Polycyclic musks in the Ruhr catchment area—transport, discharges of waste water, and transformations of HHCB, AHTN and HHCB-lactone
Kai
Bester
*
Waste and waste water management, Institute of Environmental Analysis, University Duisburg-Essen, Universitätsstr. 15, 45141 Essen, Germany. E-mail: kai.bester@uni-essen.de
First published on 22nd November 2004
Abstract
The polycyclic musk fragrance compounds HHCB (1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethylcyclopenta-(g)-2-benzopyran; trade name, e.g. galaxolide®) and AHTN (7-acetyl-1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene, trade name, e.g. tonalide®) and the transformation product of HHCB (HHCB-lactone) were analysed in surface water samples and sewage treatment plants (STP) effluents in the Ruhr megalopolis. The STPs were the dominant source for these pollutants. In the part of the river where the drinking water is extracted from the river, about 60 ng L−1 HHCB, 10 ng L−1 AHTN and 20–30 ng L−1 HHCB-lactone were found as typical riverine concentrations, while none of the compounds were detected near the spring of the river. On the other hand sewage treatment plant effluents exhibited concentrations up to 600 ng L−1. The STP’s effluent resulted in elevated concentrations in some parts of the river and in the lakes into which they discharge. As some of the plants emit HHCB-lactone with a significantly changed enantiomeric pattern, biotransformation of HHCB to HHCB-lactone occurs in some waste water treatment plants operating with activated sludge. In those parts of the river where no relevant discharges of waste water or fresh water takes place neither the concentration nor the pattern changes significantly. This holds true especially for the HHCB versus HHCB-lactone ratios which indicates degradation less than 15% of the HHCB inventory in the river Ruhr itself. In other rivers, such as the Rhine, higher levels of HHCB-lactone in comparison to HHCB were detected (ratio 1 ∶ 1).
Introduction
About 2000 t a−1 polycyclic musk compounds especially HHCB (marketed e.g., as galaxolide®) and AHTN (e.g. tonalide®) are used in Europe as fragrances for consumer products such as washing powders, shampoos, perfumes etc.1 Most of the applications of these compounds lead to direct discharges into the sewage system, thus sewage treatment plants (STPs) have to cope with these compounds. The removal which occurs in such plants is mostly due to sorption of these lipophilic compounds to sludge, but some transformation also occurs.2 As these plants eliminate some but not all of the respective material, HHCB, AHTN and the respective transformation product (HHCB-lactone) (structural formulae in Fig. 1) will be discharged into the rivers as shown by Bester,2 Simonich et al.3,4 and Eschke et al.5,6 Thus it is crucial for the evaluation of these polycyclic musks to know about degradation and persistence of the respective compounds in the rivers. This might become an issue, at least on cost terms, in those areas where the supply of drinking water relies mostly on surface waters, such as the Ruhr area with its about 7 million inhabitants. This is even more important as Seinen et al.7 found that these compounds exhibit some estrogenic activity. Drinking water in this region is mostly supplied by processing surface water from the river Ruhr. The concentrations of the parent compounds compounds (i.e. HHCB and AHTN, but not of HHCB-lactone) were monitored with limited spatial resolution some years ago by Eschke et al.5,6 A good overview on HHCB and AHTN in the aquatic environment was given in 1999 by Rimkus.8 The transformation product of HHCB (i.e. HHCB-lactone) was not available as a standard compound until 2002. Thus neither quantitative data on HHCB-lactone nor its origin are available from previous studies. This transformation product has been identified by Franke et al.9 in surface waters as this group synthesised this compound themselves, and by Kallenborn et al.10 in 2001 in fish samples, as well as Kuper et al.11 in sewage sludge. The fate and persistence of this compound in the environment is not yet known.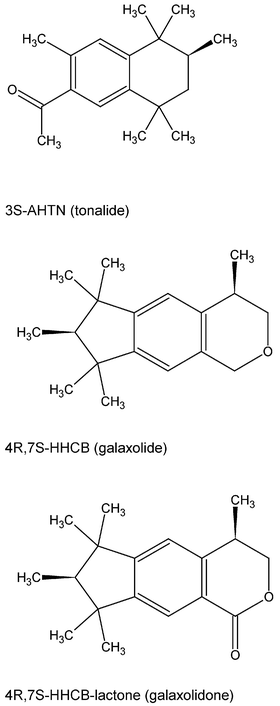 | ||
| Fig. 1 Structural formulae of AHTN, HHCB and the primary transformation product HHCB-lactone. | ||
Whether metabolisation of HHCB and AHTN or other means of elimination exists in riverine ecosystems is currently being discussed. Buerge et al.12 found photo-elimination rate constants of 0.15 d−1 and 4.6 d−1, respectively for saturated solutions of HHCB and AHTN in lake water of an oligotrophic Swiss lake. This would lead to half-lifes of 4.6 and 0.15 d for HHCB and AHTN, respectively. No transformation products were given in that study, though. To answer the question of transformation or persistence in riverine and eutrophic lake ecosystems, diverse samples of surface waters from the river Ruhr and several tributaries as well as sewage treatment plant (STPs) effluents have been taken in the river Ruhr catchment area. The sample extracts were analysed by non-chiral GC-MS, while some metabolisation data were obtained by means of chiral gas chromatography, which has been used by Franke et al.9 and Gaterman et al.13 to determine metabolic processes of HHCB and AHTN in fish. HHCB-lactone was not included in these studies.
As the persistence of these compounds in riverine ecosystems has been debated in science, by the regulatory authorities and the manufacturers it was the aim of this work to study input, transportation and possible elimination by means of metabolisation in the river Ruhr.
