Exposure to multiple doses of UVB radiation reduces the numbers of epidermal Langerhans cells and lymph node dendritic cells in mice
Joanna C.
Macve
a,
Roddie C.
McKenzie
b and
Mary
Norval
*a
aMedical Microbiology, University of Edinburgh Medical School, Teviot Place, Edinburgh, UK EH8 9AG
bClinical and Molecular Virology, Royal Dick Veterinary School, University of Edinburgh, Summerhall, Edinburgh, UK EH9 1QH
First published on 28th August 2003
Abstract
Immune suppression following UVB irradiation is partly attributed to the effects of the exposure on antigen-presenting cells. Following a single UVB irradiation, there is a decrease in epidermal Langerhans cell numbers; this is accompanied by an increase in the number of dendritic cells (DC) in lymph nodes draining the irradiated site. We investigated whether a similar effect occurred following multiple UVB exposures. Mice were irradiated on their ears and shaved dorsal skin twice a week for 3 weeks. After the final exposure, the number of ATPase+ Langerhans cells in epidermal sheets prepared from the ears was found to be decreased by 33% compared to unirradiated controls. The number of DC in the draining lymph nodes (DLN) did not increase as might have been expected; rather, a significant decrease of approximately 30% in DC numbers in the DLN of UVB-irradiated mice compared with unirradiated controls occurred. This decrease in antigen-presenting cells in both the epidermis and the DLN may be an important contributing factor to the immune suppression that follows multiple UVB exposures.
Introduction
Exposure to ultraviolet radiation (UVR) causes suppression of T-cell responses, such as the contact hypersensitivity (CH) response, in both mice and humans.1,2 During contact sensitisation, hapten-bearing Langerhans cells (LC), normally resident in the epidermis, migrate to the draining lymph nodes (DLN) to induce an immune response.3 There is evidence that UVR-induced suppression of immune responses may be due, at least partly, to effects on antigen-presenting cells of the skin. LC are both decreased in number and altered morphologically following UV irradiation.4 UV exposure of human skin results in increased numbers of LC in suction blister fluid, indicating that UV-induced migration takes place.5 In another study, UV irradiation [1 minimal erythemal dose (MED)] of the skin of human volunteers increased the lymph flow and cell output in an afferent lymph vessel.6 Moodycliffe et al.7 demonstrated that the number of dendritic cells (DC) in the skin DLN of unsensitised mice increased as a result of irradiation with 1 MED UVB, with a peak at 42 h after the exposure. Taken together, these studies suggest that, following a single dose of UV, some LC migrate from the epidermis through the afferent lymph and accumulate in the DLN. How this change relates to the immune suppression that follows UV exposure is unclear. One consequence could be a decrease in the numbers of LC in the skin available to take up antigen. Alternatively, some LC migrating from UV-irradiated skin show DNA damage5 which might lead to altered antigen presentation by these cells. In vitro UV exposure of LC affects the expression of co-stimulatory molecules such as CD86 (B7.2) and intercellular adhesion molecule-1;8,9 a decrease in these molecules could result in a tolerogenic, rather than an immunogenic, signal being generated. Finally, it has been demonstrated that following UV irradiation, there is an influx of inflammatory cells, including macrophages, into the skin;10 presentation of antigen by these cells gives rise to a suppressive, rather than an active, immune response.11Although the effects of a single, frequently erythemogenic, UV exposure on immune responses are useful for examining the complex processes involved in UV-induced immune suppression, multiple exposures over a period of weeks are perhaps more relevant to the sunlight exposure naturally received by humans. Such a chronic protocol can also remove the need to use high doses of UV. Whereas a single dose of 1250 J m−2 was not sufficient to reduce epidermal LC numbers in C3H mice,12 the numbers were decreased following exposure 3 times weekly to a dose of 500 J m−2 for 1–3 weeks.13 In the latter study, tanning of the UV exposed mice was seen after 3 weeks exposure, and it is not known at present whether such an adaptive response could affect the induction of suppression.
The purpose of the present study was to assess whether a 3 week UV protocol had similar effects to an acute UV exposure on the migration of LC in unsensitised mice. Following exposure of C3H mice to UVB for 3 weeks, the numbers of both epidermal LC and DC in LN draining the skin were counted. The expression of co-stimulatory molecules on the DC was also assessed. The in vivo CH response and the ability of LN cells to undergo spontaneous proliferation in vitro were tested as measures of immune function.
