Synthesis and structural characterization of highly chlorinated C70, C70Cl28
Sergey I.
Troyanov
*a,
Natalia B.
Shustova
a,
Ilya N.
Ioffe
a,
Andrew P.
Turnbull
b and
Erhard
Kemnitz
c
aChemistry Department, Moscow State University, Leninskie Gory, 119992, Moscow, Russia. E-mail: sergej.troyanov@rz.hu-berlin.de; Fax: +49 030 20937277; Tel: +49 030 20937429
bProtein Structure Factory, c/o BESSY GmbH, Albert-Einstein-Str. 15, 12489, Berlin, Germany
cInstitute of Chemistry, Humboldt University Berlin, Brook-Taylor-Str. 2, 12489, Berlin, Germany
First published on 19th November 2004
Abstract
Chlorination of [70]fullerene with SbCl5, VCl4 or PCl5 yielded C70Cl28 comprising three isomers, all containing four isolated benzenoid rings in the fullerene cage. This demonstrates, for the first time for C70 derivatives, a stabilization effect due to planar aromaticity.
To date, halogenated derivatives of C70 remain much less studied than those of C60. Fluorination of C70 yielded complex mixtures of highly fluorinated products; HPLC separation and subsequent characterization by means of 19F NMR, mass spectrometry and IR spectroscopy revealed the presence of several C70Fx fluorides with x ranging from 34 to 44.1 C70 bromination in organic solvents was previously supposed to yield C70Br14;2 however, a more recent study demonstrated that the only product of bromination of C70 in o-C6H4Cl2 or liquid bromine is C70Br10.3 The only known C70 chloride, C70Cl10, was synthesized by the reaction between C70 and ICl in benzene. 13C NMR data4 suggested that its molecular structure comprises a belt of chlorine atoms which splits the π-system into two isolated halves, and this arrangement was later confirmed in the structural study of C70Ph10 obtained via substitution of chlorine atoms with phenyl or methyl groups.5
Recently, we suggested a novel class of reagents for deep chlorination of fullerenes: higher chlorides of the variable valency elements.6 The most promising results were obtained using SbCl5 and VCl4, which allow the selective synthesis of Th-C60Cl24 with high yield and isomeric purity, as proven on the basis of a comparison between the experimental and calculated IR spectra. Having been applied to the chlorination of C70, these chlorinating agents were found to lead to the formation of a compound with the molecular formula C70Cl28, as preliminarily suggested on the basis of the elemental analysis data.6 In the work presented here we describe the preparation of C70Cl28 and its characterization by means of X-ray single crystal diffraction studies, IR spectroscopy and quantum-chemical calculations.
C70Cl28 samples with reproducible IR spectra (KBr pellet) and elemental composition as determined by chemical analysis (Cl : C = 27.8–28.1), were prepared in sealed glass ampoules by reacting C70 with excess liquid VCl4 (140–160 °C, 7 days), SbCl5 (200 °C, 1 day) or PCl5 (180–200 °C, 2 days). Alternatively, a similar result can be achieved by chlorination of C70Br10 with SbCl5 at 120–140 °C for 7 days. Due to the high internal pressure when heated (ca. 6–10 atm), ampoules with an inner diameter of no more than 5–7 mm were used, each of which was placed into a metallic tube for safety reasons. Removal of the inorganic components of the reaction mixture was carried out by sublimation in vacuo (SbCl5, SbCl3, PCl5, PCl3) or by washing off with 15% HCl followed by drying the product in vacuo over P2O5. This procedure yielded a yellowish-brown polycrystalline powder of C70Cl28 with low solubility in organic solvents. The synthesized compound was found to be stable when exposed to air at room temperature. According to the thermal analysis data, its decomposition into C70 and gaseous Cl2 in an inert atmosphere occurs in the temperature range 320–380 °C. A typical IR spectrum of C70Cl28 is presented in Fig. 1. The most prominent absorption bands are observed in the region of deformational carbon cage vibrations and C–Cl stretching vibrations at 413, 431, 453, 470, 592, 724, 782, 814, 839, 847, 886, 915, 948, 1105, and 1161 cm−1.