Experimental
The river Ruhr and its main tributaries, as well as discharges from significant sewage treatment plants were sampled within three days in a dry period with no rainfall in September 2002. The river’s spring is located in the moderately populated area called Sauerland. After leaving the Sauerland area (station no. 45, Fig. 2) the Ruhr flows through several lakes during its passage through the Ruhr megalopolis. In this area significant amounts of water are extracted for drinking water production. The sample stations are the same as those described in Andresen et al.14 Finally the river joins the river Rhine in Duisburg. The lakes through which the river passes are shallow artificial systems (about 1–5 m depth) and serve to maintain reasonable flow rates for the drinking water production plants, but they also serve to clarify the river as particulate matter is given a chance to settle on the lake’s bed. Hydraulic retention time in the river/lake system is 200 h (∼8 d) between stations 50 and 66 and 140 h (∼6 d) between stations 58 and 66.15 Additionally, these lakes serve as leisure areas. Swimming and bathing does not take place in this part of the river systematically, though. At the sampled stations (inflow and effluent of the lakes) the water was mostly uniformly mixed, though parts of the lakes themselves are not completely mixed most of the time. It should be noted that huge efforts are undertaken to keep the river Ruhr as clean as possible; thus major waste water streams are preferably discharged into the river Emscher, and STPs were put into operation decades ago, to protect the river Ruhr from the waste water (of about 2 million inhabitants) that is still discharged into it.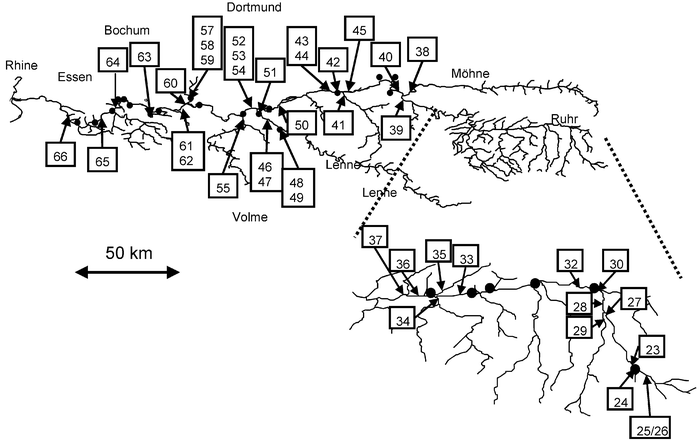 | ||
| Fig. 2 Sample stations in the river Ruhr system with the respective sewage treatment plants indicated as •. Also indicated is the river Rhine, the river Ruhr, and its tributaries Möhne, Lenne and Volme as well as the cities Essen, Bochum and Dortmund. | ||
The sampling stations for this study are shown in Fig. 2, while their characteristics are given in Table 1. In most cases the Ruhr was sampled up- and downstream from a potential place of discharge (or tributary) and wherever possible the discharge (or tributary) itself was sampled. Sometimes the plume of the discharge was sampled as well. A plume is the part of the river in which the discharge and the river mix, thus they do not necessarily represent the river. Plumes sometimes give important information especially where a discharge is not directly accessible.
| Code | Characteristics | km | Date |
|---|---|---|---|
| 23 | Effluent of STP Niedersfeld (4000 inhabitants) | 211 | 17.09.02 |
| 24 | Plume of STP Niedersfeld | 211 | 17.09.02 |
| 25/26 | Ruhr upstream of STP Niedersfeld | 213 | 17.09.02 |
| 27 | Ruhr upstream of tributary Neger | 17.09.02 | |
| 28 | Ruhr downstream of tributary Neger, Concrete plant (no visible effluent) | ||
| 29 | Tributary Neger | 17.09.02 | |
| 30 | River Ruhr | 17.09.02 | |
| 31 | Field blank | 17.09.02 | |
| 32 | River Ruhr | 17.09.02 | |
| 33 | Dam/Lock, river Ruhr (Heinrichstal) | 17.09.02 | |
| 34 | Tributary Henne | 17.09.02 | |
| 35 | Tributary Gebke | 17.09.02 | |
| 36 | STP Meschede 1 (downstream of Tributaries Henne and Gebke) | 17.09.02 | |
| 37 | STP Meschede 2 | 17.09.02 | |
| 38 | Tributary Möhne | 140 | 17.09.02 |
| 39 | Downstream of STP Wildshausen-Arnsberg (98 000 inhabitants ) upstream of Möhne | 145 | 17.09.02 |
| 40 | Downstream of tributary Möhne | 138 | 17.09.02 |
| 41 | Tributary Hönne | 117 | 17.09.02 |
| 42 | Ruhr downstream of tributary Hönne, upstream of STP Menden-Bösperde (120 000 inhabitants) | 116 | 17.09.02 |
| 43 | Plume of STP Menden-Bösperde | 115 | 17.09.02 |
| 44 | Plume of STP Menden-Bösperde | 114 | 17.09.02 |
| 45 | River Ruhr upstream of tributary Hönne and STP Menden-Bösperde | 118 | 17.09.02 |
| 46/47 | Tributary Lenne (Motorway) (downstream STP Hagen Fley 70 000 inhabitants, upstream Hagen Boele 150 000 inhabitants) | 93 | 19.09.02 |
| 48 | Effluent of STP Hagen Fley (70 000 inhabitants) | 92 | 19.09.02 |
| 49 | Tributary Lenne upstream of STP Hagen Fley (70 000 inhabitants) | 94 | 19.09.02 |
| 50 | River Ruhr at Schwerte upstream of tributary Lenne and STPs Hagen | 95 | 19.09.02 |
| 51 | Effluent STP Hagen Boele (15 000 inhabitants) | 92 | 19.09.02 |
| 52/53/54 | Ruhr downstream of STP Hagen-Vorhalle (230 000) and tributary Lenne | 90 | 19.09.02 |
| 55 | Tributary Volme | 86 | 19.09.02 |
| 56 | Ruhr upstream of STP Ölbachtal, downstream of STP Witten (120 000 inhabitants) | 69 | 19.09.02 |
| 57/58/59 | Effluent of STP Ölbachtal (160 000 inhabitants) | 68 | 19.09.02 |
| 60 | Lake Kemnaden, bight into which the effluent of STP Ölbachtal discharges (leisure boat harbour) | 67 | 19.09.02 |
| 61 | Lake Kemnaden after introduction of the effluent of STP Ölbachtal downstream of no. 60 | 66 | 19.09.02 |
| 62 | Lake Kemnaden after introduction of the effluent of STP Ölbachtal downstream of no. 61 downstream of the dam of lake Kemnaden | 65 | 19.09.02 |
| 63 | Ruhr downstream of lake Kemnaden downstream of STP Hattingen (75 000 inhabitants) | 60 | 19.09.02 |
| 64 | Ruhr downstream of STP Burgaltendorf, Steele and Rellinghausen (serving 36 000, 54 000 and 51 000 inhabitants, respectively | 56 | 19.09.02 |