Materials and methods
Mice
Female C3H/HeN (H-2k) mice aged 6–10 weeks were obtained from the Medical Faculty Animal Area, University of Edinburgh; they were housed in a room where ambient light was regulated on a 12 h light/dark cycle and had free access to food and water. Room lights were shielded such that any contaminating UV wavelengths were filtered out. All experiments were performed according to the ethical guidelines of the University of Edinburgh and the Home Office.UV exposure
Mice were irradiated under a bank of two Philips TL12 lamps, which emit a broad spectrum of UVB radiation between 280 and 360 nm, with a peak at about 305 nm, and produced an irradiance of 150 J min−1 m−2 at a distance of 20 cm from the source. The output of the lamps was determined using a filtered photodiode meter, which was calibrated against measurements made with a UV-visible spectroradiometer (Optronic Laboratories model 724) across the spectral range 250–450 nm. The backs of the mice were shaved at least 24 h before the first irradiation, and the mice irradiated with 1500 J m−2 twice a week (Tuesday and Friday) for 3 weeks. Mice were placed in a Perspex box for the irradiation, with no more than 4 mice per box to avoid shielding by littermates. Control mice were shaved but not irradiated.Epidermal Langerhans cells
UVB-irradiated and unirradiated control mice were killed by cervical dislocation 24 h after the final irradiation, and their ears removed and split. The dorsal side was floated in 0.76% tetrasodium ethyleneamine tetraacetic acid for 2 h at 37 °C, and the epidermal sheets removed. The number of LC in the epidermal sheets was determined by staining for adenine triphosphatase (ATPase), using adenine diphosphate (ADP) as a substrate.14 Following staining, the sheets were rinsed in tap water and mounted onto a glass slide under a cover slip in 50% glycerol. The number of ATPase+ cells in 10 fields per epidermal sheet was counted (1 field = 0.1 mm2), with 4 sheets per group.Lymphoproliferation of draining lymph node cells and dendritic cell enrichment
Mice were killed by cervical dislocation 42 h after the final UV irradiation or were unirradiated (8 mice per group). Their auricular, axillary and inguinal LN were collected and pooled in RPMI medium (Gibco BRL, Paisley, UK) containing 10% foetal bovine serum (Gibco BRL) and 10 mM Hepes buffer (Sigma, Poole, UK). Single cell suspensions were prepared by mechanical disaggregation through a nylon cell strainer (Fred Baker Scientific, Runcorn, UK) and washed once. Viable cells were counted by Trypan Blue exclusion. For the lymphoproliferation assay, cells were resuspended to 2 × 106 cells ml−1 and 200 µl of this seeded into wells of a 96-well round-bottom culture plate (Iwaki, Asahi Techno Glass, Tokyo, Japan) (5 replicates per group). Cells were radioactively pulsed by adding 0.7 µCi 3H-methyl thymidine (Amersham Life Science, Amersham, UK) per well and incubated for 24 h at 37 °C in a humid atmosphere of 5% CO2 in air. Cells were harvested onto filter mats and 3H-thymidine incorporation measured in counts per minute using a scintillation counter (Canberra Packard, Zurich, Switzerland). The mean count of the 5 replicates for each group was calculated.The LN cell suspensions were enriched for DC using the method of Macatonia et al.15 Briefly, 4 × 107 LN cells in 8 ml medium were underlaid with 2 ml metrizamide (14.5%, Sigma, UK). Following 15 min centrifugation at 600g, the interface layer was collected, washed and resuspended in a minimal amount of medium. The number of DC was assessed by morphological examination by light microscopy; at least 5 counts for each group were made and the mean number of DC per LN was calculated.
Phenotyping of DC by flow cytometry
DC-enriched LN cell suspensions were prepared as above and 4 × 105 cells incubated on ice for 45 min with an isotype control antibody (rat anti-human CD8, Serotec, Oxford, UK) or monoclonal antibodies recognising Ia or CD86 (rat anti-mouse, Serotec). The cells were washed once with 1 ml RPMI–Hepes, resuspended in RPMI–Hepes and incubated with an affinity-purified F(ab')2 goat anti-rat IgG-FITC conjugate (100 µl of 1 ∶ 100 dilution; Serotec) for 45 min on ice. Cells were washed again, resuspended in 1 ml phosphate-buffered saline and analysed using a Coulter XL flow cytometer. Cells were identified first using forward scatter and side angle light scatter to quantify their size and granularity. Gates were placed around the entire cell population and around cells with low forward and side angle scatter (lymphocytes) and around the larger cells. The events within each region were displayed on histograms of log fluorescence intensity (x-axis) against cell count (y-axis). Isotype controls were routinely set at 1% and a minimum of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events was accumulated in the region around the entire cell population.