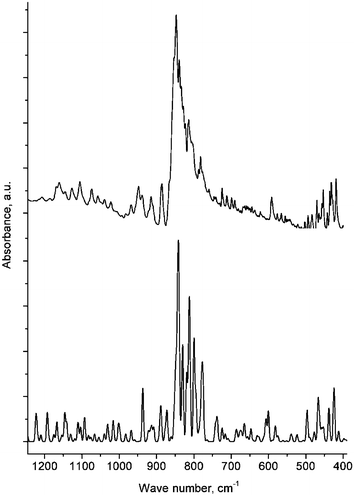 | ||
| Fig. 1 Experimental (above) and calculated (below) IR spectra of C70Cl28. | ||
Previously, it had been theoretically predicted that the attachment of 6–12 bulky X atoms to the C70 cage should occur around the equator, leading to the predominant formation of 1,4-pairs.7 The known C70X10 structures,3,4 which have nine 1,4-contacts and one 1,2-contact between the X groups in the equatorial X10 belt, constitute a good example of such trends. However, our DFT calculations of the relative stability of various possible C70Clx isomers (x ≥ 24) have demonstrated that the principles governing the addition patterns for highly chlorinated molecules are quite different.† It appeared that the main factor affecting stability was the number of isolated benzenoid cycles in the molecule. The stabilizing effects of formation of such aromatic fragments were found to surpass the negative steric effects of the 1,2-contacts of chlorine atoms except for the cases where some chlorine atoms have three such contacts. The maximal number of benzenoid rings of a closed shell derivative of C70, provided all the addends have no more than two 1,2-contacts with the others, is four, and their location is unique. As a result, among the numerous isomers of C70Cl28 considered in the calculations, those containing four benzenoid rings were found to be 80–100 kJ mol−1 more stable than the structures having only three rings, and around 200 kJ mol−1 more stable than the isomers with a minimal number of 1,2-contacts and no aromatic fragments. Here we see an obvious analogy with the addition patterns observed in some highly fluorinated fullerene molecules such as C60F36 (T isomer)8 and C74F38,9 though our calculations predict the benzenoid cycles in C70Cl28 to be less planar, i.e. less aromatic.
An X-ray single crystal diffraction study was carried out for the C70Cl28·1.76Br2 crystals grown from liquid bromine.‡ The experimentally determined structure of the C70Cl28 molecule has crystallographically imposed C2 symmetry which most likely originates from the superposition of two orientations of one isomer having C1 symmetry (Fig. 2a) and two isomers possessing C2 symmetry. (Fig. 2b and 2c). All three isomers contain four benzenoid rings with their addition patterns exhibiting minor differences, which relate to positions labeled as “1”, “2”, and “3”. The fractional experimental occupancies of these positions, namely 0.50 for 1, 0.17 for 2, and 0.33 for 3, can be accounted for by the statistically disordered distribution of isomers a (0.33), a′ (0.33), b (0.17), and c (0.17) in the crystal structure. The experimentally determined molecular structure with the location of the Cl atoms corresponding to isomer b is presented in Fig. 3. DFT calculations demonstrate that isomer a is approximately 9 kJ mol−1 more stable than isomer c and about 40 kJ mol−1 more stable than isomer b; at the same time, among more than 30 isomers of C70Cl28 containing four benzenoid rings, many possess comparable stability, lying in the range between isomers a and b. This implies that the observed isomers are partially kinetic products, therefore, formation of other isomers is, in principle, possible.
 | ||
| Fig. 2 Schlegel diagrams of experimentally observed isomers of C70Cl28 (a–c) and the possible structurally related isomer of C70Cl30 (d). The numbers 1–3 denote the positions differently occupied in the C70Cl28 isomers. | ||
 | ||
| Fig. 3 Top (left) and side (right) views of the b isomer of C70Cl28. The numbers 1–3 denote the same positions as in Fig. 2. | ||
The aromatic rings (marked with circles in Fig. 2 and with dashes in Fig. 3) reveal observable deviations from planarity in contrast to the analogous cycles in C60F36 or C60F18.8,10 This is most probably due to a lower number of addends and, consequently, sp3 carbons in the adjacent cycles, because the degree of planarity of a conjugated fragment is directly connected with buckling of its surroundings, which originates from the elongation of C–C bonds upon changing hybridization from sp2 to sp3. Generally, C–C bonds can be separated into four major groups according to their nature. These are isolated double or conjugated bonds connecting non-aromatic sp2 carbons (average length 1.38 Å observed/1.38 Å calculated for the isomer b), aromatic bonds (1.39 Å/1.40 Å), sp2–sp3 bonds (1.50 Å/1.51 Å) and finally, considerably elongated sp3–sp3 bonds (1.61 Å/1.62 Å). For comparison, the average length of sp3–sp3 bonds in C60F18 is 1.60 Å.10 The C–Cl bonds (1.82 Å/1.83 Å ) are also elongated compared to the typical value for conventional chlorocarbons (1.77 Å), which suggests that they represent relatively weak bonds. DFT-simulated IR spectra of the isomers a–c were found to be rather similar, especially in the most prominent region near 800 cm−1, and revealed good qualitative agreement with the experimental data (Fig. 1).