| 65 | Westend of lake Baldeney, downstream of STP Kupferdreh (73 000 inhabitants) | 37 | 19.09.02 |
| 66 | Downstream of STP Kettwig and STP Werden (22 000 and 29 000 inhabitants) | 18 | 19.09.02 |
Water (surface and waste water) was sampled manually as grab samples by using 1 L glass bottles with Teflon seals purchased from Schott, Mainz, Germany. Grab samples are the only appropriate way of sampling if a part of a river of some hundred km length has to be sampled with a reasonable spatial resolution. It is hard to obtain total mass balances from a river which is changing drastically due to natural conditions. However, the river Ruhr is heavily regulated for drinking water supply. As surface water is often covered with a biogenic or anthropogenic lipophilic film which may accumulate lipophilic compounds, the sample bottles were passed with a closed stopper through the surface, opened and closed under the surface of the water at depth of typically 30 cm and taken out of the water with closed stopper. The samples were stored at 4 °C and extracted with 10 ml toluene after adding an aliquot of 100 μl internal standard (IS) solution (with 100 ng D15 musk xylene) on the following day. The method validation data are given in Table 2. This IS was chosen as there were no interferences, and deuterated musk xylene does not undergo any reaction itself, as has been experienced for deuterated AHTN. AHTN is produced via proton exchange which may be reversed giving the original undeuterated product as experienced by the author as well as by Buerge et al.12 Thus deuterated musk xylene is considered a better IS compared to deuterated AHTN. The same amount of IS was used for all samples. The organic phase was separated from the aqueous one and the residual water was removed from the organic phase by freezing the samples overnight at −20 °C. The samples were concentrated using a rotary evaporator at 40 °C and 60 mbar to 1 ml. No further clean up was necessary for quantification with GC-MS. Blanks were obtained by two different operations: one empty sample bottle was present during the sample trip, to control contamination from the preparation of the bottles, and the transport (field blank). It was washed with ethyl acetate at the end of the sampling. To check laboratory and solvent contamination a 1 L sample of Millipore water was extracted the same way as the surface water samples.
The sample extracts were analysed by GC-MS (Trace, supplied by Thermo-Finnigan, Dreieich, Germany) equipped with a PTV injector. The PTV (1 μl injection volume) was operated in PTV splitless mode with the following temperature program: 90 °C [0.1 s] → 14.5 °C s−1 → 280 °C [1.0 min] → 14.5 °C s−1 → 320 °C [10 min] (cleaning phase). The GC separation was performed utilising a DB-5MS column (J&W Scientific, Folsom, CA, USA), length: 30 m, ID: 0.25 mm, film thickness: 0.25 μm and a temperature programme of: 90 °C [2 min] → 10 °C min−1 → 280 °C [15 min] with 1.5 ml min−1 He as carrier gas. The photomultiplier of the mass spectrometer was operated with a voltage of 500 V. The mass spectrometer was operated in selected ion monitoring (SIM) mode with 67 ms dwell time, while the transferline was held at 250 °C which is sufficient to transfer all compounds from the gas chromatograph into the MS as the vacuum builds up in the transferline. The ion source was operated at 200 °C. The chromatogram of mass fragment 243 (M − CH3) atomic mass units (amu) of HHCB and AHTN was used for quantification while mass 258 (amu) (molecular ion) was used for verification. For HHCB-lactone, mass fragment 257 (amu) and the molecular ion at 272 (amu) were used, respectively. The internal standard D15 musk xylene was analysed at 294 (amu) (M − CD3) and 312 (amu) (M), respectively. The calibration was performed as a 7 step internal standard calibration with linear regression from 1 ng ml−1 to 1000 ng ml−1 (extract concentrations). A 1/x weighting was used for the calibration to have a similar weighting for the small and the high concentrations, which is crucial if a calibration is performed for more than one order of magnitude.
The chiral chromatography was performed utilising a 25 m 0.25 mm ID column, with a film of heptakis-(2,3-di-O-methyl-6-O-t-butyldimethyl-silyl)-β-cyclodextrin in OV1701 (obtained as FS-Hydrodex β-6TBDM from Macherey-Nagel, Düren, Germany). Film thickness was 0.2–0.3 μm according to the manufacturer. This capillary column was used on a Trace Plus GC-MS obtained from Thermo-Finnigan. The transferline was operated at 210 °C as the chiral column's temperature limit is given as 230 °C by the manufacturer. The ion source was kept at 180 °C. The same samples were analysed without further clean up. The chiral separations were performed with a temperature programme: 110 °C [1 min] → 5 °C min−1 → 132 °C [140 min] → 1.5 °C min−1 → 194 °C [25 min] → 5 °C min−1 → 230 °C [10 min]. On the first plateau (132 °C) the enantiomers of AHTN as well as the enantiomers and diastereomers of HHCB were separated. On the second plateau (194 °C) the separation the enantiomers and diastereomers of HHCB-lactone was performed. Helium was used as carrier gas with a flow rate of 0.7 ml min−1.
