C 2 C70F38 is aromatic, contains three planar hexagons, and has equatorial addends
Peter B.
Hitchcock
a,
Anthony G.
Avent
a,
Natalia
Martsinovich
a,
Pavel A.
Troshin
b and
Roger
Taylor
*a
aChemistry Department, University of Sussex, Brighton, UK BN1 9QJ. E-mail: R.Taylor@sussex.ac.uk; Fax: 44 1273 677196; Tel: 44 1273 6768602
bChemistry Department, Moscow State University, Moscow 119899, Russia
First published on 19th November 2004
Abstract
Single crystal X-ray analysis shows the main (C2) isomer of C70F38 to contain three planar delocalised aromatic hexagons (two equivalent and one centred on the C2 axis), together with seven C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (three pairs and one straddling the C2 axis); C70F38 is the first high addition level [70]fullerene derivative to be fully characterised, is the first to have equatorial addends, and is calculated to have high stability.
C bonds (three pairs and one straddling the C2 axis); C70F38 is the first high addition level [70]fullerene derivative to be fully characterised, is the first to have equatorial addends, and is calculated to have high stability.
Understanding the addition patterns of fullerenes and their origins is an integral part of fullerene chemistry. For [60]fullerene, numerous papers describe not only numerous fully characterised cycloadducts, but also the structures of derivatives (especially those containing H, F, Cl, Br) at a range of addition levels, enabling conjectures of the stepwise addition pathways.1
The situation for [70]fullerene is more complex,2 and involves two main manifolds: additions/cycloadditions to the end caps giving e.g. C70H2/4, C70Me2, C70benzyl2, C70(OH)2, C70O, and zig-zag equatorial addition giving C70Me4/6/8/10, C70Ph2/4/6/8/10, C70H8/10, C70Cl10, C70Br10, C70(O2t-Bu)10, C70Ph9OH, C70Ph8(OH)2. Low addition-level products have also been obtained in reactions with benzyne, bromomalonate, Vaska's complex, and 2,2-bis(2,6-diisopropylphenyl)hexamethyltrisilane. In no reaction has equatorial addition ever been observed. (Reported equatorial silylation of [70]fullerene was based on 1H NMR data, consistent however with equatorial straddling).3
Information on high levels of [70]fullerene radical-addition is sparse, and these have been obtained only in hydrogenation (which gave mainly uncharacterised C70H36/38 together with some C70H40–44),4 and fluorination by MnF3 (in which we isolated twenty-one highly fluorinated [70]fullerenes (mainly C70F36/38 with some C70F34–42), and many oxide derivatives.5 However, the very complex 19F NMR spectra precluded structural identification.
A feature of these additions is the absence of intermediate addition levels and the dominance of C70X36 and C70X38 (X = H, F). This led us to propose that C70H36 must be an aromatic compound, having benzenoid patches in the structure.6 Aromatic structures were also proposed by Gerst et al., and by Book and Scuseria.7 However, subsequent MNDO calculations indicated that a structure having isolated double bonds would, for steric reasons, be much more stable than an aromatic one.8 Clare and Kepert have calculated structures for C70X36/38 (X = H, F)9 and predicted a number of structures for C70F38, including some having three aromatic rings and seven isolated double bonds. Since the 36 and 38 addition levels are clearly closely related, our target was to obtain a crystal structure to resolve both the aromaticity question and subsequently to deduce the formation route. We have now succeeded in our objective, and provide the first full characterisation of a highly addended [70]fullerene.
Fluorination of [70]fullerene by different fluorinating reagents was explored briefly, using a 5–10-fold weight excess of fluorinating reagent at ca. 520 °C; [70]fullerene is much less reactive than [60]fullerene. Each of BaPbF6,10 MnF3, and CeF4 showed C70F38 as the main product, with BaPbF6 giving the highest relative yield. CeF4 gave only C70F36 and C70F38, and very poor overall yields together with some unreacted fullerene. The other two reagents showed the presence of C70F40, whilst CoF3 gave significant quantities of C70F40–50. Because of the extreme demands of the HPLC separations,5 the products were combined and processed together. Numerous derivatives were obtained as before,5 and also some new ones (due probably to the different fluorinating reagents, and the larger scale); this will be described in a full paper. As before, the main C70F38 isomer was no. 14,5 which crystallised from toluene.
