A novel method for hydrogen production from liquid ethanol/water at room temperature
Yasushi
Sekine
*a,
Kohei
Urasaki
a,
Shinjiro
Asai
a,
Masahiko
Matsukata
a,
Eiichi
Kikuchi
a and
Shigeru
Kado
b
aDepartment of Applied Chemistry, School of Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku, Tokyo, 169-8555, Japan. E-mail: ysekine@waseda.jp; Fax: +81-3-5286-3114; Tel: +81-3-5286-3114
bDepartment of Mechanical Engineering and Science, Tokyo Institute of Technology, 2-12-1 O-okayama, Meguro, Tokyo, 152-8555, Japan
First published on 19th November 2004
Abstract
We demonstrated that non-catalytic ethanol steam reforming proceeds efficiently and selectively without coking at the conditions of room temperature and atmospheric pressure using low-energy pulsed (LEP) discharge in combination with carbon fiber electrodes.
Proton exchange membrane fuel cell (PEMFC) technology has attracted much attention recently because of its high energy efficiency and clean exhaust gases. Because of its small size, light weight, fast start-up, and rapid response, PEMFC is suitable for transportation applications or small-scale power generation. Various methods to produce and supply hydrogen, which powers PEMFC, have been proposed. Above all, hydrogen production by use of steam reforming of ethanol is a very attractive way because ethanol can be produced from various renewable sources. Unfortunately it is difficult to utilize the catalytic ethanol steam reforming process for a hydrogen production system supplying to PEMFC, which combines such features as high energy efficiency, small size, simplicity, low cost, quick start-up and shut down, and long term durability. In addition, we considered that utilization of this novel method by LEP discharge1–6 for steam reforming of ethanol offers some attractive advantages compared with a conventional catalytic reforming system. Our novel system requires no external heat supply and heat exchanger, so quick start-up, miniaturization and simplification of the hydrogen production system are expected. Moreover all problems caused by deactivation and coking of ethanol steam reforming catalyst can be prevented.
Our proposed system for steam reforming of ethanol uses LEP discharge and a carbon fiber electrode. LEP discharge is a non-equilibrium plasma. When exposed to discharge, a large amount of electrons can be irradiated in a very short time, less than 1 µs, at constant intervals of about several milliseconds. Reactant molecules can be decomposed by electron impact. Because of the extremely short irradiation time, discharge can be kept stable under atmospheric pressure and the supplied energy can be held down as compared with thermal plasmas. Moreover, the gas phase temperature does not increase despite very high electron temperature; thereby, useless side-reactions can be suppressed. This method has been utilized effectively for acetylene synthesis, dry reforming, and other applications.
Fig. 1 shows the system setup for this novel reforming system. A DC power supply (HARb-40R30; Matsusada Precision, Inc.) was used to produce the non-equilibrium pulsed discharge. All products were analyzed using gas chromatography with TCD and FID (GC14-B; Shimadzu Corp.), and GC-MS (QP1100EX; Shimadzu Corp.). The waveforms of current and voltage were observed by digital oscilloscope (LeCroy Corp.). A stainless steel rod was used as a cathode and a bundle of carbon fibers was used as an anode. The bottom of the carbon fiber electrode was immersed in a tank filled with liquid ethanol aqueous solution. This mixture was pumped up to the top of the bundle of carbon fibers by capillary force, so the fuel is supplied automatically into the discharge region. Thereby, the reaction can be performed at room temperature. No heater is required to vaporize the reactant molecules or any pump to supply liquid into the reactor. The amount of liquid pumped by the carbon fibers is controllable by changing the length or number of carbon fibers. Use of more numerous or shorter fibers would increase the amount of liquid pumped up into the discharge region. Using 84,000 (ca. 4-mm diameter) 7-cm long carbon fibers, the amount of fuel supplied into the discharge region was about 1.5 mmol min−1.
