Supramolecular reagents: versatile tools for non-covalent synthesis†
Christer B.
Aakeröy
*,
John
Desper
and
Joaquin F.
Urbina
Department of Chemistry, Kansas State University, Manhattan, KS, 66506, USA. E-mail: aakeroy@ksu.edu
First published on 25th April 2005
Abstract
The design and modular synthesis of three ternary co-crystals with desired connectivity, assembled using tailor-made pyridyl/benzimidazol-1-yl-based supramolecular reagents, are described.
Many elegant studies have shown that extended supramolecular architectures can be synthesized with the aid of complementary intermolecular interactions.1 When such assemblies are constructed from neutral molecular building blocks they are typically homomeric, which is consistent with the observation that “most crystals are built from identical (or enantiomeric) copies of the same molecule”.2 Despite an abundance of papers dealing with design and assembly of organic extended networks with desirable connectivities and shapes, it remains exceedingly difficult to bring more than two different molecular species into one crystalline lattice in a predictable manner, without making or breaking covalent bonds.3–5 In fact, our recent work with isonicotinamide remains the only reported successful strategy for the reliable construction of ternary supermolecules.6–11 In a typical reaction, isonicotinamide is allowed to react with two carboxylic acids resulting in ternary supermolecules with two primary supramolecular synthons;12 (1) the heteromeric carboxylic acid⋯pyridine hydrogen bond and (2) the heteromeric amide⋯acid hydrogen bond. The supramolecular targets are assembled using a synthetic strategy based upon a hierarchical view of intermolecular forces; the stronger acid (as determined by pKa values) interacts preferentially with the best hydrogen-bond acceptor (the pyridine nitrogen atom), and the weaker acid binds to the amide moiety.13
Despite its success, isonicotinamide is not an ideal supramolecular reagent. First, it is capable of forming self-complementary amide⋯amide and amide⋯pyridine hydrogen bonds which makes it inherently difficult to combine it with molecules that lack moieties that can compete successfully with the hydrogen-bonding capabilities of the amide functionality. Second, since the two binding sites on isonicotinamide are attached to the same delocalized backbone, it is not possible to tune the electronics of the two sites independently, which reduces versatility.
In order to bring supramolecular synthesis in the solid state (crystal engineering) to a new level of complexity we are now developing supramolecular reagents (SRs) that can be refined in such a way that they offer more opportunities for structural selectivity and specificity.
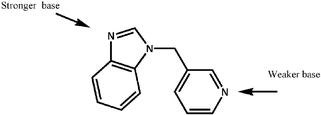
This paper describes the rationale behind, and synthesis of, a family of pyridyl/benzimidazol-1-yl-based SRs, and the subsequent modular assembly of ternary co-crystals (1∶1∶1). Our approach is based upon hydrogen-bond interactions in the context of the best-donor/best-acceptor, second-best donor/second-best acceptor concept.6,14,15 The SRs in this study are built around asymmetric bis-heterocycles where two binding sites (hydrogen-bond acceptor sites) are linked by a methylene bridge in order to provide increased solubility in a range of solvents. The binding sites have significantly different basicities,16 which means that their abilities to accept hydrogen bonds differ. They also lack strong hydrogen-bond donors and, consequently, any self-complementary intermolecular interaction will be weak and less likely to disrupt the desired heteromeric interactions, Scheme 1.
 | ||
| Scheme 1 | ||
Even though pKa/pKb values do not provide direct measures of hydrogen-bond strength, hydrogen-bond abilities and free energies of complexation have been correlated with pKa values, and within closely related classes of compounds such comparisons frequently yield correct qualitative results.17 Finally, the basicity of each heterocycle can be independently altered through suitable covalent substituents on each ring, which thereby provides an effective handle for fine-tuning differences in intermolecular reactivity between the two binding sites. The latter is highly significant as it creates a supramolecular reagent with the potential for a high degree of versatility and transferability. Ditopic bis-benzimidazoles/bis-imidazoles are also known to form co-crystals with a variety of carboxylic acids.18
The ability of these supramolecular reagents to form ternary supermolecules with predictable connectivity was put to the test by allowing each SR to react with pairs of carboxylic acids with different pKa values19 in a 1∶1∶1 ratio.20
The crystal structure of 1 consists of two crystallographically inequivalent sets of ternary supermolecules with identical connectivity, Fig. 1.21
 | ||
| Fig. 1 One of two ternary supermolecules in the crystal structure of 1 (both have the same connectivity). The best hydrogen-bond donor binds to the best hydrogen-bond acceptor and the second-best donor binds to the second-best acceptor. | ||
The primary synthons in this structure are (a) the O–H⋯N hydrogen bonds from the stronger acid (3,5-dinitrobenzoic acid) to the most basic nitrogen atom located on the benzimidazol-1-yl ring, O31⋯N13, 2.5762(17) Å, and O71⋯N53, 2.5455(18) Å, and (b) the O–H⋯N hydrogen bonds from the weaker acid (4-nitrobenzoic acid) to the less basic nitrogen atom located on the pyridyl moiety, O41⋯N21, 2.6081(18) Å and O81⋯N61, 2.6057(19) Å.
