The enhanced electrochemiluminescence of luminol on the nickel phthalocyanine modified electrode
Jian Wang*a, Guonan Chena and Jinling Huang*b
aDepartment of Chemistry, Fuzhou University, Fuzhou, Fujian 350002, P.R. China. E-mail: jwang@fzu.edu.cn; Fax: 86-591-87892632
bDepartment of Chemistry, Fuzhou University, Fuzhou, Fujian 350002, P.R. China. E-mail: hjl@fzu.edu.cn; Fax: 86-591-83709114
First published on 17th November 2004
Abstract
A glassy carbon electrode (GCE) modified with nickel(II) tetrasulfophthalocyanine (NiTSPc) and Nafion® was used for the investigation of the catalytic oxidation of luminol. The modified electrode was found to much more effectively improve the emission of electrochemiluminescence(ECL) of luminol in a solution containing hydrogen peroxide. The enhanced ECL signal corresponded to the catalytic oxidation of both luminol and H2O2 by NiTSPc. Attached Ni(II) on GCE was oxidised to Ni(III) and then used as the catalyst for the chemiluminescence of luminol. The enhanced stability of the ECL signal with Nafion® would mainly result from the prevention of the dissolution of NiTSPc and the adsorption of the oxidation product of luminol on the electrode surface. The proposed method enables a detection limit for luminal of 6.0 × 10−8 mol L−1 to be achieved in the presence of H2O2 in the neutral solution. The enhanced ECL intensity had a linear relationship with the concentration of luminol in the range of 1.0 × 10−7–8.0 × 10−6 mol L−1
1 Introduction
In recent years, several publications have detailed the rich chemistry of phthalocyanines (Pcs) in diverse areas such as gas sensing, information display, wastewater treatment and catalytic oxidation.1 Numerous methods for preparing Pcs' polymer systems have been described, including simple attachment of Pcs to a polymeric matrix by electrostatic or covalent means, direct synthesis of ring-linked Pc polymers, chemical polymerisation, etc. Metal phthalocyanines polymerized onto carbon or platinum electrodes have shown interesting electrocatalytic properties which can be used for analytical applications. In this respect, phthalocyanine films have been found to be useful for the chemical sensing of some substances, such as N-acetylcysteine and 6-mercaptopurine,2 antioxidants,3 reduced glutathione(GSH),4 chlorophenol,5 nitrite,6 and serotonin.7In this work, we explore the possible usage of the phthalocyanines in the ECL of luminol. It is known that the chemiluminescence of luminol can be catalysted by enzymes, such as peroxidase and catalase, metal ions and organometallic compounds in alkaline solution. NiTSPc polymerized on GCE apparently retains phthalocyanine's macrocycle structure, in which the Ni atom plays a role similar to that in Ni(OH)2.8 Ni(II),which had been reduced by a chemiluminescence process, was oxidized to Ni(III) again as the Ni was attached to the electrode surface. The modified electrode was stable enough for repetitive use and the light emission could also be detected in the neutral solutions with the present system.
2 Experimental
2.1 Reagent
All the reagents were of analytical grade: the water used for the preparation of solutions was doubly distilled. 3-Aminophthalhydrazide (luminol, Aldrich Chem Co.), and nickel(II) phthalocyanine tetrasulfonic acid tetrasodium salt (NiTSPc, Aldrich Chem Co.) were used without further purification. Nafion® (5 wt% aqueous alcoholic solution, obtained from Aldrich) was prepared at 0.1% by dilution in ethanol. A stock solution of 0.20% H2O2 (v/v) was prepared by diluting 5.5 ml of 30% (v/v) H2O2 to 250 ml with water.2.2 Apparatus
The experimental equipment for ECL measurement includes a BPCL Ultra-Weak Chemiluminescence Analyzer controlled by a personal computer with BPCL program (Institute of Biophsics, Academia Sinica, China) and a electrochemical analyzer (CHI660a, Shanghai Chenghua Instrument Co., China).A conventional three-electrode system was used as the electrolytic system, which was composed of a GCE as a working electrode, a platinum wire as the counter electrode and a Ag/AgCl (sat. KCl) electrode as the reference electrode. A commercial 5 ml cylindroid's glass cell was used as an ECL cell. Before each measurement, the working electrode was fixed in the same position and directly faced the window of the photomultiplier tube. The working electrode was pretreated by polishing its surface with aqueous slurries of alumina powders (1.0 µm and 0.3 µm α-Al2O3) on polishing cloth and then carefully washed with water to give a smooth and clean electrode surface. The ECL cell was washed with 0.2 M nitric acid and water in sequence before use.
