One-step synthesis of alkoxyamines for nitroxide-mediated radical polymerization
Robert B.
Grubbs
*,
Jakub K.
Wegrzyn
and
Qing
Xia
Department of Chemistry and Center for Nanomaterials Research, 6128 Burke Laboratory, Dartmouth College, Hanover, NH, USA. E-mail: Robert.B.Grubbs@Dartmouth.edu; Fax: +1 603 646 3946; Tel: +1 603 646 9096
First published on 29th November 2004
Abstract
An alkoxyamine that is an effective initiator for the controlled polymerization of styrene and isoprene has been prepared by the reaction of 2-methyl-2-nitrosopropane with two equivalents of radicals derived from 1-bromoethylbenzene.
Controlled and living free radical polymerization methodologies have proven to be extremely useful for the preparation of novel polymeric architectures that are unattainable through other living polymerization techniques. Atom transfer radical polymerization (ATRP) and related metal-mediated polymerization methods have found much use because of the commercial availability of effective initiators and catalysts.1,2
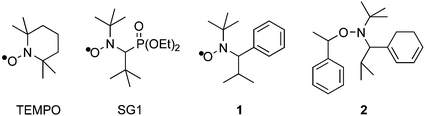
Nitroxide-mediated radical polymerization has also proven to be a powerful technique for control over polymer architecture,3 though its general utility has been somewhat hindered by the limited availability of appropriate nitroxides and alkoxyamines. For example, though rubbery diene blocks are useful components of multi-block copolymer-based materials, successful living radical polymerization of dienes has only been achieved with systems involving nitroxide 1, including those utilizing the unimolecular alkoxyamine initiator 2.4,5
While alkoxyamines that initiate controlled polymerizations are typically prepared either as isolated compounds or in situ from the corresponding nitroxides and carbon-centered radicals, several groups have endeavored to make these useful compounds from other precursors. For example, Bergbreiter's group has described the preparation of allyloxyamines by the Meisenheimer rearrangement of allylamine oxides.6
Other elegant and intriguing strategies for one-pot formation of active alkoxyamines from nitric oxide precursors and standard radical initiators have led to some successes in controlled radical polymerization of standard monomers, though due to the proven sensitivity of nitroxide-mediated polymerization to nitroxide structure, the polymers prepared from these one step methods typically show broader molecular weight distributions than those prepared in the presence of more typically used nitroxides such as TEMPO, SG1 (or DEPN),7 and 2,2,5-trimethyl-4-phenyl-3-azahexane-3-nitroxide (1).8–11
Building upon this prior work, and noting the approximate structural symmetry in α-hydridoalkoxyamines such as 2, where benzylic carbons are attached to both the nitrogen and oxygen atoms of the alkoxyamine, we have sought to prepare related alkoxyamines such as 3 by the double addition12,13 of α-alkylbenzyl radicals to 2-methyl-2-nitrosopropane, eqn. (1).
 | (1) |
In order to prepare alkoxyamine 3 as an analog of alkoxyamine 2, 2-methyl-2-nitrosopropane (t-BuNO) must react with two equivalents of 1-phenylethyl radical. 1-Bromoethylbenzene in the presence of the Cu(I)Br/Cu(0)/PMDETA catalytic system was used as a convenient source of the necessary radicals in a reasonable concentration at room temperature. Copper(0) serves to regenerate active Cu(I) species by disproportionation with the Cu(II) species formed upon abstraction of bromine from 1-bromoethylbenzene; similar catalyst systems have found use in ATRP with 1-haloethylbenzenes as initiators.1,2tBuNO and 1-bromoethylbenzene (2.2 equivalents) were dissolved in toluene under N2 and treated with Cu(I)Br, Cu(0), and PMDETA (0.25 : 1 : 0.25 equivalents relative to 1-bromoethylbenzene) (Scheme 1). Loss of 1-bromoethylbenzene from the reaction mixture was monitored by following the decrease in intensity of the peak corresponding to the benzylic proton at δ 5.23 and the corresponding appearance of the peak corresponding to the benzylic peak in the product at δ 4.63 in the crude 1H NMR mixture.