000 events was accumulated in the region around the entire cell population.
Annexin V binding of DC-enriched LN cell populations was determined by incubation of the cells for 3 min at room temperature with FITC-conjugated annexin V (Boehringer, Ingelheim, Germany) diluted 1 ∶ 500 in Hanks' balanced salt solution containing 5 µM CaCl2. Cells were analysed immediately by flow cytometry, as described above.
Measurement of CH response
The CH response to oxazalone was measured in irradiated and unirradiated mice by a standard technique, as outlined previously.16 Six mice per group received a sensitising dose of 50 µl 1% oxazalone in an acetone–olive oil (4 ∶ 1) vehicle on their shaved backs, 3 days after the final UV exposure. An unirradiated negative control group received 50 µl of the vehicle alone. Eight days after the sensitisation step, ear thicknesses were measured and all the mice were challenged with 25 µl per ear of 0.25% oxazalone on the dorsal surface. Ear swelling was measured 24 h later and the mean ear increase for each mouse was calculated, followed by the mean increase for each group of mice.Statistics
Statistical significance between groups was determined using the two-tailed student's t-test for unpaired data. A probability of less than (p <) 0.05 of no difference between the groups was considered significant.Results
Exposure to UVB for 3 weeks reduces epidermal LC numbers
It has been reported that a single exposure to 5000 J m−2 UVB (TL-12) reduces the number of LC in the epidermis by around 28%.12 This depletion was demonstrated to be dose dependent, with a dose of 1250 J m−2 being insufficient to cause a significant drop in LC numbers. To determine the effect of multiple UV doses over a period of 3 weeks on epidermal LC numbers, mice were irradiated twice a week for 3 weeks with 1500 J m−2 broad-band UVB. The ears were removed 24 h after the final irradiation and the frequency of LC in the epidermis determined by counting the number of ATPase+ cells with dendritic morphology. Fig. 1 shows that the number of epidermal LC decreased by approximately 33%, a significant difference compared to the unirradiated control mice.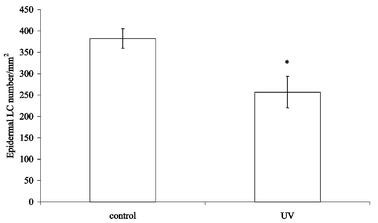 | ||
| Fig. 1 Effect of 3 weeks UVB exposure on epidermal LC numbers. Mice (2 per group) were irradiated twice a week for 3 weeks and their ears removed 24 h after the final UV exposure. Control mice were not irradiated. The epidermal sheets were removed, fixed and stained using ADP-lead; the number of ATPase+ DC was counted in 40 fields of 1 mm2 each. Results are expressed as the mean of 40 fields, error bars show the SEM. The asterisk indicates a significant (p < 0.05) difference between the irradiated and unirradiated groups. | ||
Exposure to UVB for 3 weeks decreases the number of DC in the DLN
Moodycliffe et al.7 demonstrated that a single exposure of the ears of C3H mice to 1440 J m−2 broad-band UVB induced an increase in the number of DC in the auricular LN, which was maximal at around 42 h following the irradiation. In the present study, mice were shaved and exposed to a single dose of 1500 J m−2 UVB. This resulted in an increase in the number of DC in the LN draining the irradiated dorsal surface from 4575 to 6595 per LN. To determine whether the same effect was seen in mice following multiple UV exposures, mice were exposed to 1500 J m−2 UVB twice a week for 3 weeks and LN draining both the ears and back were pooled and enriched for DC. The results of 4 separate experiments are shown in Table 1. The number of DC in the LN from irradiated mice was consistently less than the number in the LN from control mice. The mean for each group was calculated and a t-test showed that exposure of mice to UV for 3 weeks significantly (p < 0.05) decreased the number of DC in the DLN.To assess whether the decrease in DC numbers in the LN of irradiated mice was due to increased cell death, the level of annexin V binding by the DC-enriched cell populations was measured. DC from control and irradiated mice demonstrated similar levels of annexin V binding (Fig. 2), with an average (from two experiments) of 72% positive cells for control DC and 74% for DC from irradiated mice.