The single-crystal X-ray structure† (Figs. 1 and 2), reveals the structure to be aromatic, having three planar delocalised benzenoid rings, together with seven isolated double bonds. Their location is most easily seen from the Schlegel diagram (Fig. 3), which also gives the numbering. The view from the end caps (Fig. 2) shows a ‘triangular’ shape imparted by the presence of the three planar hexagons. Two of the benzenoid rings (Ar) are equivalent, whilst the third (Ar*) is unique and sits on the (non-crystallographic) C2 axis. The double bonds comprise three equivalent pairs (C4–5/C40–41; C12–13/C68–69; C15–16/C43–60), and the unique C20–21 straddling the C2 axis. A unique structural feature is the presence of two equatorial fluorines; equatorial addends on [70]fullerene have never been seen before.
 | ||
Fig. 1 ORTEP drawing (20% ellipsoids) for C2 C70F38, viewed down the C2 axis; the facing planar central hexagon is Ar* and the rear central C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond is C20–C21 (see Fig. 3). C bond is C20–C21 (see Fig. 3). | ||
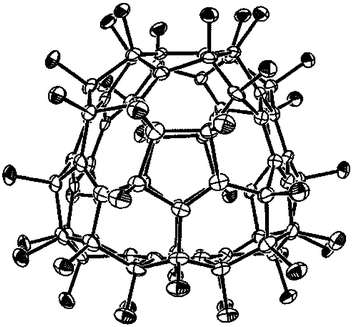 | ||
| Fig. 2 ORTEP drawing (20% ellipsoids) for C2 C70F38 viewed from an end cap, showing the ‘triangular’ shape imparted by the planar regions. | ||
 | ||
| Fig. 3 Schlegel diagram for C2 C70F38 showing the aromatic rings and the isolated double bonds (• = F). The C2 axis bisects the C20–C21 bond and passes through the centre of the aromatic ring Ar*. | ||
The bond lengths (Table 1) are the average values for symmetry equivalent bonds given in terms of the lowest locant numbers. The average bond lengths (Å) in the three aromatic rings are 1.381(5), 1.384(5) and 1.382(5), confirming their aromaticity, cf. the values found in C1 C60F36
(1.371, 1.378, 1.389),11T C60F3612 and C60F1813
(both 1.373). The C15![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C16/C43
C16/C43![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C60 double bonds are exceptionally short (1.313 Å average), attributed to compression produced by the neighbouring fluorines (cf. the short isolated double bonds in both C1 C60F3611 and C60F48).14 As with C1 C60F36 there is an exceptionally long FC–CF bond of 1.642 Å
(27,28; 52,66), which arises as in the previous example, where a carbon is surrounded by three CF groups. The planarity of the benzenoid rings is shown by the valence angle sums for the aromatic carbons, which average 359.0° for Ar/Ar and 359.6° for Ar*.
C60 double bonds are exceptionally short (1.313 Å average), attributed to compression produced by the neighbouring fluorines (cf. the short isolated double bonds in both C1 C60F3611 and C60F48).14 As with C1 C60F36 there is an exceptionally long FC–CF bond of 1.642 Å
(27,28; 52,66), which arises as in the previous example, where a carbon is surrounded by three CF groups. The planarity of the benzenoid rings is shown by the valence angle sums for the aromatic carbons, which average 359.0° for Ar/Ar and 359.6° for Ar*.
