom leads to even further improvement: not only is the polarity in 12 increased again but also the virtual clearing point. The small increase in γ1 is tolerable.
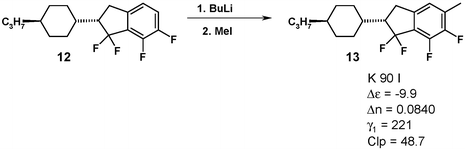
Compound 12 can easily be functionalized via ortho-metallation and was alkylated with methyl iodide. The resulting product 13 exhibits even higher polarity although the concomitant increase in γ1 now is too high to be useful.
The X-ray crystal structure of 13 (Fig. 1) shows no unusual features.17 A B3LYP/6-31G(d)//B3LYP/6-31G(d) calculation yields a very similar geometry but predicts a value of only −6.2 for Δε. The reason for this discrepancy is not yet clear and further work is necessary.
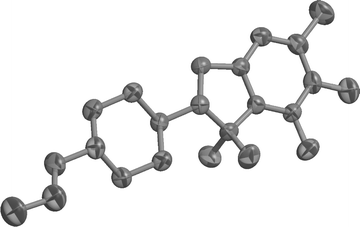 | ||
| Fig. 1 X-Ray crystal structure of 13 (ORTEP). | ||
In summary, we have described the synthesis and physical properties of a new class of materials for nematic liquid crystal mixtures which offer high absolute values for the dielectric anisotropies, high clearing temperatures and low rotational viscosities.
Acknowledgements
This work was supported by the Bundesministerium für Bildung und Forschung (FKZ 01 BM 904). We thank Dr Melanie Klasen-Memmer and Volker Reiffenrath for experimental support and discussions, and Ingrid Svoboda (Materials Science, Technical University of Darmstadt) for the X-ray crystal structure determination.References
- (a) P. Kirsch and M. Bremer, Angew. Chem., 2000, 112, 4384 CrossRef; (b) P. Kirsch and M. Bremer, Angew. Chem., Int. Ed., 2000, 39, 4216 CrossRef CAS.
- A. Takeda, S. Kataoka, T. Sasaki, H. Chida, H. Tsuda, K. Ohmuro, Y. Koike, T. Sasabayashi and K. Okamoto, SID ’98 Digest, 1998, 1077 Search PubMed.
- Y. Ishii, S. Mizushima and M. Hijikigawa, SID ’01 Digest, 2001, 1090 Search PubMed.
- K. H. Kim, K. Lee, S. B. Park, J. K. Song, S. Kim and J. H. Souk, Asia Display, 1998, 383 Search PubMed.
- (a) M. Klasen, M. Bremer, A. Götz, A. Manabe, S. Naemura and K. Tarumi, Jpn. J. Appl. Phys., 1998, 37, L945 CrossRef CAS; (b) M. Bremer and K. Tarumi, Adv. Mater., 1993, 5, 842 CAS.
- (a) K. Tarumi, M. Bremer and B. Schuler, Inst. Electron., Inf. Commun. Eng., Trans., Sect. E, 1996, E79-C, 1035 Search PubMed; (b) K. Tarumi, M. Bremer and T. Geelhaar, Annu. Rev. Mater. Sci., 1997, 27, 423 Search PubMed; (c) P. Kirsch, V. Reiffenrath and M. Bremer, Synlett, 1999, 389 CrossRef CAS.
- S. M. Kelly, Liq. Cryst., 1991, 10, 261 CAS.
- G. W. Gray, M. Hird, D. Lacey and K. J. Toyne, J. Chem. Soc., Perkin Trans. 2, 1989, 2041 RSC.
- K. Miyazawa, T. Kato, M. Itoh and M. Ushioda, Liq. Cryst., 2002, 29, 1483 CrossRef CAS.
- K. Miyazawa and A. De Meijere, Mol. Cryst. Liq. Cryst., 2001, 364, 529 CAS.
- K. Tarumi, M. Bremer and V. Reiffenrath, Ger. Offen., DE 19900517 (Chem. Abstr., 1999, 131, 123055) Search PubMed.
- The phase transition temperatures are given in °C, the γ1 values in mPa s. K = crystalline, Sx = smectic X, N = nematic, I = isotropic. Clp, Δn and γ1 were determined by linear extrapolation from a 10% w/w solution in the commercially available Merck mixture ZLI-4792 (TNI = 92.8 °C, Δε = 5.3, Δn = 0.0964). The extrapolated values are corrected empirically for differences in the order parameter. Δε was extrapolated from ZLI-2857 (TNI = 82.3 °C, Δε = −1.4, Δn = 0.0776). For the pure substances the mesophases were identified by optical microscopy, and the phase transition temperatures by differential scanning calorimetry (DSC).
- V. Snieckus, Chem. Rev., 1990, 90, 879 CrossRef CAS.
- S. Bräse and A. De Meijere, in A Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E. Negishi, John Wiley & Sons, New York, 2002, pp. 1223–1254 Search PubMed.
- K. A. Jolliffe, Aust. J. Chem., 2001, 54, 75 CrossRef CAS.
- M. Hird, K. J. Toyne, A. J. Slaney, J. W. Goodby and G. W. Gray, J. Chem. Soc., Perkin Trans. 2, 1993, 2337–2350 RSC.
- Crystal data for 13: P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a
= 8.961(1)
Å, b
= 9.253(1)
Å, c
= 11.405(2)
Å, α
= 109.38(1)°, β
= 103.19(1)°, γ
= 96.75(1)°, V
= 849.1(2)
Å3, Z
= 2, T
= 299(2) K. 7986 reflections were measured (2θmax
= 52.74°). 3434 unique reflections (Rint
= 0.0306). Full matrix least squares refinement with 282 parameters converged to R1 = 0.0550 and wR2 = 0.1181. CCDC 229584. See http://www.rsc.org/suppdata/nj/b4/b414312d/ for crystallographic data in .cif or other electronic format.
, a
= 8.961(1)
Å, b
= 9.253(1)
Å, c
= 11.405(2)
Å, α
= 109.38(1)°, β
= 103.19(1)°, γ
= 96.75(1)°, V
= 849.1(2)
Å3, Z
= 2, T
= 299(2) K. 7986 reflections were measured (2θmax
= 52.74°). 3434 unique reflections (Rint
= 0.0306). Full matrix least squares refinement with 282 parameters converged to R1 = 0.0550 and wR2 = 0.1181. CCDC 229584. See http://www.rsc.org/suppdata/nj/b4/b414312d/ for crystallographic data in .cif or other electronic format.
Footnote |
| † Electronic supplementary information (ESI) available: experimental details for the synthesis of fluorinated indanes. See http://www.rsc.org/suppdata/nj/b4/b414312d/ |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2005 |