2.3 Procedure
3 Results and discussion
3.1 Electrode characterization
 | ||
| Fig. 1 Cyclic voltammograms of the electrodeposition of NiTSPc film on a GCE by consective potential cycling in a fresh solution containing 4.0 × 10−3 mol L−1 NiTSPc and 0.20 mol L−1 NaOH in the potential range from −0.2 V to 0.8 V (versus Ag/AgCl) at a scan rate of 100 mV s−1. A = Second 20 cycles, B = fourth 20 cycles, C = sixth 20 cycles. | ||
It is generally agreed that both the attachment of the NiTSPc to the electrode surface and the increase in film thickness take place via the formation of oxo-bridges.9–11 Trevin et al. studied the electropolymerized films of Ni-salen, Ni-porphyrine and Ni-cyclam, and found evidence for the formation of nickel hydroxide-like materials.12 The anodic peak at 0.58 V for GCE has been attributed to the Ni(II)/Ni(III) process,whereas the cathodic peaks at 0.45 V in the negative scan corresponded to the reverse process.
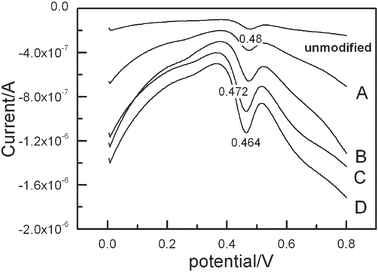 | ||
| Fig. 2 Differential-pulse voltammograms (amplitude 0.05 V, pulse period 0.2 s) on the different polyNiTSPc modified GCE in a borate buffer (pH = 7.4) with luminol and H2O2. The polymers with different thickness obtained by controlling the number of the electropolymerizing scans. A = 20 cycles, B = 40 cycles, C = 60 cycles, D = 80 cycles. | ||
Fig. 3 shows the relationship between the ECL intensity and the number of electropolymerization scans. The light emission on the unmodified GCE was quite small in comparison with NiTSPc modified GCE: the maximum ECL was about 19.4 times of that at the bare electrode. These results indicated that poly[NiTSPc]-GCE effectively enhanced ECL of luminol.
 | ||
| Fig. 3 ECL response of luminol on the different polyNiTSPc modified GCE measured with cyclic voltammetry. The polymers with different thickness obtained by controlling the number of the electropolymerizing scans. A = 20 cycles, B = 40 cycles, C = 60 cycles, D = 80 cycles, E = 100 cycles, F = 120 cycles. | ||
3.2 The effect of Nafion®
Nafion, a cation-exchange polymer, was usually used for modification of the electrode surface. Modification of the electrodes with Nafion® has important advantages in analytical chemistry. Nafion® has cation-exchange properties due to its sulfonated group, so the surface of the modified electrode only allows the transfer of the cations. We found that, when the poly[NiTSPc] modified electrode uncoated with Nafion® was used, the amperometric signals of luminol decreased with repetitive measurements gradually, as shown in Fig. 4. This could be explained as the oxidation products of luminol were adsorbed on the surface of poly[NiTSPc] modified electrode, which would reduce the effective surface and affected the reproducibility of the voltammetric measurements. After coating the poly[NiTSPc] modified electrode with Nafion®, the successive measurements could be performed with slight decrease in reproducibility because of the Nafion® blocking the adsorption sites and inhibit the solution of NiTSPc.![Electrogenerated chemiluminescence of luminol obtained at: (a), an uncoated poly[NiTSPc] GCE; (b), a Nafion® coated poly[NiTSPc] GCE. The number of polymerization scans was 80.](/image/article/2005/AN/b410598b/b410598b-f4.gif) | ||
| Fig. 4 Electrogenerated chemiluminescence of luminol obtained at: (a), an uncoated poly[NiTSPc] GCE; (b), a Nafion® coated poly[NiTSPc] GCE. The number of polymerization scans was 80. | ||
It is very important for a modified electrode to be stable for a prolonged time. It was found in our work that no significant change of response for the Nafion-poly[NiTSPc]-GCE was observed after the electrode was stored in borate buffer for 5 hours. The electrode retained 95% of its initial response. Such stability is acceptable for most practical applications.
In spite of the modification of Nafion®, a little portion of the oxidised products of the luminol may still remain on the electrode surface, reducing the effective surface. So a cleaning method must be optimized to regenerate the surface after each measurement. The best results were obtained using a borate buffer solution of pH 7.4 and applying successive scans (five cycles) between −0.2 and 0.8 V by means of cyclic voltammetry. With this method of regeneration of the polymer surface, the substances were desorbed completely and the electrode had more measurement reproducibility. Then, a series of 10 measurements could be performed without any decrease in reproducibility.
3.3 Analytical characteristics
Concentration of H2O2 in the reagent solution is an important factor influencing signal magnitude. Fig. 5 shows the increase of the signal with the increase of concentration of H2O2. In this paper, the intermediate concentration (5.0 × 10−4 mol L−1) used for luminol determination was selected. | ||
| Fig. 5 Dependence of the concentration of H2O2 on enhanced electrogenerated chemiluminescence intensity. Luminol: 1.0 × 10−5 mol L−1, borate buffer: pH = 7.4. The number of polymerization scans: 80. | ||
The enhanced ECL intensity had a linear relationship with the concentration of luminol in the range of 1.0 × 10−7 to 8.0 × 10−6 mol L−1
(see Fig. 6). The regression equation was
| IECL = 5.32 + 1.47 × 108C (R = 0.9987) |
 | ||
| Fig. 6 Calibration curve for luminol. H2O2: 5.0 × 10−4 mol L−1, borate buffer: pH = 7.4. The number of polymerization scans: 80. | ||
In order to check the precision of the method, ten replicate measurements of the 1.0 × 10−5 mol L−1 luminol solutions were carried out. The relative standard deviation was 3.2%. As a check, the reproducibility was calculated using different modified electrodes. With this in mind, measurements of peak current of ten Nafion-poly[NiTSPc]-GCEs prepared according to the procedure mentioned above were performed using the same solution of 1.0 × 10−5 mol l−1 luminol. A precision value of 3.8%, expressed as RSD, was obtained.
3.4 Mechanism
The mechanisms of the ECL of luminol in the presence of H2O2 in weak alkaline solution have been reported,13,14 and some metal ions and organmetallic compounds have been reported to be able to enhance the chemiluminescence reaction of luminol via a multi-step electrode reaction.15–19 It is well known that the chemiluminescence of luminol is generated from the excited state of 3-aminophthalate (AP*).20,21In this work, the mechanism could be accepted for the electrocatalytic reactions involving NiPc. The reason for the enhancement of ECL of luminal at the NiTSPc modified electrode is the effective generation of luminol−. and HO2−. (HO.) from H2O2. The oxidation of luminol on GCE were considered as follows.
| Ni(II) → Ni(III) + e− | (1) |
| Ni(III)Pc + luminol → Ni(II)Pc + oxidation products of luminol | (2) |
The adsorbed Ni(II) took part in catalytic oxidation of the luminol anion (LH–) and was similar to other metal ions in solutions. The catalyst is regenerated according to eqn. (2). Electrocatalytic activity of NiPc complexes is known to be depenedent on the Ni(III)/Ni(II) couple for many reactions.5,22 When the electrode potential was more positive than the redox potential of Ni(II)/Ni(III) (about 450 mV), the Ni(III) catalyzed the oxidation of luminol: the reduced Ni(II) was reoxidized to Ni(III) on the modified electrode immediately. Then, light emission continued to be observed when the electrode potential was more positive than the redox potential of luminol.