The crystal structure determination of 2 also reveals a ternary 1∶1∶1 supermolecule with the same connectivity as found in 1, Fig. 2.22
 | ||
| Fig. 2 The ternary supermolecule in the crystal structure of 2. The best hydrogen-bond donor binds to the best hydrogen-bond acceptor and the second-best donor binds to the second-best acceptor. | ||
The primary synthons comprise (a) an O–H⋯N hydrogen bond between the stronger acid, 3,5-dinitrobenzoic acid, and the most basic heterocyclic moiety, O47⋯N13, 2.652(3) Å, and (b) another O–H⋯N interaction between the weaker acid, 3-N,N-dimethylaminobenzoic acid, and the second-best hydrogen-bond acceptor, the pyridyl moiety, O37⋯N21, 2.665(3) Å.
The crystal structure of 3 contains the desired 1∶1∶1 supermolecule with the expected connectivity, Fig. 3.23
 | ||
| Fig. 3 The ternary supermolecule in the crystal structure of 3. The best hydrogen-bond donor binds to the best hydrogen-bond acceptor and the second-best donor binds to the second-best acceptor. | ||
The best acceptor, the benzimidazol-1-yl moiety, forms an O–H⋯N hydrogen bond with the best donor, the stronger acid, O47⋯N13, 2.553(3) Å. The second-best acceptor, the pyridyl moiety, binds to the weaker acid via an O–H⋯N hydrogen bond, O37⋯N21, 2.641(3) Å.
All three structures, 1–3, contain ternary supermolecules constructed through the deliberate use of directional intermolecular synthetic operations.24 Each SR has two binding sites that differ primarily in their basicity but neither site is otherwise biased or predisposed towards interacting preferentially with either of the two competing carboxylic acids. The differences in basicity are translated into supramolecular reactivity and selectivity that subsequently carry over into the solid state, which demonstrates that supramolecular assembly can be controlled by fine-tuning individual binding sites. This raises the possibility that a solution to the problem of making non-covalent one-pot synthesis “sequential” may be to devise modular assembly processes based upon a hierarchy of intermolecular interactions derived from molecular properties and structural trends.
It should be emphasized that ternary co-crystals are extremely rare and that the supramolecular reagents presented in this study contribute to high-yielding reactions; in a supramolecular sense, this translates to a high frequency of occurrence of a particular intermolecular binding pattern in the presence of potentially disruptive intermolecular interactions.
SRs of the type presented herein will at some point undoubtedly generate results that do not acquiesce to the proposed assembly principles. However, through covalent synthesis we have unlimited opportunities for modulating the electronic and geometric details of each binding site on a supramolecular reagent such that a variety of chemical functionalities can be targeted for binding. In this way we can build a team of SRs where each member is capable of affecting the assembly of new supermolecules with a high degree of specificity and reliability, thereby clearing a path towards practical and transferable guidelines for versatile supramolecular synthesis. We are currently probing the limits and limitations of this hierarchical approach to non-covalent synthesis by examining the structural reactivity of libraries of supramolecular reagents containing multiple binding sites with easily adjustable differences in hydrogen-bond donating/accepting capabilities.
We are grateful for financial support from NSF (CHE-0316479).
Notes and references
- C. B. Aakeröy, Acta Crystallogr., Sect. B: Struct. Sci., 1997, 53, 569 CrossRef; B. Moulton and M. J. Zaworotko, Chem. Rev., 2001, 101, 1629 CrossRef CAS; G. R. Desiraju, Acc. Chem. Res., 2002, 35, 565 CrossRef CAS; M. W. Hosseini, CrystEngComm, 2004, 6, 318 RSC; L. R. MacGillivray, CrystEngComm, 2004, 6, 77 RSC; L. Brammer, Chem. Soc. Rev., 2004, 33, 476 RSC; C. B. Aakeröy and A. M. Beatty, Aust. J. Chem., 2001, 54, 409 CrossRef CAS; J.-M. Lehn, Supramolecular Chemistry, VCH, Weinheim, 1995 Search PubMed; D. L. Caulder and K. N. Raymond, Acc. Chem. Res., 1999, 32, 975 Search PubMed; D. N. Reinhoudt and M. Crego-Calama, Science, 2002, 295, 2403 CrossRef CAS; J.-M. Lehn, Science, 2002, 295, 2400 CrossRef CAS; D. Braga, G. Desiraju, J. S. Miller, A. G. Orpen and S. L. Price, CrystEngComm, 2002, 4, 500 CrossRef CAS; D. Braga, L. Maini, M. Polito and F. Grepioni, Struct. Bond., 2004, 111, 1 RSC; G. Lewis and A. G. Orpen, Chem. Commun., 1998, 1873 CAS; L. J. Prins, D. N. Reinhoudt and P. Timmerman, Angew. Chem. Int. Ed., 2001, 40, 2382 RSC; S. C. Zimmerman and P. S. Corbin, Struct. Bond., 2000, 96, 63 CrossRef CAS.
- J. D. Dunitz, in Perspectives in Supramolecular Chemistry: The Crystal as a Supramolecular Entity, ed. G. R. Desiraju, Wiley, Chichester, 1995, pp. 1–30 Search PubMed.
- Many binary co-crystals based on principles of molecular recognition have, however, been reported e.g.: S. Shan, E. Batchelor and W. Jones, Tetrahedron Lett., 2002, 43, 8721 Search PubMed; R. D. Bailey Walsh, M. W. Bradner, S. Fleischman, L. A. Morales, B. Moulton, N. Rodriguez-Hornedo and M. J. Zaworotko, Chem. Commun., 2003, 186 CrossRef CAS; L. R. MacGillivray, J. L. Reid and J. A. Ripmeester, J. Am. Chem. Soc., 2000, 122, 7817 RSC; P. Vishweshwar, R. Thaimattam, M. Jaskolski and G. R. Desiraju, Chem. Commun., 2002, 1830 CrossRef CAS; J. A. Zerkowski, J. C. MacDonald and G. M. Whitesides, Chem. Mater., 1997, 9, 1933 RSC; J. J. Kane, R.-F. Liao, J. W. Lauher and F. W. Fowler, J. Am. Chem. Soc., 1995, 117, 12003 CrossRef CAS; J.-M. Lehn, M. Mascal, A. DeCian and J. Fischer, J. Chem. Soc., Chem. Commun., 1990, 479 CrossRef CAS; Ö. Almarsson and M. J. Zaworotko, Chem. Commun., 2004, 1889 RSC.
- G. R. Desiraju and J. A. R. P. Sarma, J. Chem. Soc., Chem. Commun., 1983, 45 RSC; C. Huang, L. Leiserowitz and G. M. Schmidt, J. Chem. Soc., Perkin Trans. 2, 1973, 503 RSC; F. Pan, W. S. Wong, V. Gramlich, C. Bosshard and P. Gunter, Chem. Commun., 1996, 2 Search PubMed; V. R. Pedireddi, W. Jones, A. P. Chorlton and R. Docherty, Chem. Commun., 1996, 997 RSC; P. Vishweshwar, A. Nangia and V. M. Lynch, J. Org. Chem., 2002, 67, 556 CrossRef CAS; C. B. Aakeröy, A. M. Beatty, M. Nieuwenhuyzen and M. Zou, Tetrahedron, 2000, 56, 6693 CrossRef; S. H. Dale, M. R. J. Elsegood, M. Hemmings and A. L. Wilkinson, CrystEngComm, 2004, 6, 207 RSC; J. F. Remenra, S. L. Morisette, M. L. Peterson, B. Moulton, J. M. MacPhee, H. R. Guzman and Ö. Almarsson, J. Am. Chem. Soc., 2003, 125, 8456 CrossRef CAS.
- C. B. Aakeröy, J. Desper and B. A. Helfrich, CrystEngComm, 2004, 6, 19 RSC.
- C. B. Aakeröy, A. M. Beatty and B. A. Helfrich, Angew. Chem. Int. Ed., 2001, 40, 3240 CrossRef CAS.
- C. B. Aakeröy, A. M. Beatty, B. A. Helfrich and M. Nieuwenhuyzen, Cryst. Growth Des., 2003, 3, 159 CrossRef.
- Koefler's complex is a solvate (pyridine), and its preparation was not based upon an explicit supramolecular strategy. J. Bernstein, H. Regev and F. H. Herbstein, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 1170 Search PubMed.