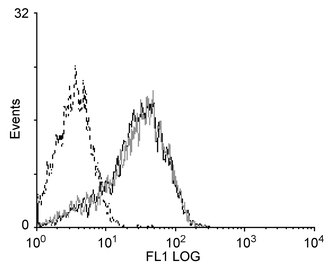 | ||
| Fig. 2 Effect of 3 weeks UVB exposure on annexin V binding to DC. Mice (8 per group) were irradiated twice a week for 3 weeks and their LN removed 42 h after the final exposure. Control mice were not irradiated. Following DC enrichment of DLN cells, the enriched populations were stained with FITC-conjugated annexin V. The fluorescence intensity of the gated DC population is shown for control (continuous black line) and irradiated mice (grey line). The fluorescence intensity of DC from unirradiated mice stained with a control antibody is also shown (dotted black line). | ||
Exposure to UVB for 3 weeks has no effect on DC surface molecules
Lappin et al.17 demonstrated that, in mice that were contact sensitised, prior exposure to a single dose of UVB had no effect on the expression of several cell surface molecules on DC enriched from DLN, compared with unirradiated mice. We assessed whether chronic UV exposure affected the expression of cell surface markers involved in antigen presentation in unsensitised mice. DLN cells from unirradiated mice and from mice that had been irradiated over a 3 week period were enriched for DC, and the expression of Ia and CD86 determined by flow cytometry. No difference was seen in the expression of either Ia [Fig. 3(a)] or CD86 [Fig. 3(b)] between mice exposed to multiple doses of UV and unirradiated controls. This experiment was repeated twice with similar results.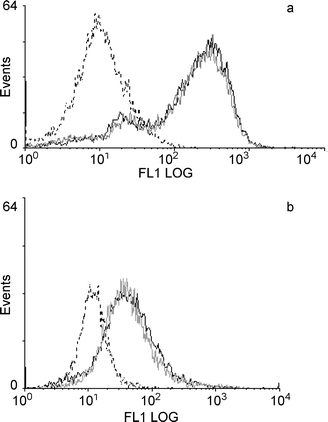 | ||
| Fig. 3 Effect of 3 weeks UVB exposure on expression of cell surface markers on DC. Mice (8 per group) were irradiated twice a week for 3 weeks and their LN removed 42 h after the final exposure. Control mice were not irradiated. Following DC enrichment of DLN cells, the enriched populations were stained for the presence of Ia (a) and CD86 (b), and analysed by flow cytometry. The fluorescence intensity of the gated DC population is shown for control (continuous black line) and irradiated mice (grey line). The fluorescence intensity of DC from unirradiated mice stained with a control antibody is also shown (dotted black line). | ||
Exposure to UVB for 3 weeks has no effect on the spontaneous proliferation of DLN cells
LN cells that are removed from normal mice have a low spontaneous proliferation in vitro; application of a contact sensitiser to the skin results in increased proliferation of cells from LN draining the skin.18 To assess whether UV irradiation had any effect on the spontaneous proliferation of DLN cells, mice were irradiated twice a week for 3 weeks and their LN removed 42 h after the final irradiation. No difference was seen in the spontaneous proliferation of cells from UV-irradiated mice compared with unirradiated controls (Fig. 4). This experiment was repeated 3 times with similar results. In one experiment, proliferation of LN cells stimulated with the mitogen concanavalin A (2.5 µg ml−1 for 42 h)19 was measured; the stimulation index (proliferation in response to concanavalin A divided by proliferation without the mitogen) was 37 for both control and irradiated mice.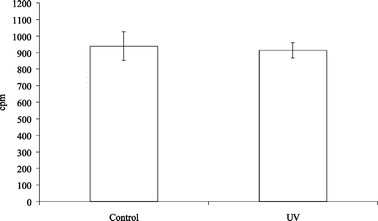 | ||
| Fig. 4 Effect of 3 weeks UVB exposure on the spontaneous proliferation of DLN cells. Mice (8 per group) were irradiated twice a week for 3 weeks and LN draining the irradiated sites removed 42 h after the final exposure. Control mice were not irradiated. The level of proliferation was assessed using 3H-thymidine incorporation. Error bars show the SEM. | ||
Exposure to UVB for 3 weeks suppresses the CH response
Suppression of the CH response following multiple UVB exposures has been demonstrated previously.13 To assess the effect of the UV exposure protocol used in the present study on the ability of mice to mount an immune response, the CH response to oxazalone was measured. As shown in Fig. 5, mice irradiated with multiple UVB doses showed a significantly suppressed CH response compared with unirradiated controls.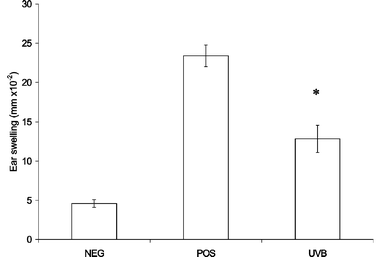 | ||
| Fig. 5 Effect of 3 weeks UVB exposure on CH response to oxazalone. Irradiated (UVB) or unirradiated (POS) mice (4–6 per group) were sensitised with oxazalone on the dorsal skin 3 days after the final UVB exposure, and challenged 8 days later on the ears. Unirradiated negative control mice (NEG) were challenged but not sensitised. Error bars show the SEM. The asterisk indicates a significant (p < 0.05) difference between the irradiated and unirradiated groups. | ||
Discussion