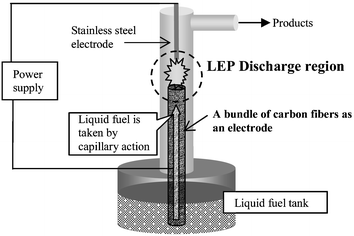 | ||
| Fig. 1 Schematic image of novel reformer with LEP discharge and carbon fibers. | ||
Steam reforming of ethanol by means of LEP discharge in combination with a carbon fiber electrode was performed under various output energies of the power supply and various ethanol concentrations. Fig. 2 shows those results. In any output energy of the power supply, steam reforming of ethanol proceeded and hydrogen was obtained as a main product. Both the hydrogen formation rate and ethanol consumption rate increased with an increase in output energy of the power supply. Carbon deposition or wax formation was negligible; also, the discharge state was kept stable for a long time. The carbon fiber properties and its ability for capillary action were also stable. Gases such as carbon monoxide, carbon dioxide, C2 compounds (mainly acetylene) and methane were produced in addition to hydrogen. The selectivity to products was not influenced by output energy of the power supply. On the other hand, the selectivity to products was changed dramatically in the case of changing the mixing ratio of ethanol to water. With an increase in ethanol concentration, the selectivity to C2 compounds increased and the selectivity to carbon dioxide decreased. On the other hand, the selectivity to methane was not influenced greatly. The selectivity to methane did not exceed 20%. Slight carbon deposition was observed when the ethanol concentration was 100%. The formation of C2 compounds and methane decreased the amount of hydrogen obtained. However, the selectivities to methane and C2 compounds were relatively small except in the case of high ethanol concentration. Therefore, the influence of formation of methane and C2 compounds on hydrogen formation was very small and the selectivity to hydrogen was high, more than 80%. Moreover, the selectivity to C2 compounds was controllable by changing the concentration of ethanol and water as shown in Fig. 2. The selectivities to products were almost identical in both a previous vapor phase reactor system2 and this novel system. We consider that the same reaction proceeded at the discharge region in both reactors. Therefore, the discharge energy loss in the case of the vapor phase reactor was utilized partly to vaporize the ethanol and water in the case of the liquid phase reactor.
 | ||
| Fig. 2 Ethanol concentration and selectivity to products; carbon based, ambient temperature, atmospheric pressure. | ||
The amount of hydrogen produced was almost identical in both the vapor phase reactor and liquid phase reactor, as shown in Fig. 3. Using LEP discharge in combination with a carbon fiber electrode, we improved the energy efficiency because the liquid phase reactor does not require a heater, which is typically necessary to vaporize the mixture of water and ethanol and prevent their condensation. Moreover, no pump is required to transport the liquid into the reactor. Taking account of the fact that no heating furnace was required, we consider that the process scale can be reduced drastically. The liquid phase reactor has another merit. The carbon fiber electrode pumps up the liquid by capillary force because of the concentration gradient from the bottom to the top of the bundle of carbon fibers. Therefore, the supplying rate of liquid pumped up by the carbon fiber electrode equals the rate of ethanol consumption by the discharge. The outlet gas does not include unreacted ethanol, which is usually included except for a 100% conversion rate, because the excess amount of liquid is not supplied in the case of a vapor phase reactor.
 | ||
| Fig. 3 Energy consumption and ethanol conversion, H2 formation: open symbols: novel system, solid symbols: former conventional LEP reformer. | ||
The energy consumption and energy efficiency in this novel system are shown in Table 1. Energy efficiency was calculated by Eqn. 1
| Energy efficiency = (Σ Eoutput/Σ Einput)*100 | (1) |
| Formula |
Formation/Consumption
[mmol/min] |
Q
LHV
[MJ/mol] |
E
LHV
[kJ/min] |
|---|---|---|---|
| C2H5OH | 0.10 | 1.36 | 0.129 |
| H2O | 0.00 | 0.000 | |
| Electricity | (0.25 W) | 0.015 | |
| E input | 0.144 |
Its energy efficiency reached up to 89% at LHV (Lower Heating Value) based calculation.
In summary, the LEP discharge in combination with a carbon fiber electrode has various excellent features from the point of view of energy efficiency, saving the process scale and so on, in comparison to the vapor phase reactor. For that reason, we consider that this process would be very useful for a room temperature hydrogen formation system.
Nomenclature
Q LHV; Calorific value per unit based on LHV [MJ/mol]
E LHV; Calorific value based on LHV [kJ/min]
E input/Eoutput; Summation of ELHV [kJ/min]
Notes and references
- S. Kado, K. Urasaki, Y. Sekine and K. Fujimoto, Chem. Commun., 2001, 415–416 RSC.
- Y. Sekine, K. Urasaki, S. Asai, M. Matsukata, E. Kikuchi and S. Kado, Energy Fuels, 2004, 18(2), 455–459 CrossRef CAS.
- S. Kado, K. Urasaki, Y. Sekine, K. Fujimoto, T. Nozaki and K. Okazaki, Fuel, 2003, 82, 2291–2297 CrossRef CAS.
- S. Kado, K. Urasaki, H. Nakagawa, K. Miura and Y. Sekine, ACS Symp. Ser., 2003, 852, 303–313.
- S. Kado, Y. Sekine, T. Nozaki and K. Okazaki, Catal. Today, 2004, 89(1–2), 47–55 CrossRef CAS.
- S. Kado, Y. Sekine, K. Urasaki, K. Okazaki and T. Nozaki, Stud. Surf. Sci. Catal., 2004, 147, 577–582 CAS.
| This journal is © The Royal Society of Chemistry 2005 |