- A three-component co-crystal based on isomorphous replacement with acridine in a 2∶3 2,2′-dihydroxybiphenyl phenazine co-crystal has been reported. This approach relies on molecular complementarity and recognition and may be effective in the design of other ternary systems. T. Smolka, R. Boese and R. Sustmann, Struct. Chem., 1999, 10, 429 Search PubMed.
- A 3∶1∶1 ternary co-crystal has been reported previously although no explicit design strategy was evident. D. E. Lynch, G. Smith, K. A. Byriel and C. H. L. Kennard, J. Chem. Soc., Chem. Commun., 1992, 300 Search PubMed.
- The term “ternary supermolecule” indicates a discrete species with predictable and desirable connectivity, constructed from three different molecular species and assembled via directional non-covalent forces. Thus, ternary systems formed by ionic interactions (salts) or by incorporation of solvent molecules within a lattice (e.g. clathrates, solvates, or inclusion compounds) are distinctly different from the supermolecules that we discuss in this paper.
- G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311 CrossRef CAS.
- To date, more than ten ternary co-crystals based around isonicotinamide or nicotinamide with two different carboxylic acids are known, and the stronger acid binds to the pyridine moiety, and the weaker acid binds to the amide functionality in each case.
- M. C. Etter, Acc. Chem. Res., 1990, 23, 120 CrossRef CAS; M. C. Etter, J. Phys. Chem., 1991, 95, 4601 CrossRef CAS.
- Approaches such as this are also predicated upon the idea that a small number of specific intermolecular interactions can provide a significant part of the stabilization energy of molecular crystals; P. Dauber and A. T. Hagler, Acc. Chem. Res., 1980, 13, 105–112 Search PubMed.
- An estimate for the differences in basicity between the pyridyl and the benzimidazol-1-yl moieties were obtained by calculating pKa values for the conjugated acids of the two hydrogen-bond acceptors in each SR. 3-(Benzimidazol-1-yl)methylpyridine: pKa = 4.71 ± 0.10 and 5.72 ± 0.30 for pyridyl and benzimidazol-1-yl moieties, respectively. 3-(2-Methylbenzimidazol-1-yl)methylpyridine: pKa = 4.72 ± 0.10 and 5.85 ± 0.18 for pyridyl and benzimidazol-1-yl moieties, respectively. The calculations were performed using ACD/Solaris version 4.76, Advanced Chemistry Development, Inc., Toronto, ON, Canada, http://www.acdlabs.com, 1994–2005 Search PubMed.
- C. Laurence and M. Berthelot, Perspect. Drug Discovery Des., 2000, 18, 39 Search PubMed; M. H. Abraham, Chem. Soc. Rev., 1993, 22, 73 RSC; S. Shan, S. Loh and D. Herschlag, Science, 1996, 272, 97 CrossRef CAS.
- C. B. Aakeröy, J. Desper, B. Leonard and J. F. Urbina, Cryst. Growth Des., 2005 DOI:10.1021/cg049682i.
- 3,5-Dinitrobenzoic acid (pKa = 2.8); 4-nitrobenzoic acid (pKa = 3.44); 3-N,N-(dimethylamino)benzoic acid (pKa = 4.30). Dissociation constants of organic acids in aqueous solution, ed. G. Kortüm, W. Vogel and K. Andrussow, Butterworth, London, 1961 Search PubMed.
- Due to the fact that all these co-crystallization reactions may yield crystals of each reagent by itself, five possible binary co-crystals (the SR plus one of the two carboxylic acids in either 1∶1 or 1∶2 stoichiometry, or a heteromeric acid⋯acid co-crystal), or the desired 1∶1∶1 ternary product, it is important to carry out analysis of individual crystallites. The combination of TLC and NMR, when performed on individual crystals, will give information about which of the three species are present as well as their relative ratios. Single-crystal X-ray crystallography is required in order to determine the connectivity of the supermolecule (and hence the selectivity of the supramolecular reagent).
- Crystal data for 1,† 3-(benzimidazol-1-yl)methylpyridine, 3,5-dinitrobenzoic acid, 4-nitrobenzoic acid,: C27H20N6O10, M
= 588.49 amu, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a
= 7.7602(11)
Å, b
= 15.269(2)
Å, c
= 22.456(3)
Å, α
= 82.064(3)°, β
= 81.610(3)°, γ
= 78.915(3)°, V
= 2566.7(6)
Å3, Z
= 4, Dc
= 1.523 g cm−3, μ(Mo Kα)
= 0.119 mm−1, crystal size 0.37 × 0.21 × 0.06 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer
using Mo Kα radiation. A total of 30029 reflections (1.56° < θ < 30.14°) were processed of which 9331 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out with the SHELXL-9725 software package release 97-2. Final residuals for I > 2σ(I) were R1
= 0.0544 and wR2
= 0.1090 (GOF = 0.886).