| Bond | Bond | Bond | |||
|---|---|---|---|---|---|
| a These are the average values for symmetry equivalent bonds, and given as lowest locants; esd values are all 0.013 ± 0.001 Å. | |||||
| C1–2 | 1.592 | C10–C26 | 1.377 | C25–C44 | 1.499 |
| C1–6 | 1.580 | C11–C12 | 1.507 | C26–C27 | 1.505 |
| C1–9 | 1.498 | C11–C28 | 1.591 | C27–C28 | 1.642 |
| C2–3 | 1.555 | C12–C13 | 1.328 | C27–C46 | 1.547 |
| C2–12 | 1.543 | C13–C14 | 1.498 | C28–C29 | 1.536 |
| C3–4 | 1.509 | C13–C30 | 1.478 | C29–C30 | 1.585 |
| C3–14 | 1.568 | C14–C15 | 1.501 | C29–C48 | 1.481 |
| C4–5 | 1.343 | C15–C16 | 1.313 | C30–C31 | 1.565 |
| C4–17 | 1.498 | C15–C32 | 1.508 | C31–C32 | 1.557 |
| C5–6 | 1.470 | C16–C17 | 1.492 | C31–C49 | 1.496 |
| C5–19 | 1.498 | C16–C34 | 1.480 | C32–C33 | 1.615 |
| C6–7 | 1.574 | C17–C18 | 1.552 | C33–C34 | 1.587 |
| C7–8 | 1.508 | C18–C19 | 1.578 | C33–C51 | 1.601 |
| C7–21 | 1.500 | C18–C36 | 1.510 | C46–C47 | 1.481 |
| C8–9 | 1.378 | C19–C20 | 1.546 | C47–C48 | 1.382 |
| C8–24 | 1.398 | C20–C21 | 1.335 | C47–C63 | 1.375 |
| C9–10 | 1.355 | C24–C25 | 1.392 | C48–C49 | 1.391 |
| C10–11 | 1.478 | C25–C26 | 1.395 | ||
Clare and Kepert proposed nineteen possible structures for C70F38 based on AM1 calculations, five of which had three aromatic rings and seven isolated double bonds,9 though none correspond to the characterised structure. Previously, we isolated seven other isomers of C70F38 (all C1) and it seems probable that these arise merely from 1,3-migrations of fluorines giving alternative locations of the double bonds (there are fourteen possibilities), producing C1 structures. We hope to investigate this aspect further. We have calculated (AM1) the heat of formation of C2 C70F38 and find it to be ca. 24 and 19 kcal mol−1 lower than the values for the most stable structures (nos. 1 and 2, respectively) in Table 4 of the Clare and Kepert paper.
The other highly fluorinated species C70F34/36 and C40/50 are probably also aromatic, with more or fewer double bonds respectively, but retaining the locations of the benzenoid hexagons. The present structure thus presents a basis for extensive further calculations.
The 19F NMR spectrum that we obtained previously for this isomer showed nineteen lines (confirmed here), two of which were upfield multiplets at δ −164.59 and −168.19. Our previous work with fluorinated fullerenes showed that upfield multiplets arise from fluorines attached to carbons having three CF neighbours, and are therefore assigned to fluorines at C28/C66, and C33/C45, which are c- and d-type15 carbons, respectively. Since c-type carbons appear more downfield, we provisionally assign the δ −164.59 peak to F28/66. We hope to be able to assign all of the NMR peaks, and hence establish the peak positions of fluorines in specific locations in C70Fn, and thereby solve all of the other structures.
Lastly, C2 C70F38 does not contain the motif present in C70Cl10etc., suggesting that addition commences at the end caps, though isolation of intermediate structures would be necessary to support this conjecture.
Notes and references
- R. Taylor, Lecture Notes on Fullerene Chemistry: A Handbook for Chemists, Imperial College Press, London, 1999, ch. 4, 7, 9, 11 Search PubMed.
- Leading references are collated in: R. Taylor, J. Fluorine Chem., 2004, 125, 359 Search PubMed.
- T. Akasaka, E. Mitsihida, W. Ando, K. Kobayashi and S. Nagau, J. Am. Chem. Soc., 1994, 116, 2627 CrossRef CAS.
- A. D. Darwish, A. K. Abdul-Sada, G. J. Langley, H. W. Kroto, R. Taylor and D. R. M. Walton, J. Chem. Soc., Perkin Trans. 2, 1995, 2359 RSC.
