A new route to nanorods of cadmium sulfide
Paul
Christian
and
Paul
O'Brien
*
The School of Materials Science and School of Chemistry, University of Manchester, Oxford Road, Manchester, UK M13 9PL. E-mail: paul.o'brien@man.ac.uk; Fax: +44 (0)161 2754616; Tel: +44 (0)161 2754652
First published on 21st April 2005
Abstract
We report a novel synthesis of luminescent CdS nanorods which, unusually, are predominantly of the cubic phase.
There is considerable contemporary interest in the growth of materials with critical dimensions in the order of nanometres. In particular, cadmium chalcogenides have attracted substantial interest. There are many reports on the synthesis of CdSe1 and CdTe2 nanorods, and of deposition routes to CdS nanorods,3 but there are few which detail the solution-based synthesis of CdS nanorods.4,5 Of these, even fewer describe in detail the growth habit of the crystal, and usually without any supporting structural (XRD) data.5 Those CdS or CdSe rods reported in the literature are predominantly hexagonal, the unique c-axes of which form their long axes.6 In each, the {100} plane lies perpendicular to the long axis and the {002} plane parallel to it, both of which can be observed in high-resolution TEM (HR-TEM) images.6e,7 The new synthesis described in this communication is of particular interest as it uses readily-available reagents and provides a convenient route to luminescent nanorods. Furthermore, we have also been able to demonstrate a relationship between growth conditions and both the morphology and habitat of the nanodimensional crystal by using a combination of powder XRD (P-XRD) and HR-TEM.
The procedure involved injecting solutions of anhydrous cadmium acetate and sulfur in octylamine into a reaction mixture in hexadecylamine already primed with these reagents.† The CdS particles were isolated by precipitation with methanol, washing with toluene and reprecipitation with methanol before analysis. The results are summarised in Table 1.
XRD analysis of the materials (Fig. 1) shows that the phase of the CdS depended on the reaction conditions. The data in Table 1 are crucial in interpreting these results. At low concentrations, where we would expect closer to equilibrium reaction conditions, the thermodynamically-stable hexagonal phase of CdS was unequivocally the form observed. On simply increasing the concentration by 70%, the characteristic pattern for the hexagonal phase collapsed. Whilst the assignment of phase from powder data is equivocal in this size range,8 we strongly suggest that this result is most consistent with cubic or polytypical material. This is since the particles in sample B are similar in size to those in A. Furthermore as the overall conditions of the reactions are similar to each other, we might expect annealing to lead to broadly similar degrees of crystallinity for A and B under both sets of reaction conditions (Table 1 and Fig. 2). Hence we suggest a change in the predominant phase to the cubic form results from a more rapid reaction due to the more concentrated solution. Similar dependencies of phase on reaction conditions have previously been reported for bulk zinc sulfide systems.9
 | ||
| Fig. 1 P-XRD patterns showing the hexagonal phase of A and cubic phase of sample B. The inset is at higher resolution. | ||
 | ||
| Fig. 2 TEM images of samples A–D. Scale bar = 85 nm. | ||
At low concentrations (A), large numbers of spherical particles were observed with diameters of 3.5 nm and a band gap of 446 nm; whereas at high concentrations (D), a rod-like morphology was predominant with rod diameters of 7 nm, aspect ratios of 1 ∶ 3.5. and a band gap of 481 nm. All of the samples were found to luminesce when excited at 380 nm and the quantum yield varied from 2.3–0.1% with no apparent trend‡. It was interesting to note that there were rods in all the samples. However, rods were only the predominant product at higher concentrations (Fig. 3 and Table 1).
 | ||
| Fig. 3 Photoluminescence spectra for samples A–D, excited at 380 nm (* indicates Raman scattering from solvent). | ||
Due to the absence of a peak at 48.5°, the XRD patterns for samples C and D were not consistent with the hexagonal phase (Fig. 4). There were also diffraction peaks at 23° and 52° which are consistent with the cubic form.
There are a few reports of nanorods in the cubic form.10 One study has shown that the twinning of cubic CdS crystals can result in a rod structure.5 However in the present case, close inspection of the HR-TEM images and the lattice spacings demonstrate a more complex picture. The cubic and hexagonal forms of CdS share similar lattice spacings for the {111} and {002} planes of ∼3.36 Å. However the {100} plane for the hexagonal form is significantly larger (3.59 Å). Analysis of the lattice spacing of sample D by HR-TEM demonstrates the unperturbed 3.34 Å lattice spacing perpendicular to the long axis of the rod (Fig. 5 top). This is consistent with both the cubic {111} and hexagonal {002} planes (Fig. 5 centre). However, lattice planes running parallel to the long axis show significant variations. Measurements at several points show a combination of lattice spacings of 3.33 and 3.52 Å (±0.05 Å). These observations have lead us to conclude that most of the rods are in fact a mixture of the cubic and hexagonal phases. However, given the lack of a {103} plane peak in the P-XRD (Fig. 4), we suggest that the cubic phase is the dominant form in samples B–D.