 | ||
| Scheme 1 | ||
After 51 h at rt, the heterogeneous reaction mixture was flushed through basic alumina to remove a majority of the residual catalyst, then purified by flash chromatography (9 : 1 hexanes–dichloromethane) to give 3 as a 1.2 : 1 mixture of diastereomers in 68% yield.†
Alkoxyamine 3 can be used to control the polymerization of styrene. For example, heating 3 and styrene (200 equivalents) at 125 °C under N2 for 19 h (Conversion (1H NMR) = 90%) led to polystyrene with a molecular weight close to that predicted based upon conversion (Mn,SEC = 17.1 kg mol−1; Mn,calc = 19.1 kg mol−1) and a narrow molecular weight distribution (Mw/Mn = 1.14).
Most interestingly, alkoxyamine 3 has also proven effective for the controlled polymerization of isoprene (Table 1). For example, alkoxyamine 3 and isoprene (200 equivalents) were heated under N2 at 125 °C for 16 h to give polyisoprene (Conversion (gravimetry) = 25%; SEC: Mn = 5 kg mol−1, Mw/Mn = 1.28) with the narrow molecular weight distribution typical of a nitroxide-mediated polymerization and a microstructure (90% 1,4-addition; determined by 1H NMR) typical for free radical polymerization.‡
| [I]/[3] | Conv.b (%) | M n (theor.)c/kg mol−1 | M n (SEC)d/kg mol−1 | M w/Mnd | |
|---|---|---|---|---|---|
| a All polymerizations run at 125 °C for 16 h under a N2 atmosphere. b Conversion determined gravimetrically. c Calculated based upon conversion. d Determined by SEC vs. polystyrene standards. | |||||
| a | 200 | 25 | 3.72 | 5.0 | 1.28 |
| b | 400 | 21 | 5.98 | 9.4 | 1.26 |
| c | 500 | 16 | 5.74 | 8.9 | 1.27 |
Nitroxide-mediated polymerization of acrylates is generally well-controlled only in the presence of a small amount of excess nitroxide.4,5 Studies of acrylate polymerization with 3 were hampered by the apparent instability of nitroxide 4, which was prepared by the addition of methylmagnesium bromide to N-tert-butyl α-phenylnitrone,4,5 but appeared to decompose (evident by a color change from orange to green) over several days at 0 °C (Scheme 2). Further studies will be necessary, perhaps with a more stable nitroxide as the free nitroxide, in order to elucidate the applicability of alkoxyamine 3 to the polymerization of acrylates, acrylamides, and related monomers.
 | ||
| Scheme 2 | ||
We are currently carrying out more detailed studies into polymerization kinetics and the feasibility of block copolymer preparation with this initiation system. It is our hope that the relative ease with which these useful alkoxyamines can be prepared will facilitate future research into nitroxide-mediated polymerization.
Acknowledgement is made to the NSF (CAREER DMR-0239697) and the Donors of The Petroleum Research Fund, administered by the American Chemical Society, for partial support of this research. R.B.G. thanks the Research Corporation for a Research Initiation Award and 3M for a Non-Tenured Faculty Award.
Notes and references
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS.
- M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689 CrossRef CAS.
- C. J. Hawker, A. W. Bosman and E. Harth, Chem. Rev., 2001, 101, 3661 CrossRef CAS.
- D. Benoit, E. Harth, P. Fox, R. M. Waymouth and C. J. Hawker, Macromolecules, 2000, 33, 363 CrossRef CAS.
- D. Benoit, V. Chaplinski, R. Braslau and C. J. Hawker, J. Am. Chem. Soc., 1999, 121, 3904 CrossRef CAS.
- D. E. Bergbreiter and B. Walchuk, Macromolecules, 1998, 31, 6380 CrossRef CAS.
- S. Grimaldi, J.-P. Finet, F. Le Moigne, A. Zeghdaoui, P. Tordo, D. Benoit, M. Fontanille and Y. Gnanou, Macromolecules, 2000, 33, 1141 CrossRef CAS.