AHTN and D15 musk xylene were purchased from Ehrenstorfer, Augsburg, Germany. HHCB and HHCB-lactone were received as pure standard compounds as a gift from International Flavours and Fragrances (IFF), Hilversum, Netherlands. The purity was re-checked by GC-MS. Toluene and other solvents were purchased residue grade (z.R.) from Merck, Darmstadt, Germany.
Results and discussion
The methods proved to be sensitive, precise and robust for these analytes. The recovery rates were 75–100%, the relative standard deviations 8–23%, and limits of quantification obtained as three times the blank are shown in Table 2. These benchmarks are well within the regulations of the codex alimentarius (requiring more than 75% recovery) or other regulations concerning recovery rates. Laboratory blank concentrations (Millipore water) are 1.1 ng L−1 HHCB, 0.37 ng L−1 AHTN and 3.0 ng L−1 HHCB-lactone in this experiment, while the field blanks were below the limit of detection. For the determination of the LOQs the laboratory blanks were used. Full data are given in Table 2.| Quantifier mass/amu | Verifier mass/amu | RT/min | RR [%] | SD [%] | RSD [%] | LOQ/ng L−1 | |
|---|---|---|---|---|---|---|---|
| RT = retention time, RR = recovery rate, SD = standard deviation, RSD = relative standard deviation, LOQ = Limit of quantification determined as 3 times the blank concentration. The recovery rates were determined at 1, 3, 10, 30, 100, 300 and 1000 ng L−1, each in triplicate. | |||||||
| AHTN | 243 | 258 | 9.52 | 78 | 7 | 9 | 1 |
| HHCB | 243 | 258 | 9.39 | 75 | 6 | 8 | 3 |
| HHCB-lactone | 257 | 272 | 14.80 | 100 | 23 | 23 | 9 |
The concentrations of HHCB, AHTN and HHCB-lactone found in this study are shown in Fig. 3. They ranged from the limit of quantification <3 ng L−1 to 600 ng L−1 for HHCB, while they were <1 ng L−1 to 120 ng L−1 for AHTN and <10 ng L−1 to 300 ng L−1 for HHCB-lactone in the river Ruhr. Concentrations exceeding 100 ng L−1 were regularly found in the STPs effluents or plumes of effluents entering this river. Concentrations of 100 ng L−1 to 600 ng L−1 HHCB, 30 ng L−1 to 300 ng L−1 HHCB-lactone and 20 ng L−1 to 300 ng L−1 AHTN were found in those STP effluent samples (sample no.: 23, 24, 36, 43, 57 ff). Some tributaries such as the Lenne exhibited elevated levels, too. The typical concentrations in the river Ruhr in areas where drinking water is extracted are about 60 ng L−1 HHCB, 10 ng L−1 AHTN and 20–30 ng L−1 HHCB-lactone. The lowest concentrations <LOQ were found near the spring of the river Ruhr. The highest concentrations were found in the effluent of the sewage treatment plants such as Bochum-Ölbachtal (stations 57–58). However, not all STPs effluents exhibited elevated levels, e.g., at STP Hagen (Station no. 51) the concentrations of polycyclic musk fragrances in the effluent were similar to those determined in the river Ruhr.
![Concentrations [ng L−1] of the polycyclic musk compounds HHCB, AHTN and the transformation product HHCB-lactone in the river Ruhr, some sewage treatment plant effluents, and the tributaries Möhne, Hönne, Lenne, Volme (river Ruhr samples are indicated with R). Maximum HHCB values are 600 ng L−1 in the effluent Ölbachtal. Sample 31 is a field blank.](/image/article/2005/EM/b409213a/b409213a-f3.gif) | ||
| Fig. 3 Concentrations [ng L−1] of the polycyclic musk compounds HHCB, AHTN and the transformation product HHCB-lactone in the river Ruhr, some sewage treatment plant effluents, and the tributaries Möhne, Hönne, Lenne, Volme (river Ruhr samples are indicated with R). Maximum HHCB values are 600 ng L−1 in the effluent Ölbachtal. Sample 31 is a field blank. | ||
Considering the tributaries Neger (No. 29), Henne (No. 34), Gebke (No. 35), Möhne (No. 38), Hönne (No. 41) and Volme (No. 55) similar or lower concentrations than in the river Ruhr were determined. On the other hand samples from the tributary Lenne (46–49), which receive effluents from a small STP nearby, exhibited elevated levels of these analytes. In the main part of the river, i.e., the most populated parts (no. 50–66), the concentrations were about 60 ng L−1 HHCB, about 30 ng L−1 HHCB-lactone and about 10 ng L−1 AHTN without significant changes. No significant inflow can be detected from these data downstream of station no. 61, though several small STPs discharge into this part of the river (Table 1). Upstream of station no. 61 the waste water of more than 1.4 million inhabitants is discharged into the Ruhr while between no. 61 and 66 the treated waste water of 340 000 inhabitants is discharged into the river. This is less than 25% of the amounts discharged in the upper parts of the river and is near the significance level (two times the standard deviation) of the analytical method. The fact that these STPs are spread over some distance may possibly be the reason why these STPs were not identified as significant sources too. The main flow of waste water in the northwestern part of the Ruhr megalopolis (including most parts of the cities of Essen and Bochum etc.) goes to the river Emscher.