 | ||
| Fig. 4 P-XRD patterns showing the cubic phase of samples B–D. | ||
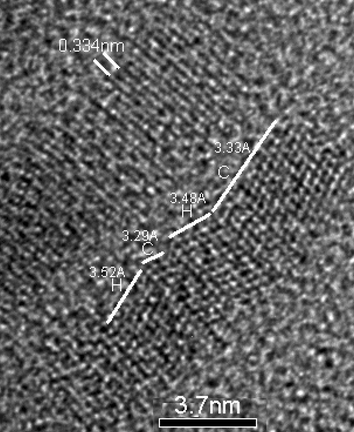 | ||
| Fig. 5 A HR-TEM image of a rod from sample D showing lattice spacing and hexagonal (H) and cubic (C) phases. | ||
These results are best rationalised as being due to a change in control of the reaction from close to thermodynamic at the lower concentrations to a kinetic regime at higher temperatures. The striking feature is that the rods formed are fully consistent with a predominately cubic rather than hexagonal form.
We would like to thank the EPSRC for funding this work, Peter Kenway for his help and expertise with the microscopy and Prof. G. Lorimer for his useful advice.
Notes and references
- C.-C. Chen, C.-Y. Chao and Z.-H. Lang, Chem. Mater., 2000, 12, 1516 CrossRef CAS.
- S. D. Bunge, K. M. Krueger, T. J. Boyle, M. A. Rodriguez, T. J. Headley and V. L. Colvin, J. Mater. Chem., 2003, 13, 1705 RSC.
- H. Zhang, X. Ma, J. Xu and D. Yang, J. Cryst. Growth, 2004, 263, 372 CrossRef CAS; X. Wu and Y. Tao, J. Cryst. Growth, 2002, 242, 309 CrossRef CAS; O. Conde, A. G. Rolo, M. J. M. Gomes, C. R. Ricolleau and D. J. Barber, J. Cryst. Growth, 2003, 247, 371 CrossRef CAS.
- Y. Li, X. Li, C. Yang and Y. Li, J. Mater. Chem., 2003, 13, 2641 RSC; P. S. Nair, T. Radhakrishnan, N. Revaprasadu, G. A. Kolawole and P. O'Brien, Chem. Commun., 2002, 564 RSC.
- J. A. Ascencio, P. Santiago, L. Rendon and U. Pal, Appl. Phys. A, 2004, 78, 5 CrossRef.
- (a) J. Joo, H. B. Na, T. Yu, J. H. Yu, Y. W. Kim, F. Wu, J. Z. Zhang and T. Hyeon, J. Am. Chem. Soc., 2003, 125, 11100 CrossRef CAS; (b) Y. Li, X. Li, C. Yang and Y. Li, J. Mater. Chem., 2003, 13, 2631 Search PubMed; (c) X. Ge, Y. Ni and Z. Zang, Radiat. Phys. Chem., 2002, 64, 223 CrossRef CAS; (d) P. S. Nair, T. Radhakrishnan, N. Revaprasadu, G. A. Kolawole and P. O'Brien, J. Chem. Soc., Chem. Commun., 2002, 64 RSC; (e) B. A. Simmons, S. Li, V. T. John, G. L. McPherson, A. Bose, W. Zhou and J. He, Nano Lett., 2002, 2, 263 CrossRef CAS.
- C. Mao, J. Qi and A. M. Belcher, Adv. Funct. Mater., 2003, 13, 648 CrossRef CAS.
- B. O. Dabbousi, J. Rogrigues-Viego, F. V. Mikulec, J. R. Heine, H. Mattoussi, R. Ober, K. F. Jensen and M. G. Bawendi, J. Phys. Chem., 1997, 101, 9463 Search PubMed.
- S. D. Scott and H. L. Barnes, Geochim. Cosmochim. Acta, 1972, 36, 1275 CrossRef CAS.
- J.-U. Kim, S.-H. Cha, K. Shin, J. Y. Jho and J.-C. Lee, Adv. Mater. (Weinheim, Ger.), 2004, 16(5), 459 CrossRef CAS.
Footnotes |
| † Anhydrous cadmium acetate was dissolved in octylamine (10 ml). A stoichiometric amount of sulfur was dissolved in octylamine (10 ml). Hexadecylamine (100 g) was de-gassed under reduced pressure for 1 h at 140 °C then brought up to atmospheric pressure under nitrogen. The reaction mixture was held at 140 °C and the sulfur-containing solution (1 ml) injected followed by the cadmium acetate solution (1 ml). The reaction was left to proceed for 15 min, after which the remaining reagents were added at a rate of 3.17 ml h−1 over approximately 3 h. The reaction mixture was then maintained at 140 °C for a further 10 h. After cooling, the particles were precipitated by the addition of dry methanol and isolated by centrifugation. The product was then redissolved in toluene and reprecipitated with methanol. XRD data were recorded on a Brucker D8 diffractometer. Photoluminescence data were collected using a Horiba Fluorolog-3 (FL3-22). TEM images were recorded on a Philips CM200 at 200 kV and a Tecni FEG-TEM 300 kV microscope. |
| ‡ Quantum Yields were measured in chloroform against diphenylanthracene; A 0.1% , B 0.1%, C 2.3%, D 0.4%. |
| This journal is © The Royal Society of Chemistry 2005 |
