One- and two-photon photostability of 9,9-didecyl-2,7-bis(N,N-diphenylamino)fluorene
Kevin D.
Belfield
*a,
Mykhailo V.
Bondar
b,
Olga V.
Przhonska
b and
Katherine J.
Schafer
a
aDepartment of Chemistry and School of Optics/CREOL, University of Central Florida, P.O. Box 162366, Orlando, FL 32816-2366, USA
bInstitute of Physics, Prospect Nauki, 46, Kiev-28, 03028 Kiev, Ukraine
First published on 25th September 2003
Abstract
The quantum yields of the photochemical reactions of 9,9-didecyl-2,7-bis(N,N-diphenylamino)fluorene have been determined in hexane and CH2Cl2 under one-photon (linear) and near-IR two-photon (nonlinear) absorption conditions. The photochemical decomposition proceeds by a first-order reaction and is independent of the type of excitation (one- or two-photon). In hexane solution, the quantum yields of the photoreactions are in the range (2–5) × 10−4 and increase dramatically to 10−2 in CH2Cl2. The predominant mechanisms of the photoreactions and the photoproducts products which result were investigated via UV-visible absorption, fluorescence, and excitation anisotropy spectral methods.
1 Introduction
Photochemical properties of new two-photon absorbing (2PA) organic materials are the subject of great interest due to a number of important applications, such as non-destructive fluorescence imaging,1 two-photon microfabrication,2,3 optical power-limiting devices,4 and photodynamic cancer therapy.5 In this paper, we report an investigation of the photochemical properties of a 2PA fluorene derivative6,7 that possesses strong nonlinear optical absorptivities. Some aspects of the photochemical properties of this class of compounds under low intensity irradiation in acetonitrile have been described previously.8Fluorene and its derivatives were investigated earlier via one-photon excitation as important toxic pollutants in the ecosystem.9,10 The effect of solvent and substituent groups on the photooxidation of fluorene has also been reported.11 Barbas et al. investigated the photochemistry of fluorene at a silica gel/air interface.12 Nonetheless, relatively little is known about the photochemical reactions of these compounds, although it is well recognized that the photostability of organic materials is a major impediment in the development of a number of technologies requiring nonlinear optical organic materials. Herein, we aim to address this for an important two-photon absorbing dye, 9,9-didecyl-2,7-bis(N,N-diphenylamino)fluorene, through comparative photochemical studies under one- and two-photon excitation in order to understand the nature of its photodecomposition and photostability.
2 Experimental
The photochemical properties of the symmetrical fluorene derivative 9,9-didecyl-2,7-bis(N,N-diphenylamino)fluorene (1) were investigated in spectroscopic grade hexane and CH2Cl2 at room temperature under one- and two-photon excitation over a wide concentration range (3 × 10−6 ≤ c/M ≤ 1.3 × 10−3). The structure of 1 is shown in Fig. 1(a). Spectral parameters of 1 and its photoproducts were measured with a Cary 3 UV-visible spectrophotometer (absorption spectra) and Photon Technologies, Inc. (PTI) Quantamaster spectrofluorimeter (fluorescence, excitation, and anisotropy spectra) in 10 mm fluorometric quartz cuvettes for dye concentrations of c ≤ 3 × 10−6 M. | ||
| Fig. 1 (a) Structure of the fluorene derivative 1. (b) Normalized absorption (1, 2), fluorescence (1′, 2′), and anisotropy (3) spectra of 1 in hexane (1, 1′), CH2Cl2 (2, 2′), and silicone oil (3). | ||
One-photon excitation of 1 was accomplished via UV lamp irradiation (UVGL-25; excitation wavelength, λexc ≈ 360 nm with FWHM ≈ 15 nm; irradiation intensity, I ≈ 4 mW cm−2) in 10 × 10 × 35 mm quartz cuvettes. The entire volume of the cuvette was irradiated simultaneously. The quantum yields of the photoreactions under one-photon excitation, Φ, were measured by an absorption method based on the temporal changes in the absorption spectrum under irradiation, and calculated using eqn. 1,13