Samples for comparison were taken from the river Rhine near Düsseldorf and from the river Lippe in the vicinity of the Ruhr megalopolis. In the Rhine concentrations of around 20 ng L−1 HHCB, 4 ng L−1 AHTN and about 20 ng L−1 HHCB-lactone were determined. The samples from the river Lippe gave concentrations of 75 ng L−1 HHCB, 12 ng L−1 AHTN and 30 ng L−1 HHCB-lactone. These concentrations agree well with those published by Dsikowitzky et al.16 who found concentrations of about 100 ng L−1 for HHCB and 50 ng L−1 for AHTN in a similar part of the river in samples taken in winter 2000. In 1995 the concentrations in the river Elbe were about 100 ng L−1 with similar concentrations for HHCB and AHTN as shown by Bester et al.17 and by Franke et al.9 Nowadays HHCB dominates significantly over AHTN and HHCB-lactone in all samples taken, thus a change of usage pattern is obvious.
Considering temporal changes, the Ruhr and some tributaries were sampled in 1993 for HHCB and AHTN by Eschke et al.,5,6 while at that time neither analysis of the metabolite HHCB-lactone nor enatioselective analysis was possible. While the effluent of STP Ölbachtal has not changed significantly over a ten year time period, the typical river concentrations seem to have changed, as they were approximately 500 ng L−1 HHCB and ∼300 ng L−1 AHTN in 1993 while now they are 60 ng L−1 and 10 ng L−1, respectively. The STP situation in this region has changed considerably, as a multitude of older and smaller STPs have been decommissioned, and waste water is increasingly treated in larger more centralised facilities which utilise current technology.
Additionally, the water mass flow is not constant in rivers like the Ruhr. During this new study the waterflow in the Ruhr was less than the average. The data from the older studies are not available. Thus it seems that the riverine values have decreased considerably. This finding may also be attributed to the efforts of manufacturers of laundry detergents to replace polycyclic musk compounds as well as nitroaromates.
Heberer et al.18 published data on HHCB and AHTN in water samples in Berlin, Germany from surface and discharge waters. In that study the concentrations ranged from 20 ng L−1 to 12 500 ng L−1 (HHCB) and from 30 ng L−1 to 5800 ng L−1 (AHTN). Berlin’s surface waters are continuously heavily polluted by sewage treatment plant discharges. The highest concentrations were found in STP effluent samples, though. The typical riverine concentrations were similar to those obtained in this study. For HHCB-lactone there are no quantitative data published.
Transformation data
To gain some insight into the persistence and possible transformation of HHCB, the ratio of HHCB-lactone versus HHCB was calculated from all current Ruhr samples with significantly elevated concentrations. The respective graph is shown in Fig. 4. During this study the technical product, but not the analytical standard, of HHCB was found to contain about 10% HHCB-lactone, which is also found in the STP influent as shown in Table 3, in which data from several days 24 h composite sampling are averaged.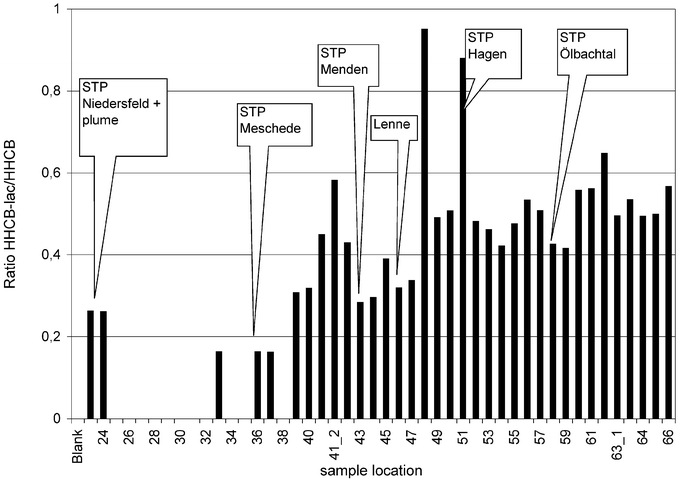 | ||
| Fig. 4 Ratio HHCB-lactone vs. HHCB. No ratios were calculated from values near the blank concentration. | ||
| Introduction/ng L−1 | Discharge/ng L−1 | Elimination [%] | ||
|---|---|---|---|---|
| STP-A | ||||
| 22.10.02 | HHCB | 2836 | 635 | 78 |
| AHTN | 678 | 161 | 76 | |
| HHCB-lactone | 341 | 257 | 25 | |
| HHCB-Lac/HHCB | 0.12 | 0.40 | ||
| STP-B | ||||
| 20.10.02 | HHCB | 5049 | 871 | 83 |
| AHTN | 1182 | 160 | 87 | |
| HHCB-lactone | 438 | 233 | 47 | |
| HHCB-Lac/HHAB | 0.09 | 0.27 | ||
| STP-Dortmund | ||||
| Median | HHCB | 1900 | 700 | 63 |
| AHTN | 580 | 210 | 64 | |
| HHCB-lactone | 230 | 370 | Generation | |
| HHCB-Lac/HHAB | 0.12 | 0.53 |
It should be noted that the diverse STP discharges that were sampled gave different ratios of HHCB-lactone to HHCB. It seems that each STP has its own metabolic profile. In STP effluent samples the ratio ranged from 0.17 (no. 36) to 0.95 (no. 48, 51). For comparative purposes the STP technology which is used in the STPs from which the samples were obtained is given in Table 4. It seems that each STP has its individual emission pattern that cannot be calculated from theoretical data.
| STP | Sample no. | Effluent/m3 d−1 | Type | RT/h | BODa removal [%] | CODa removal [%] | Nitri-fication | Denitrification | Ratio mun [%] | C HHCB/ng L−1 | C HHCB lac/ng L−1 | ER Lac (SS/RR) | C AHNT/ng L−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AS: activated sludge, RT: hydraulic residence time, n.a.: data not available, No: does not exist in the respective plant, Ratio mun: ratio municipal vs. industrial contribution.a Data obtained from the waterworks company. | |||||||||||||
| Niedersfeld | 24 | 3800 | AS, clarifier | 14 | 92 | 86 | No | No | 100 | 264 | 69 | 0.883 | 38 |
| Arnsberg- Wildhausen | 20 800 | AS, clarifier | 15 | 97 | 89 | Yes | Yes | 74 | n.a. | n.a. | n.a. | n.a. | |
| Menden Bösperde | 43 | 38 900 | AS, trickling filter | 3.5 | 92 | 83 | No | No | 75 | 106 | 30 | 0.930 | 19 |
| Hagen-Fley | 48 | 12 900 | AS, clarifier | 3.3 | 98 | 78 | No | No | 100 | 42 | 40 | n.a. | 25 |
| Hagen-Boele | 51 | Not yet fully established | 55 | 58 | 51 | n.a. | 18 | ||||||
| Bochum-Ölbachtal (mean) | 57 | 70 300 | AS, clarifier | 22 | 98 | 93 | Yes | Yes | 63 | 451 | 198 | 0.783 | 84 |
In samples from the river Ruhr with high concentrations (downstream from Schwerte (no. 50)) this ratio is more or less a constant of 0.5 in all riverine samples during the passage through the lakes until station 66 is reached. Some tributaries such as the river Lenne exhibit slightly lower ratios, though. The distance from sample no. 57–66 is about 50 km, and the river passes several lakes such as lake Kemnaden (3 × 106 m3), lake Baldeney (8.3 × 106 m3), and lake Kettwig (1.5 × 106 m3). Hydraulic residence time in this part of the river is about 6 d. The treated waste water from about 1.5 million inhabitants is discharged upstream of this point, while only those from 0.34 million is discharged downstream of station (no. 60). Therefore it was suggested that, if degradation of HHCB in the river took place, this would be the most probable place it could occur and be determined. However, in this region neither the concentration nor the ratio of HHCB versus HHCB-lactone changed significantly, thus indicating a rather conservative transport of these compounds to the mouth of the river without significant transformation from HHCB to HHCB-lactone. This is supported by the fact that the concentrations remain constant, too. These ratios have been analysed with about 15% uncertainty. This means the transformation of HHCB to HHCB-lactone in the river was less than 15% of the riverine inventory of HHCB. On the other hand, in STPs HHCB can be transformed to some degree into the lactone (Fig. 3, as well as in ref. 2). In the upstream part of the river Ruhr the concentrations are too low to lead to clear conclusions.
As a comparison of the Ruhr data similar experiments for the Rhine gave a ratio of 1.0 for HHCB vs. HHCB-lactone while for the river Lippe this ratio was 0.4. In the river Elbe HHCB and HHCB-lactone were tentatively determined by Franke et al.9 and Meyer.19 In this river a ratio of 0.3 HHCB-lactone in comparison to HHCB was reported. It may be that these different ratios of HHCB vs. HHCB-lactone in the different rivers (Ruhr, Elbe, Rhine, Lippe) are due to the different sewage treatment plant technology which is used. This is also indicated in this set of data, as different STPs do not discharge the same ratios. Here ratios varied from 0.2 (Niedersfeld) and 0.9 (Hagen-Fley) (Fig. 4). Currently it is not possible to correlate STPs emission concentration or the respective ratios with the technology applied in the respective plant. This has also been a difficulty for other authors3 or in the understanding of another dataset on polycyclic musks in sludge.2 Considering AHTN there is no significant change of concentrations in the river from station no. 50–66. In this part of the river AHTN concentrations are 19% of HHCB with 2% standard deviation. A slight increase of concentrations would be expected, as the STPs in that part of the river could contribute about 22% of the total load. This could still be covered by the measurement uncertainty. Anyway if degradation of AHTN does occur, the estimation based on these data is that it will be less than 22% on passage through the lower part of the river, even though the sampling took place in bright warm summer weather in a dry period without any rainfall, with conditions favourable to photo- and biodegradation.
Assuming the river to be at steady state during this experiment, the following calculations can be performed.
Generally the equation for an elimination rate constant for first order kinetics is as indicated in eqn. 1. k being the reaction rate constant, C0 the concentration at time = 0, C the concentration at the given time t.
 | (1) |
![[greater than or equal, slant]](https://www.rsc.org/images/entities/char_2a7e.gif) 40 d.
40 d.
A comparison with the elimination rate constants of Buerge et al.12 resulted in C0/C values of 2.5 for HHCB and about 1011 for AHTN or half-lives of 4.6 and 0.15 d, respectively. These Swiss data obviously cannot be used to describe the river Ruhr situation, and can thus not be applied in assessing the fate of these compounds in this ecosystem. Especially, the large difference between AHTN and HHCB as described by Buerge et al.12 cannot be seen in the dataset of the river Ruhr. Possibly the rather eutrophic Ruhr is too different from the rather oligotrophic Swiss lakes, or the high concentrations applied in the Buerge et al. study do not compare well to the relatively low concentrations found in this study.
Enantioselective determination for the verification of transformation data
Formation of enantiomeric ratios differing from 1 starting from technical racemates are used as a strong clue for biodegradation.20 In the past there was some discussion whether there is transformation of HHCB at all, and if yes, whether this is biodegradation or (photo) oxidation. Thus enantioselective analysis was used to obtain data on the mechanism of transformation.Additionally the chiral measurements on the other hand can be more precise, as they are relative measurements.20 In Fig. 5 the separation of the enantiomers and diastereomers of HHCB-lactone is shown, while the elution order determined by Meyer19 is used. As HHCB-lactone has two chiral centres, four peaks are separated. The distribution of enantiomeric ratios of HHCB-lactone in the samples is shown in Fig. 6. HHCB-lactone has two enantiomeric pairs: (4S,7R) vs. (4R,7S) as well as (4S,7R) vs. (4R,7S).