The results of this study show that, following a 3 week UVB radiation protocol, the number of epidermal LC decreased and this decrease was accompanied by a decrease in DC numbers in the DLN, in contrast to the increase that occurred following a single UV exposure.The effect of chronic UVB exposure on epidermal LC numbers has been reported previously.13 The 3 week UV protocol used in the current study also led to local suppression of the contact hypersensitivity response to oxazalone. A correlation between UV-induced LC depletion and down-regulation of contact hypersensitivity responses has been demonstrated previously,1 suggesting a role for LC in UV-induced immune suppression. The decrease in epidermal LC numbers following UV irradiation could be due to apoptosis of these cells, resulting from UV-induced DNA damage. In vitro irradiation of human LC with low dose UVB resulted in increased apoptotic cell death.8 Following ex vivo UVB exposure of human skin biopsies, the number of LC in the epidermis decreased compared to unirradiated controls. This decrease could not be attributed to migration however, as the number of LC found in the culture medium decreased following UV irradiation of the skin.20 On the other hand, a number of studies has provided evidence that the decrease in LC numbers following UV exposure is due to the migration of LC from the skin, rather than through the induction of apoptosis. Experiments involving cannulation of draining afferent lymph vessels have demonstrated an increase in lymph flow and cell output after exposure of human volunteers to UVR,6 and an increase in the number of LC in afferent lymph draining the skin of UV-irradiated sheep.21 In another human study, suction blisters were raised in skin and the number of LC detected in the blister fluid increased following UVB exposure.5 Of these, 20–30% showed evidence of UV-induced DNA damage, indicating that they were derived from the UV-exposed epidermis; additionally, very few apoptotic LC were observed in the epidermis and dermis of the irradiated skin. Following a single UV irradiation, mouse cells staining positive for cyclobutane pyrimidine dimers (a specific marker for the commonest form of UV-induced DNA damage) were present in DLN; at least some of these cells had characteristic DC markers, suggesting that they were LC that had migrated from the irradiated skin.22
In the current study, as reported previously by Moodycliffe et al.,7 the number of DC in the DLN increased in mice that had received a single dose of UVR, confirming that exposure to UV causes migration of DC. By contrast, following a chronic 3 week UV protocol, the number of DC in DLN decreased compared to unirradiated mice. In the study by Moodycliffe et al.,7 the increase in DC numbers in the DLN of irradiated mice reached a maximum at 42 h after the exposure, and then decreased rapidly to the levels found in unirradiated mice by around 60 h. This decrease may be due to the re-circulation of the DC; alternatively, the DC that have migrated from the UV-exposed skin may apoptose upon reaching the LN. In vitro studies have demonstrated that UVB-irradiated LC only show increased apoptosis following at least 2 days in culture,8,9 leading to the suggestion that LC migrating from the skin apoptose once they have reached the DLN. Similarly, following skin painting with an irritant, DC accumulate in the DLN, but disappear rapidly after 2 days,23 an effect attributed to the death of the DC, since no DC have been observed leaving LN via the efferent lymphatics.24 This is in contrast to DC migrating from the skin in unsensitised mice, which demonstrate a turnover of around 30 days.23 The death of DC in sensitised mice could result from interaction with antigen-specific T cells, as splenic DC showed increased apoptosis when cultured in vitro with antigen-specific T cells.25 No increase in the spontaneous proliferation of cells from the DLN of irradiated mice was seen in the present study, indicating that the DC did not stimulate antigen-specific T cells upon arrival; this is to be expected, since the mice were not sensitised. No difference in the level of cell death in DC isolated from unirradiated and irradiated mice was indicated using the marker annexin V; however, a high level of staining was seen in both groups, possibly as a consequence of the in vitro manipulation of the cells. It may be that the decrease in both epidermal LC and LN DC numbers in irradiated mice resulted from cell death that occurred earlier in the UVB protocol; alternatively some of the migrating LC could have died in the draining lymphatics.