The highest degree of chlorination satisfying the “four rings and no triple Cl–Cl contacts” condition, is represented by the single isomer of C70Cl30 (see Fig. 2d). According to our DFT results, the enthalpy of consecutive chlorination with Cl2 remains negative only up to C70Cl28. However, C70Cl30 is still rather stable and use of strong chlorinating agents can make its formation thermodynamically favorable. Further chlorination of C70Cl30, which would result in the destruction of the benzenoid rings and an increase in the Cl–Cl adjacency, is unlikely due to high endothermicity.
INI is thankful to Russian Presidential grant MK-2734.2004.3.
Notes and references
- R. Taylor, A. K. Abdul-Sada, O. V. Boltalina and J. K. Street, J. Chem. Soc., Perkin Trans. 2, 2000, 1013 RSC.
- G. Waidmann and M. Jansen, Z. Anorg. Allg. Chem., 1997, 623, 623 CAS.
- S. I. Troyanov, A. A. Popov, N. I. Denisenko, O. V. Boltalina and E. Kemnitz, Angew. Chem., Int. Ed., 2003, 42, 2395 CrossRef CAS.
- P. R. Birkett, A. G. Avent, A. D. Darwish, H. W. Kroto, R. Taylor and D. R. M. Walton, J. Chem. Soc., Chem. Commun., 1995, 683 RSC.
- A. G. Avent, P. R. Birkett, A. D. Darwish, H. W. Kroto, R. Taylor and D. R. M. Walton, Tetrahedron, 1996, 52, 5235 CrossRef; H. Al-Matar, A. K. Abdul-Sada, A. G. Avent, R. Taylor and X.-W. Wei, J. Chem. Soc., Perkin Trans. 2, 2002, 1251 RSC.
- S. I. Troyanov, N. B. Shustova, A. A. Popov, M. Feist and E. Kemnitz, Zh. Neorg. Khim., 2004, 49, 1413 Search PubMed.
- S. J. Austin, P. W. Fowler, J. P. B. Sandall, P. R. Birkett, A. G. Avent, A. D. Darwish, H. W. Kroto, R. Taylor and D. R. M. Walton, J. Chem. Soc., Perkin Trans. 2, 1995, 1027 RSC; B. W. Clare and D. L. Kepert, J. Mol. Struct.: THEOCHEM, 1999, 491, 249 CrossRef CAS.
- P. B. Hitchcock and R. Taylor, Chem. Commun., 2002, 2078 RSC.
- A. A. Goryunkov, V. Yu. Markov, I. N. Ioffe, R. D. Bolskar, M. D. Diener, I. V. Kuvychko, S. H. Strauss and O. V. Boltalina, Angew. Chem., Int. Ed., 2004, 43, 997 CrossRef CAS.
- I. S. Neretin, K. A. Lyssenko, M. Yu. Antipin, Yu. L. Slovokhotov, O. V. Boltalina, P. A. Troshin, A. Yu. Lukonin, L. N. Sidorov and R. Taylor, Angew. Chem., Int. Ed., 2000, 39, 3273 CrossRef CAS.
Footnotes |
| † The program PRIRODA with the implemented original basis set of TZ2P quality (D. N. Laikov, Chem. Phys. Lett., 1997, 281, 151) and the PBE exchange–correlation functional (J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865) were used. |
| ‡ Crystal data: C70Cl28·1.76Br2, monoclinic, P2/n, a = 13.003(1) Å, b = 17.362(1) Å, c = 15.020(1) Å, β = 102.458(3)°, V = 3311.0(4) Å3, Dc = 2.12 g cm−3, Z = 2, T = 100 K. Data collection on a MAR345 image plate using synchrotron radiation at the BESSY storage ring (λ = 0.9184 Å), PSF BL 14.2 of the Free University of Berlin, Germany. One Cl atom was found to be disordered over three positions with site occupancies of 0.50, 0.33, and 0.17. Some small peaks assigned as additional Cl atoms with partial (0.12) occupancies originated from a complex overlap of three molecules with different shapes. The positions of two solvate Br2 molecules are also partially occupied. Anisotropic refinement with 5425 reflections and 527 parameters yielded a conventional R1 (F) = 0.086 for 3960 reflections with I > 2σ(I) and wR2 (F2) = 0.259 for all reflections. CCDC 247934. See http://www.rsc.org/suppdata/cc/b4/b412448k/ for crystallographic data in .cif or other electronic format. |
| This journal is © The Royal Society of Chemistry 2005 |