 | ||
| Fig. 5 Enantioselective separations of the enantiomers and diastereomers of HHCB-lactone in a STPs effluent sample. Elution order following Meyer.19 | ||
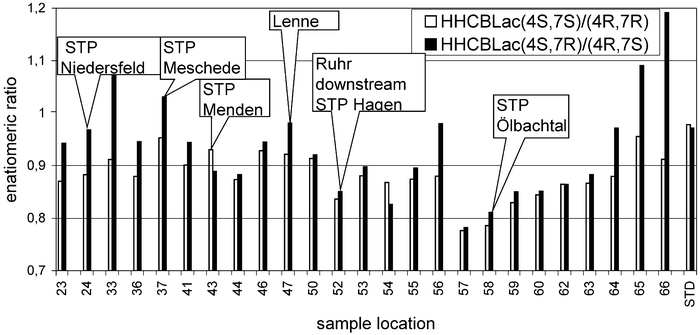 | ||
| Fig. 6 Enantiomeric ratios of HHCB-lactone (4S,7S) vs. (4R,7R) and (4S,7R) vs. (4R,7S) in selected river Ruhr water as well as STPs effluent. | ||
The enantiomeric ratios (ERs) measured for HHCB-lactone enantiomers (4S,7S) vs. (4R,7R) in the standard solutions were 0.98 with 0.015 (1.5%) RSD, similar to those determined for the waste water inflow of STPs. The samples obtained from the river Ruhr upstream of Schwerte (no. 50) exhibited ERs of about 0.9 while the samples from the effluent of STP Ölbachtal exhibited an ER of about 0.8 (57–59). It seems that in some STPs, though not in all, HHCB may be transformed to some extent to HHCB-lactone by biological processes. Thus further proof for biotransformation is found.
Considering the ratio of the other enantiomeric pair (4S,7R) vs. (4R,7S) the analytical standard exhibits an enantiomeric ratio (ER) of 0.97 (1.8% RSD) which is similar to that for raw waste water (inflow in the STPs) while the river Ruhr samples were found to have ERs of 0.9 to 1.0 upstream of sample 57. The STP Ölbachtal discharged HHCB-lactone with an ER of 0.8, thus some biotransformation of HHCB occurs during the sewage treatment process. Downstream of this situation another process seems to become important, as ratios change to as high as 1.2.
The finding that in most cases ERs are smaller than 1.0 holds for most STP effluents. On the other hand in some samples ERs higher than one (only (4S,7R) vs. (4R,7S)), have been determined. These samples originate mostly from locations with long residence times in the river (samples 33, 65 and 66). Thus this difference may indicate different biological processes, e.g. different enzymes, which are relevant in STPs and the riverine ecosytems. It should be noted, though, that overall only a small percentage (∼7%) of the HHCB in the river may be transformed to HHCB-lactone.
The enantiomeric ratios of AHTN and the respective stereoisomers of HHCB have also been determined. No significant change in these has been detected. For AHTN there is probably almost no microbial degradation, while for HHCB this is probably due to two facts:
(1) As a major fraction of the HHCB-lactone is formed in the STP, changes are more easily detected than for HHCB. If for instance 5% of the HHCB was transformed to the HHCB-lactone, a maximum change in the ERs for HHCB would be 5% which would be very hard to detect. This could typically represent e.g. 50% of the HHCB-lactone (the residue could typically originate from product impurities), thus changes in the ERs of transformation products are much more easy to detect.
(2) The chiral separation of HHCB may sometimes be affected by co-elution problems with other impurities of the technical product, thus the precision is not as good as for the determination of the enantiomeric ratios of HHCB-lactone.
While mass balance studies such as Bester2 demonstrated the transformation of HHCB to HHCB-lactone, the question remained whether this effect was due to biotic or abiotic processes. The analysis of enantiomeric composition in this study helped to establish that biotic processes are involved in this transformation. There are only small indications for biotransformation in the river and the lakes, though. The situation in other rivers such as the river Rhine which exhibited HHCB to HHCB-lactone ratios of 1 ∶ 1 may be different, though. The concentrations in the Rhine measured in this study were too low for enantioselective analysis, as the signal to noise ratio for real world samples in chiral analysis was too low at concentrations lower than 30 ng L−1.
Conclusions
The typical river levels for HHCB, HHCB-lactone and AHTN in the region studied are about 60, 25 and 10 ng L−1, respectively. From these data it can be estimated that the river Ruhr and its total flow of about 2200 million m3 water annually, transports about 130–350 kg HHCB, 55–150 kg HHCB-lactone and 22–60 kg AHTN (depending on the annual transport patterns) into the river Rhine each year.On the other hand discharges of 0.1–0.13 g HHCB per person annually and 0.014–0.04 g AHTN per person and 0.04–0.07 g HHCB-Lac per person have been established from STPs to the rivers utilising the data given in Table 3. These data compare well to STP influent data observed by Buerge et al.12 This means the actual inputs into the sewer system are considerably higher. These STP-effluent data lead to the observation that 230–310 kg HHCB, 90–270 kg HHCB-lactone and 36–70 kg AHTN are discharged into the river. The result of this mass balance approach is that all the HHCB, HHCB-lactone and AHTN that is discharged into the river Ruhr is transported to its mouth and no significant degradation can be observed during the passage of the river in summertime. It seems the river Ruhr is in a kind of a steady state, i.e., the input to the river Ruhr is discharged to the river Rhine, without significant effect due to sedimentation or biodegradation in the river.
The concentrations of HHCB and even more so of AHTN in the river Ruhr are now less than they were a decade ago. The effluents of larger STPs which have not changed still exhibit similar high concentrations (100–600 ng L−1 HHCB), as in 1993, though. Possibly, the efficiencies of the smaller STPs have increased as there has been considerable reconstruction of STPs in this area. The decommissioning of old and small STPs in favour of larger units has had some effect as well.