Despite the decrease in DC numbers in the DLN of irradiated mice, no difference in expression of the cell surface molecules Ia and CD86 was found on DC from DLN of UV-exposed mice, compared to DC from unirradiated mice. Therefore, the DC that remain in the DLN following multiple UV exposures express normal levels of at least two of the molecules involved in antigen presentation to, and stimulation of, T cells. However this finding does not rule out the possibility that UVB may affect the expression of other DC surface molecules such as CD80, CD40 and CD11c, which were not assessed in the present study.
Although we have shown that, following exposure of mice to UV over a 3 week period, the number of LC in the epidermis decreased, there was no evidence that the LC had migrated to the DLN, since the number of DC in the DLN of chronically UV-irradiated mice was significantly decreased compared to unirradiated mice. The ultimate fate of these cells remains to be determined. As seen previously, multiple exposures to UV suppress the contact hypersensitivity response13 and result in enhanced outgrowth of implanted tumours in UV-irradiated mice.26 The decrease in both epidermal LC and DC in the DLN suggests that there is a lack of available antigen-presenting cells in chronically UV-irradiated mice, which could either lead to less effective initiation of an immune response, or make antigen presentation by other cells, such as macrophages, more important in these mice.
Acknowledgement
This work was funded by the Association for International Cancer Research.References
- G. B. Toews, P. R. Bergstresser and J. W. Streilein, Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB, J. Immunol., 1980, 124, 445–453 Search PubMed.
- T. Yoshikawa, V. Rae, W. Bruinsslot, J. W. Vandenberg, J. R. Taylor and J. W. Streilein, Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans, J. Invest. Dermatol., 1990, 95, 530–536 CrossRef CAS.
- M. L. Kripke, C. G. Munn, A. Jeevan, J. M. Tang and C. Bucana, Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization, J. Immunol., 1990, 145, 2833–2838 Search PubMed.
- W. Aberer, G. Schuler, G. Stingl, H. Honigsmann and K. Wolff, Ultraviolet-light depletes surface markers of Langerhans cells, J. Invest. Dermatol., 1981, 76, 202–210 CAS.
- W. Kolgen, H. Both, H. van Weelden, K. L. H. Guikers, C. A. F. M. Bruijnzeel-Koomen, E. F. Knol, W. A. van Vloten and F. R. de Gruijl, Epidermal Langerhans cell depletion after artificial ultraviolet B irradiation of human skin in vivo: apoptosis versus migration, J. Invest. Dermatol., 2002, 118, 812–817 CrossRef CAS.
- N. Yawalkar, M. C. Aebischer, R. Hunger, C. U. Brand and L. R. Braathen, Effects of UV irradiation with one minimal erythema dose on human afferent skin lymph in vivo, Exp. Dermatol., 1998, 7, 362–368 CAS.
- A. M. Moodycliffe, I. Kimber and M. Norval, The effect of ultraviolet B radiation, and urocanic acid isomers on dendritic cell migration, Immunology, 1992, 77, 394–399 CAS.
- F. M. Rattis, M. Concha, C. Dalbiez-Gauthier, P. Courtellemont and J. Peguet-Navarro, Effects of ultraviolet B radiation on human Langerhans cells: functional alteration of CD86 upregulation and induction of apoptotic cell death, J. Invest. Dermatol., 1998, 111, 373–379 CrossRef CAS.
- A. Tang and M. C. Udey, Effects of ultraviolet-radiation on murine epidermal Langerhans cells - doses of ultraviolet-radiation that modulate ICAM-1 (CD54) expression and inhibit Langerhans cell-function cause delayed cytotoxicity in vitro, J. Invest. Dermatol., 1992, 99, 83–89 CAS.