- C. Detrembleur, P. Teyssie and R. Jerome, Macromolecules, 2002, 35, 1611 CrossRef CAS.
- C. Detrembleur, V. Sciannamea, C. Koulic, M. Claes, M. Hoebeke and R. Jerome, Macromolecules, 2002, 35, 7214 CrossRef CAS.
- D. F. Grishin, L. L. Semyonycheva and E. V. Kolyakina, Mendeleev Commun., 1999, 250 CrossRef.
- M.-O. Zink, A. Kramer and P. Nesvadba, Macromolecules, 2000, 33, 8106 CrossRef CAS.
- A. K. Hoffmann, A. M. Feldman, E. Gelblum and W. G. Hodgson, J. Am. Chem. Soc., 1964, 86, 639 CrossRef.
- A. Maschke, B. S. Shapiro and F. W. Lampe, J. Am. Chem. Soc., 1963, 85, 1876 CrossRef CAS.
- A. Studer, K. Harms, C. Knoop, C. Mueller and T. Schulte, Macromolecules, 2004, 37, 27 CrossRef CAS.
Footnotes |
| † 2,2-Dimethyl-3-(1-phenylethoxy)-4-phenyl-3-azapentane (3). In a nitrogen-filled glovebox, 2-nitroso-2-methylpropane dimer (0.18 g, 2.1 mmol equivalent monomer) and (1-bromoethyl)benzene (0.85 g, 4.6 mmol) were dissolved in toluene (4 mL) to give a blue solution. Cu(I)Br (0.164 g, 1.15 mmol), Cu powder (0.292 g, 4.60 mmol), and PMDETA (0.197 g, 1.15 mmol) were added sequentially and the heterogeneous green reaction mixture was allowed to stir for 50 h at rt. The reaction mixture was then removed from the glovebox and filtered through alumina (basic), evaporated to dryness and purified by flash chromatography (SiO2, 9 : 1 hexanes–CH2Cl2) to give alkoxyamine 3 (0.42 g, 68%) as a clear oil (TLC: 4 : 1 hexanes–CH2Cl2, Rf = 0.33). 1H NMR (both diastereomers, CDCl3, 500 MHz) δ 7.70–7.10 (ArH, 10 H), 4.61 (m, overlapping quartets?, CH3CH(Ph)O–, 1 H, J = 6.1 Hz), 4.30 (q, CH3CH(Ph)N–, 1 H, J = 6.6 Hz, minor diastereomer), 4.24 (q, CH3CH(Ph)N–, 1 H, J = 6.6 Hz, major diastereomer), 1.62 (d, CH3–, 3H, J = 6.8 Hz, minor diastereomer), 1.53 (d, CH3–, 3H, J = 6.6 Hz, major diastereomer), 1.30 (s, CH3–, 9H, minor diastereomer), 1.01 (s, CH3–, 9H, major diastereomer). 13C NMR (CDCl3, 125 MHz) δ 148.51, 148.23, 144.32, 144.04, 128.27, 128.15, 128.09, 127.63, 127.35, 127.35, 127.27, 127.07, 126.35, 126.09, 82.06, 80.94, 60.97, 60.80, 59.18, 58.64, 27.46, 27.35, 22.60, 21.82, 19.58, 18.86 ; Anal. calcd. for C20H27NO: C, 80.76; H, 9.15; N, 4.71. Found: C, 80.54; H, 9.56; N, 4.53. |
| ‡ Polymerization procedure. For a typical polymerization, alkoxyamine 3 and monomer (styrene or isoprene) were sealed in a pressure tube under N2. The tube was placed in an oil bath thermostatted at 125 °C for the indicated length of time, then removed from the bath, allowed to cool, and evaporated to dryness. The contents of the tube were massed, purified by precipitation into methanol from dichloromethane, and analyzed by 1H NMR and SEC. |
| This journal is © The Royal Society of Chemistry 2005 |