- R. Taylor, A. K. Abdul-Sada, O. V. Boltalina and J. M. Street, J. Chem. Soc., Perkin Trans. 2, 2000, 1013 RSC.
- R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1994, 2497 RSC.
- M. Gerst, H.-D. Beckhaus, C. Rüchardt, E. E. B. Campbell and R. Tellgman, Tetrahedron Lett., 1993, 34, 7729 CrossRef CAS; L. D. Book and G. E. Scuseria, J. Phys. Chem., 1994, 98, 4283 CrossRef CAS.
- P. W. Fowler, J. P. B. Sandall, S. J. Austin, D. E. Manolopoulos, P. D. M. Lawrenson and J. M. Smallwood, Synth. Met., 1006, 77, 97 Search PubMed; P. W. Fowler, J. P. B. Sandall and S. J. Austin, Fullerene Sci. Technol., 1996, 4, 369 CAS; P. W. Fowler, J. P. B. Sandall and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1997, 419 RSC.
- B. W. Clare and D. E. Kepert, J. Mol. Struct.: THEOCHEM, 2002, 583, 19–45 CrossRef CAS.
- P. A. Troshin, O. V. Boltalina, N. V. Polyakova and Z. E. Klinkina, J. Fluorine Chem., 2001, 110, 157 CrossRef CAS.
- A. G. Avent, B. W. Clare, P. B. Hitchcock, D. L. Kepert and R. Taylor, Chem. Commun., 2002, 2370 RSC.
- P. B. Hitchcock and R. Taylor, Chem. Commun., 2002, 2078 RSC.
- I. S. Neretin, K. A. Lyssenko, M. Yu. Antipin, Yu. L. Slovokhotov, O. V. Boltalina, P. A. Troshin, A. Yu. Lukonin, L. N. Sidorov and R. Taylor, Angew. Chem., Int. Ed., 2000, 39, 3273 CrossRef CAS.
- S. I. Troyanov, P. A. Troshin, O. V. Boltalina, I. N. Ioffe, L. N. Sidorov and E. Kemnitz, Angew. Chem., Int. Ed., 2001, 40, 2285 CrossRef CAS.
- R. Taylor, J. P. Hare, A. K. Abdul-Sada and H. W. Kroto, J. Chem. Soc., Chem. Commun., 1990, 1423 RSC.
Footnote |
† Crystal data: C70F38·2.5(C7H8), M
= 1793.0, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a
= 12.1508(6), b
= 15.5160(7), c
= 18.0125(8)
Å, α
= 94.308(3), β
= 101.526(2), γ
= 106.457(2)°, V
= 3159.6 Å3, Z
= 2, Dx
= 1.89 Mg m−3, μ(Mo-Kα)
= 0.19 mm−1, T
= 173 K. Diffraction from the crystal was weak and limited. 11057 unique reflections (Rint
= 0.13) measured on a KappaCCD diffractometer. Three disordered toluene solvate molecules included with restrained geometry. Refinement on F2 using SHELX97, R1 = 0.110 for 5490 reflections with I > 2σ(I), wR2 = 0.324 for all reflections. CCDC 247743. See http://www.rsc.org/suppdata/cc/b4/b412599a/ for crystallographic data in .cif or other electronic format.
(no. 2), a
= 12.1508(6), b
= 15.5160(7), c
= 18.0125(8)
Å, α
= 94.308(3), β
= 101.526(2), γ
= 106.457(2)°, V
= 3159.6 Å3, Z
= 2, Dx
= 1.89 Mg m−3, μ(Mo-Kα)
= 0.19 mm−1, T
= 173 K. Diffraction from the crystal was weak and limited. 11057 unique reflections (Rint
= 0.13) measured on a KappaCCD diffractometer. Three disordered toluene solvate molecules included with restrained geometry. Refinement on F2 using SHELX97, R1 = 0.110 for 5490 reflections with I > 2σ(I), wR2 = 0.324 for all reflections. CCDC 247743. See http://www.rsc.org/suppdata/cc/b4/b412599a/ for crystallographic data in .cif or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |