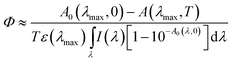 | (1) |
Two-photon excitation of 1 was performed using a Clark-MXR 2001 Ti:Sapphire amplified, second harmonic of an erbium-doped fiber ring oscillator system (output 775 nm) that pumped an optical parametric generator/amplifier (TOPAS, Light Conversion Ltd.), providing a pulse duration of τP ≈ 120 fs (FWHM) at a repetition rate of f ≈ 1 kHz, and tuning range of 560–2100 nm. The quantum yields of the photoreactions under two-photon excitation, Φ2PA, were determined by recording the temporal changes in fluorescence intensity, F(t), of 1 with the PTI Quantamaster spectrofluorimeter. A detailed description of this fluorescence method, with a comprehensive discussion of the experimental set-up, has been reported previously.13,14 In order to measure photochemical parameters by the fluorescence method over a wide range of dye concentrations (3 × 10−6 ≤ c/M ≤ 1.3 × 10−3), microcuvettes ∅1 × 0.03 mm, ∅1 × 0.1 mm and ∅1 × 0.5 mm were used.13 A dye solution was placed in a microcuvette. The microcuvette was then placed into a regular fluorescence cuvette (10 × 10 × 35 mm) containing the corresponding solvent. The entire volume of the microcuvette was irradiated simultaneously at λexc ≈ 720 nm and the average laser power was determined with a Ophir Optronics, Inc. Laserstar powermeter. The absorbance of the solutions in the microcuvette did not exceed 0.1. The quantum yields of the photoreactions under two-photon excitation (Φ2PA) were calculated using this fluorescence method and eqn. 2,13
 | (2) |
 and
and  are the temporal terms determined by the Gaussian shape of the laser pulse, and t is time in seconds. For τP
= 120 fs, α
≈ 9 × 10−14 s and β
≈ 1.28 × 10−13 s.
are the temporal terms determined by the Gaussian shape of the laser pulse, and t is time in seconds. For τP
= 120 fs, α
≈ 9 × 10−14 s and β
≈ 1.28 × 10−13 s.
3 Results and discussion
3.1 Spectral parameters
The UV-visible absorption, corrected fluorescence, and anisotropy spectra of 1 are presented in Fig. 1(b). The absorption maximum is nearly independent of solvent polarity (traces 1 and 2). The fluorescence spectrum exhibits a weak dependence on solvent polarity (traces 1′ and 2′), indicating that 1 undergoes a small change in dipole moment upon excitation. The excitation anisotropy spectrum of 1 in viscous silicone oil (trace 3) reveals the nature of the absorption band. The constant anisotropy in the spectral region λ ≥ 340 nm corresponds to the first electronic transition from the ground, S0, to the first excited, S1, electronic state. The decrease in anisotropy at λ ≈ 300 nm (short wavelength absorption maximum) corresponds to the second electronic transition, S0 → S2 (S2 is the second excited state). A comprehensive spectral investigation of 1 in various solvents has been reported.73.2 One-photon excitation
Time-dependent absorption spectra of 1 under low intensity UV lamp irradiation (UVGL-25, λexc ≈ 360 nm) in hexane and CH2Cl2 are presented in Fig. 2. A decrease in the absorbance at the absorption maximum is accompanied by absorption due to photochemical products over a broad spectral range. For the non-polar solvent hexane [Fig. 2(a)], photodecomposition processes for small changes in absorbance are characterized by a linear dependence, [A(λi,t) − A(λi,0)] ∼ t, at various wavelengths, λi, indicating the direct transformation of 1 into the photoproducts without intermediate photochemical reactions. In contrast, quite complex transformations of the photochemical products were observed in CH2Cl2 [Fig. 2(b)]. For example, a linear dependence, [A(λi,t) − A(λi,0)] ∼ t, was observed in the spectral region in the main absorption band (near λmax), but a nonlinear dependence was found in the long wavelength spectral region (480–490 nm), revealing at least two consecutive photoreactions. The quantum yields of the photoreactions (Φ) were determined using eqn. 1 for different dye concentrations in hexane and CH2Cl2, and are presented in Table 1. The values of Φ are independent of dye concentration (first-order photoreactions), reaching ca. (3.4–4.6) × 10−4 in hexane and increasing to 1.5 × 10−2 in CH2Cl2. | ||
| Fig. 2 Time-dependent absorption spectra for 1 in hexane (a) and CH2Cl2 (b) under UV lamp irradiation (λexc = 360 nm, I ≈ 4 mW cm−2). The dye concentration is 7 × 10−5 M. | ||
| c/M | Hexane | CH2Cl2 | ||||
|---|---|---|---|---|---|---|
| Φ × 104 | Φ d × 104 | Φ 2PA × 104 | Φ × 102 | Φ d × 102 | Φ 2PA × 102 | |
| 1.3 × 10−3 | 3.4 ± 0.8 | — | 3 ± 1.5 | 1 ± 0.2 | — | 1.4 ± 0.7 |
| 3 × 10−4 | 4.6 ± 1.2 | 10 ± 2 | 2 ± 1 | 1 ± 0.3 | 1.3 ± 0.3 | 0.8 ± 0.4 |
| 8 × 10−5 | 4 ± 1 | 15 ± 5 | 2.2 ± 1 | 1 ± 0.3 | 1 ± 0.2 | 1.7 ± 0.8 |
| 2 × 10−5 | 3.4 ± 0.8 | 6 ± 1.5 | 2 ± 1 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1 ± 0.5 |
| 3 × 10−6 | 3.5 ± 0.8 | 5 ± 1 | 2.3 ± 1 | 1.5 ± 0.5 | 1.5 ± 0.5 | 0.7 ± 0.3 |
The fluorescence and excitation spectra of the photochemical products of 1 were measured after low intensity irradiation in order to understand the nature of the photoreactions and to determine the main possible mechanisms. Excitation spectra for a hexane solution (Fig. 3, traces 2–4) reveal only three different fluorescent species. One of them (trace 3) can be mainly attributed to unreacted fluorene 1.7 The short and long wavelength fluorescent species (traces 2 and 4) are the photochemical products that arise from the direct transformation of 1 upon irradiation. From the excitation spectra, the long wavelength portion of the photoproducts' absorption band (trace 1, λ ≥ 430 nm) corresponds to non-fluorescent species. These longer wavelength absorbing, non-fluorescent products can be attributed to stable cation radicals produced by photoinduced electron transfer from the nitrogen atom of the diphenylamino group.14
 | ||
| Fig. 3 Normalized absorption (1), excitation (2–4), and fluorescence (5; λexc = 300 nm) spectra of the photochemical products of 1 in hexane obtained after UV lamp irradiation (λexc ≈ 360 nm; I ≈ 4 mW cm−2; irradiation time, 60 min). Observed wavelength, λobs = 340 (2), 400 (3), and 550 nm (4). The sharp peak at 275 nm is a scattering line. | ||
Photochemical decomposition of 1 in the more polar solvent CH2Cl2 is characterized by a relatively high quantum yield of the photoreaction, Φ ≈ 10−2, and the appearance of photoproducts over a broad spectral range (300–800 nm). The fluorescence and excitation spectra of these photoproducts are presented in Fig. 4. The analysis of the excitation spectra reveals products with overlapping fluorescence spectra formed in CH2Cl2 upon UV irradiation. In addition to unreacted 1 (trace 3), many different fluorescent centers are observed (traces 4–8). From Fig. 4, the long wavelength part of the photoproduct absorption spectrum (trace 1, λ ≥ 500 nm) may be assigned to a non-fluorescent, stable aminofluorenyl cation radical species.14
 | ||
| Fig. 4 Absorption (1), excitation (3–8), and fluorescence (2; λexc = 330 nm) spectra of the photochemical products of 1 in CH2Cl2 obtained after UV lamp irradiation (λexc ≈ 360 nm; I ≈ 4 mW cm−2; irradiation time, 2 min). Observed wavelength, λobs = 410 (3), 470 (4), 510 (5), 530 (6), 550 (7), and 590 nm (8). The sharp peaks at 295, 275, 265, and 255 nm are the scattering lines. | ||
A two- to four-fold increase in the quantum yields of the photoreactions (Table 1) was observed in deoxygenated hexane solutions of 1 relative to air-saturated ones. These results clearly demonstrate that oxygen concentration is a decisive factor in the photostability of 1 in non-polar media. In contrast, the photochemical behavior of 1 in the electron-accepting solvent CH2Cl2 is independent of oxygen concentration.
3.3 Two-photon excitation
High intensity irradiation of fluorene 1, using the output from an IR femtosecond laser at λexc = 720 nm, resulted in two-photon excitation of 1 at an energy corresponding to the maximum of the main absorption band (∼360 nm). Two-photon absorption cross-sections, σ2PA(λexc), of 1 in hexane and CH2Cl2 were determined by the up-converted fluorescence method15 and found to be σ2PA ≈ 5 ± 1 GM and σ2PA ≈ 20 ± 4 GM, respectively. Temporal changes in the fluorescence intensity of 1, F(t), under high intensity irradiation were measured for air-saturated solutions.13 The quantum yields of the photochemical reactions under two-photon excitation (Φ2PA) were calculated using eqn. 2 and are presented in Table 1. From these, it can be seen that the Φ2PA values are nearly the same as those obtained for low intensity UV lamp irradiation, i.e. Φ2PA ≈ Φ for hexane and CH2Cl2 solutions of 1. Therefore, it can be concluded that photodecomposition of 1 in both solvents is independent of the type of excitation (one- or two-photon) though different mechanisms predominate depending on whether irradiation is conducted in hexane or CH2Cl2. These results suggest that, in some particular cases, two-photon photochemistry can be assessed for an organic compound through careful investigation of the corresponding one-photon photochemistry.4 Conclusion
The photochemical reactions of the two-photon absorbing fluorescent dye 1 in hexane and CH2Cl2 correspond to first-order reactions under one- and two-photon excitation. The quantum yields of the photoreactions under low intensity UV lamp irradiation are (3.4–4.6) × 10−4 in non-polar hexane and increase dramatically to 1.5 × 10−2 in polar CH2Cl2. The direct transformation of 1 into photoproducts was observed in hexane solutions without intermediate photoreactions. In this case, two different fluorescent photoproducts were detected, and molecular oxygen plays an important role in the photochemical stability of 1. For the more polar (electron-accepting) solvent CH2Cl2, many different photoproducts were observed under low intensity irradiation, exhibiting absorption over a broad spectral region (visible and near-IR). In addition, the photodecomposition is independent of the presence of oxygen in the solution. Two-photon excitation of 1 in hexane and CH2Cl2 afforded nearly the same quantum yields of the photoreactions as for low intensity UV lamp irradiation. Thus, the photostability of 1 is independent of the type of excitation (one- or two-photon). The relatively low photochemical quantum yield of 1 in hexane suggests this compound has characteristics favorable for use in linear and nonlinear optical applications, aspects which are currently under investigation.Acknowledgements
We wish to acknowledge the donors of The Petroleum Research Fund of the American Chemical Society, the Research Corporation Cottrell College Science program, the National Research Council COBASE program, the National Science Foundation, and the University of Central Florida Presidential Initiative for Major Research Equipment for partial support of this work.References
- W. Denk, J. H. Strickler and W. W. Webb, Two-photon laser scanning fluorescence microscopy, Science, 1990, 248, 73–76 CAS.