4 Conclusion
It has been shown that the electrocatalytic mediated oxidation of luminol commences at a potential where a catalytic Ni(III)/Ni(II) species is electrogenerated at the GCE modified with NiTSPc. The Nafion-poly[NiTSPc]-GCEs can be used for measurements of luminol at levels higher than 6.0 × 10−8 mol L−1. If luminol based assays are carried out in alkaline solution, the quantum efficiency for generating the chemiluminescent light of luminol is known to be increased with pH and the concentration of H2O2.The reliability and stability of the electrodes offers a good possibility for extending in the future, such as the detection to routine analysis of luminol in more complex samples by coupling the Nafion–poly[NiTSPc]-GCEs system detection with liquid chromatographic systems or for monitoriing capillary eletrophoretic separation of the environmental or clinical samples.
Acknowledgements
The authors are grateful for the financial support received from the National Nature Science Funding of China (20201005 ) and Fuzhou University (2004-XY-06).References
- Phthalocyanines: Properties and Applications, eds. C. C. Leznoff and A. B. P. Lever, VCH Publications, New York, vol. I, 1989, vol. II, 1992, vol. III, 1993, vol. IV, 1996 Search PubMed.
- X. Qi, R. P. Baldwin, H. Li and T. F. Guarr, Electroanalysis, 1991, 3, 119 CAS.
- M. A. Ruiz, M. G. Blázquez and J. M. Pingarrón, Anal. Chim. Acta, 1995, 305, 49 CrossRef CAS.
- S. A. Wring, J. P. Hart and B. J. Birch, Analyst, 1991, 116, 123 RSC.
- M. S. Ureta-Zaňartu, C. Berríos, J. Pavez, J. Zagal, C. Gutiérrez and J. F. Marco, J. Electroanal. Chem., 2003, 553, 147 CrossRef CAS.
- Z. H. Wen and T. F. Kang, Talanta, 2004, 62, 351 CrossRef CAS.
- S. de Irazu, N. Unceta, M. C. Sampedro, M. Aranzazu Goicolea and R. J. Barrio, Analyst, 2001, 126, 495 RSC.
- M. S. Ureta-Zanartu, C. Berros, J. Pavez, J. Zagal, C. Gutierrez and J. F. Marco, J. Electroanal. Chem., 2003, 553, 147 CrossRef CAS.
- G. Roslonek and J. Taraszewska, J. Electroanal. Chem., 1992, 323, 285 CrossRef CAS.
- J. Bukowska, G. Roslonek and J. Taraszewska, J. Electroanal. Chem., 1996, 403, 47 CrossRef CAS.
- J. Desilvestro, D. A. Corrigan and M. J. Weaver, J. Electrochem. Soc., 1988, 135, 885 CAS.
- S. Trevin, F. Bedioui, M. G. Gomez Villegas and C. Bied-Charreton, J. Mater. Chem., 1997, 7, 923 RSC.
- K. E. Haapakka and J. J. Nankare, Anal. Chim. Acta, 1982, 138, 263 CrossRef CAS.
- S. Sakura, Anal. Chim. Acta, 1992, 262, 49 CrossRef CAS.
- Y. Sato, T. Sawaguchi and F. Mizutani, Electrochem. Commun., 2001, 3, 131 CrossRef CAS.
- C. S. Ouyang and C. M. Wang, J. Electroanal. Chem., 1999, 474, 82 CrossRef.
- R. Wilson and D. J. Schiffrin, Anal. Chem., 1996, 68, 1254 CrossRef CAS.
- R. Wilson and D. J. Schiffrin, J. Electroanal. Chem., 1998, 448, 125 CrossRef.
- C. E. Taylor, IV and S. E. Creager, J. Electroanal. Chem., 2000, 485, 114 CrossRef.
- Y. Nosaka, Y. Yamashita and H. Fukuyama, J. Phys. Chem. B, 1997, 101, 5822 CrossRef CAS.
- G. Merenyi, J. Lind, X. Shen and T. E. Eriksen, J. Phys. Chem., 1990, 94, 748 CrossRef CAS.
- T. F. Kang, G. L. Shen and R. Q. Yu, Anal. Chim. Acta, 1997, 356, 245 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2005 |