, a
= 7.7602(11)
Å, b
= 15.269(2)
Å, c
= 22.456(3)
Å, α
= 82.064(3)°, β
= 81.610(3)°, γ
= 78.915(3)°, V
= 2566.7(6)
Å3, Z
= 4, Dc
= 1.523 g cm−3, μ(Mo Kα)
= 0.119 mm−1, crystal size 0.37 × 0.21 × 0.06 mm. Data were collected at 100 K on a Bruker SMART 1000 diffractometer
using Mo Kα radiation. A total of 30029 reflections (1.56° < θ < 30.14°) were processed of which 9331 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out with the SHELXL-9725 software package release 97-2. Final residuals for I > 2σ(I) were R1
= 0.0544 and wR2
= 0.1090 (GOF = 0.886). - Crystal data for 2,† 3-(benzimidazol-1-yl)methylpyridine, 3,5-dinitrobenzoic acid, 3-N,N-(dimethylamino)benzoic acid: C29H26N6O8, M
= 586.56 amu, triclinic, space group P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a
= 8.1324(7)
Å, b
= 10.8110(9)
Å, c
= 15.3761(13)
Å, α
= 95.489(4)°, β
= 90.318(5)°, γ
= 91.503(5)°, V
= 1345.2(2)
Å3, Z
= 2, Dc
= 1.448 g cm−3, μ(Mo Kα)
= 0.108 mm−1, crystal size 0.35 × 0.35 × 0.15 mm. Data were collected at 203 K on a Bruker SMART 1000 diffractometer using Mo Kα radiation. A total of 9845 reflections (1.33 < θ < 27.51) were processed of which 3561 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out with the SHELXL-9725 software package release 97-2. Final residuals for I > 2σ(I) were R1
= 0.0628, and wR2
= 0.1840 (GOF = 0.974).
, a
= 8.1324(7)
Å, b
= 10.8110(9)
Å, c
= 15.3761(13)
Å, α
= 95.489(4)°, β
= 90.318(5)°, γ
= 91.503(5)°, V
= 1345.2(2)
Å3, Z
= 2, Dc
= 1.448 g cm−3, μ(Mo Kα)
= 0.108 mm−1, crystal size 0.35 × 0.35 × 0.15 mm. Data were collected at 203 K on a Bruker SMART 1000 diffractometer using Mo Kα radiation. A total of 9845 reflections (1.33 < θ < 27.51) were processed of which 3561 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out with the SHELXL-9725 software package release 97-2. Final residuals for I > 2σ(I) were R1
= 0.0628, and wR2
= 0.1840 (GOF = 0.974). - Crystal data for 3,† 3-(2-methylbenzimidazol-1-yl)methylpyridine, 3,5-dinitrobenzoic acid, 4-nitrobenzoic acid: C28H22N6O10, M = 602.52 amu, monoclinic P21/c, a = 7.7318(8) Å, b = 7.0062(7) Å, c = 49.395(5) Å, α = γ = 90°, β = 91.012(6)°, V = 2675.3(5) Å3, Z = 4, Dc = 1.496 g cm−3, μ(Mo Kα) = 0.116 mm−1, crystal size 0.35 × 0.35 × 0.10 mm. Data were collected at 173 K on a Bruker SMART 1000 diffractometer using Mo Kα radiation. A total of 18644 reflections (1.65° < θ < 28.26°) were processed of which 3208 were unique and significant with I > 2σ(I). Structure solution and refinement were carried out with the SHELXL-9725 software package release 97-2. Final residuals for I > 2σ(I) were R1 = 0.0613, wR2 = 0.1511 (GOF = 0.891). All hydrogen atoms in 1–3 except the carboxylic acid –COOH hydrogen atoms were located in idealized positions and allowed to refine. Coordinates for the carboxylic acid hydrogen atoms were allowed to refine.
- It is noteworthy that the crystal structure of 1 contains two crystallographically unique supermolecules, both of which display the desired connectivity.
- G. M. Sheldrick, SHELXL-97, Program for refinement of crystal structures, University of Göttingen, Germany, 1997 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: synthesis of supramolecular reagents and ternary co-crystals and CIF files (CCDC 259793–259795). See http://www.rsc.org/suppdata/cc/b5/b503718b/ |
| This journal is © The Royal Society of Chemistry 2005 |