- K. D. Belfield, X. Ren, E. W. Van Stryland, D. J. Hagan, V. Dubikovski and E. J. Meisak, Near-IR two-photon photoinitiated polymerization using a fluorene/amine initiating system, J. Am. Chem. Soc., 2000, 122, 1217–1218 CrossRef CAS.
- K. D. Belfield, K. J. Schafer, Y. Liu, J. Liu, X. Ren and E. W. Van Stryland, Multiphoton-absorbing organic materials for microfabrication, emerging optical applications and non-destructive three-dimensional imaging, J. Phys. Org. Chem., 2000, 13, 837–849 CrossRef CAS.
- L. W. Tutt and T. F. Boggess, A review of optical limiting mechanisms and devices using organic, fullerenes, semiconductors and other materials, Prog. Quantum Electron., 1993, 17, 299–338 CrossRef CAS.
- E. A. Wachter, W. P. Partridge, W. G. Fisher, H. C. Dees and M. G. Petersen, Simultaneous two-photon excitation of photodynamic therapy agents, Proc. SPIE-Int. Soc. Opt. Eng., 1998, 3269, 68–75 Search PubMed.
- K. D. Belfield, K. J. Schafer, W. Mourad and B. A. Reinhardt, Synthesis of new two-photon absorbing fluorene derivatives via Cu-mediated Ullmann condensations, J. Org. Chem., 2000, 65, 4475–4481 CrossRef CAS.
- K. D. Belfield, M. V. Bondar, O. V. Przhonska, K. J. Schafer and W. Mourad, Spectral properties of several fluorene derivatives with potential as two-photon fluorescent dyes, J. Lumin., 2002, 97, 141–146 CrossRef CAS.
- K. D. Belfield, M. V. Bondar, O. V. Przhonska and K. J. Schafer, Photostability of a series of two-photon absorbing fluorene derivatives, J. Photochem. Photobiol., A, in press Search PubMed.
- M. P. Ligocki, C. Leuenberger and J. F. Pankow, Trace organic compounds in rain–II. Gas scavenging of neutral organic compounds, Atmos. Environ., 1985, 19, 1609–1617 CrossRef CAS.
- R. M. Dickhut and K. E. Gustafson, Atmospheric washout of polycyclic aromatic hydrocarbons in the Southern Chesapeake Bay region, Environ. Sci. Technol., 1995, 29, 1518–1525 CAS.
- L. Moeini-Nombel and S. Matsuzawa, Effect of solvents and a substituent group on photooxidation of fluorene, J. Photochem. Photobiol., A, 1998, 119, 15–23 CrossRef CAS.
- J. T. Barbas, M. E. Sigman, R. Arce and R. Dabestani, Spectroscopy and photochemistry of fluorene at a silica gel/air interface, J. Photochem. Photobiol., A, 1997, 109, 229–236 CrossRef CAS.
- K. D. Belfield, M. V. Bondar, Y. Liu and O. V. Przhonska, Photophysical and photochemical properties of 5,7-dimethoxycoumarin under one- and two-photon excitation, J. Phys. Org. Chem., 2003, 16, 69–78 CrossRef CAS.
- K. D. Belfield, M. V. Bondar, A. R. Morales, O. Yavuz and O. V. Przhonska, A new blue light-emitting oligofluorene glass: synthesis, characterization and photophysical properties, J. Phys. Org. Chem., 2003, 16, 194–201 CrossRef CAS.
- M. Rumi, J. E. Ehrlich, A. A. Heikal, J. W. Perry, S. Barlow, Z. Hu, D. McCord-Maughon, T. C. Parker, H. Rockel, S. Thayumanavan, S. R. Marder, D. Beljonne and J. L. Bredas, Structure-property relationships for two-photon absorbing chromophores: bis-donor diphenylpolyene and bis(styryl)benzene derivatives, J. Am. Chem. Soc., 2000, 122, 9500–9510 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2004 |