A considerable amount of HHCB is transformed into HHCB-lactone in the STPs. This should also be taken into account when performing assessments of polycyclic musk fragrances. As the transformation process is enantioselective in some STPs, the degradation in these plants is probably due to enzymatic transformation and not abiotic oxidation. No indication of removal or biotransformation of these polycyclic musks while in the river Ruhr was detected in this study, though several lakes could serve as sedimentation sinks. Possible changes such as sorption to sediment, volatilisation, biodegradation or photodegradation should result in changes of ratios of HHCB versus HHCB-lactone as all physico-chemical parameters, such as Henry’s constant, water solubility or sorption to sediment of both compounds should differ significantly due to the higher polarity of the lactone. However, it cannot totally be excluded that the combination of several processes may mean that no changes are observed in the ratios. From the results presented in this paper, this seems very improbable, though. It therefore seems reasonable to assume that no degradation does take place in the shallow lakes or in rivers like the Ruhr itself. For an overview of risk assessment for human health the concentrations, exposure and toxicity of the respective compounds need to be compared. The estrogenic effects of HHCB and AHTN are relatively low.7 Additionally water purification will probably reduce the concentrations in drinking water considerably. However, very little published toxicological data are available on HHCB-lactone, which will make risk assessment very difficult at this stage. On the other hand large amounts of these compounds most probably reach human bodies via the skin, from perfumes or treated clothing.21 It should also be noted that the consumer has to pay considerably for water purification. All three compounds add to the load of xenobiotics that wildlife living in industrialised riverine systems has to cope with. An additional issue arises in fish caught in these rivers and consumed afterwards. The presence and fate of HHCB-lactone should be included in all assessments concerning HHCB. Toxicity data on HHCB-lactone are especially needed for further assessment.
Acknowledgements
Millipore supported this research with the lease of a Milli-Q Gradient with an Elix 3 apparatus. Franke and Meyer provided information on the elution order of HHCB-lactone. IFF supported this research with a gift of HHCB-lactone and pure HHCB. The author is indebted to A. Grundmann, who performed some of the measurements of HHCB-lactone. G. Hardes, C. Stolle and K. Fänger provided excellent technical work in the laboratory. This study was supported by the federal countries’ environmental protection agency (LUA-NRW) with contract No. 11.2–1781 MZ 72/01. Also the grant of the Ministry for the Environment and Conservation's, Agriculture and Consumer Protection of the state of North Rhine Westphalia under the project: “Einträge und Quellen von Tris (2-chlorpropyl)-phosphat und Tris (2-chlorethyl)-phosphat in Oberflächen- und Abwässern” is highly acknowledged.References
- F. Balk and R. R. Ford, Environ. Toxicol. Lett., 1999, 111, 57–79 Search PubMed.
- K. Bester, Chemosphere, 2004, 57, 863–870 CrossRef CAS.
- S. L. Simonich, T. W. Federle, W. S. Eckhoff, A. Rottiers, S. Webb, D. Sabaliunas and W. De Wolf, Environ. Sci. Technol., 2002, 36, 2839–2847 CrossRef CAS.
- S. L. Simonich, W. M. Begley, G. Debaere and W. S. Eckhoff, Environ. Sci. Technol., 2000, 34, 959–965 CrossRef CAS.
- H. D. Eschke, J. Traud and H. J. Dibowski, UWSF-Z. Umweltchem. Ökotox., 1994, 6, 183–189 Search PubMed.
- H. D. Eschke, H. J. Dibowski and J. Traud, UWSF-Z. Umweltchem. Ökotox., 1995, 7, 131–138 Search PubMed.
- W. Seinen, J. C. Lemmen, R. H. H. Pieters, E. M. J. Verbruggen and B. van der Burg, Toxicol. Lett., 1999, 111, 161–168 CrossRef CAS.
- G. G. Rimkus, Toxicol. Lett., 1999, 111, 37–56 CrossRef CAS.
- S. Franke, C. Meyer, N. Heinzel, R. Gatermann, H. Hühnerfuss, G. Rimkus, W. A. Konig and W. Francke, Chirality, 1999, 11, 795–801 CrossRef CAS.
- R. Kallenborn, R. Gatermann, T. Nygard, J. Knutzen and M. Schlabach, Fresenius Environ. Bull., 2001, 10, 832–842 CAS.
- T. Kupper, J. D. Berset, R. Etter-Holzer, R. Furrer and J. Tarrandellas, Chemosphere, 2004, 54, 1111–1120 CrossRef CAS.
- I. J. Buerge, H. R. Buser, M. D. Muller and T. Poiger, Environ. Sci. Technol., 2003, 37, 5636–5644 CrossRef CAS.
- R. Gatermann, S. Biselli, H. Hühnerfuss, G. G. Rimkus, S. Franke, M. Hecker, R. Kallenborn, L. Karbe and W. A. Konig, Arch. Environ. Contam. Toxicol., 2002, 42, 447–453 CrossRef CAS.
- J. A. Andresen, A. Grundmann and K. Bester, Sci. Total Environ., 2004 Search PubMed , in press.
- Ruhrverband, Anhaltswerte Fliesdatentabelle für die Ruhr, (Niedrigwasser) (Waterflow of the river Ruhr), 2004 Search PubMed.
- L. Dsikowitzky, J. Schwarzenbauer and R. Littke, Org. Geochem., 2002, 33, 1747–1758 CrossRef CAS.
- K. Bester, H. Hühnerfuss, W. Lange, G. G. Rimkus and N. Theobald, Water Res., 1998, 32, 1857–1863 CrossRef CAS.
- T. Heberer, S. Gramer and H. J. Stan, Acta Hydrochim. Hydrobiol., 1999, 27, 150–156 CrossRef CAS.
- C. Meyer, Screening, identification and enantioselective analysis of organic compounds in surface waters (in German), PhD thesis, University Hamburg, 2001 Search PubMed.
- K. Bester, Anal. Bioanal. Chem., 2003, 376, 302–304 CAS.
- G. Rimkus and M. Wolf, Chemosphere, 1996, 33, 2033–2043 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