- K. D. Cooper, N. Duraiswamy, C. Hammerberg, E. Allen, C. Kimbroughgreen, W. Dillon and D. Thomas, Neutrophils, differentiated macrophages, and monocyte/macrophage antigen presenting cells infiltrate murine epidermis after UV injury, J. Invest. Dermatol., 1993, 101, 155–163 CAS.
- C. Hammerberg, N. Duraiswamy and K. D. Cooper, Active induction of unresponsiveness (tolerance) to DNFB by in vivo ultraviolet-exposed epidermal cells is dependent upon infiltrating Class II MHC+ CD11b (bright) monocytic/macrophagic cells, J. Immunol., 1994, 153, 4915–4924 Search PubMed.
- M. S. Duthie, I. Kimber, R. J. Dearman and M. Norval, Differential effects of UVA1 and UVB radiation on Langerhans cell migration in mice, J. Photochem. Photobiol., B, 2000, 57, 123–131 CrossRef CAS.
- A. A. El-Ghorr, M. Norval, M. B. Lappin and J. C. Crosby, The effect of chronic low-dose UVB radiation on Langerhans cells, sunburn cells, urocanic acid isomers, contact hypersensitivity and serum immunoglobulins in mice, Photochem. Photobiol., 1995, 62, 326–332 CAS.
- M. B. Chaker, M. D. Tharp and P. R. Bergstresser, Rodent epidermal Langerhans cells demonstrate greater histochemical specificity for ADP than for ATP and AMP, J. Invest. Dermatol., 1984, 82, 496–500 CAS.
- S. E. Macatonia, A. J. Edwards and S. C. Knight, Dendritic cells and the initiation of contact sensitivity to fluorescein isothiocyanate, Immunology, 1986, 59, 509–514 CAS.
- A. M. Moodycliffe, I. Kimber and M. Norval, Role of tumour necrosis factor-α in ultraviolet B light-induced dendritic cell migration and suppression of contact hypersensitivity, Immunology, 1994, 81, 79–84 CAS.
- M. B. Lappin, I. Kimber, R. J. Dearman and M. Norval, Exposure of UVB sensitive mice to immunosuppressive doses of UVB in vivo fails to affect the accessory function or the phenotype of the draining lymph node dendritic cells, Exp. Dermatol., 1996, 5, 286–294 CAS.
- I. Kimber, J. A. Mitchell and A. C. Griffin, Development of a murine local lymph node assay for the determination of sensitizing potential, Food Chem. Toxicol., 1986, 24, 585–586 CrossRef CAS.
- A. A. El-Ghorr and M. Norval, The role of interleukin-4 in ultraviolet B light-induced immunosuppression, Immunology, 1997, 92, 26–32 CAS.
- I. B. Kremer, R. M. R. Sylva-Steenland, J. D. Bos and M. B. M. Teunissen, Despite the presence of UVB-induced DNA damage, HLA-DR+ cells from ex vivo UVB-exposed human skin are able to migrate and show no impaired allostimulatory capacity, J. Invest. Dermatol., 1997, 109, 626–631 CAS.
- G. W. Dandie, G. J. Clydesdale, F. J. Radcliff and H. K. Muller, Migration of Langerhans cells and γδ+ dendritic cells from UV-B-irradiated sheep skin, Cell. Immunol. Biol., 2001, 79, 41–48 Search PubMed.
- Y. Sontag, C. L. H. Guikers, A. A. Vink, F. R. de Gruijl, H. Van Loveren, J. Garssen, L. Roza, M. L. Kripke, J. C. van der Leun and W. A. van Vloten, Cells with UV-specific DNA damage are present in murine lymph nodes after in vivo UV irradiation, J. Invest. Dermatol., 1995, 104, 734–738 CAS.
- C. Ruedl, P. Koebel, M. Bachmann, M. Hess and K. Karjalainen, Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes, J. Immunol., 2000, 165, 4910–4916 Search PubMed.
- J. B. Smith, G. H. McIntosh and B. Morris, The traffic of cells through tissues: a study of peripheral lymph in sheep, J. Anat., 1970, 107, 87–100 CAS.
- H. Matsue, D. Edelbaum, A. C. Hartmann, A. Morita, P. R. Bergstresser, H. Yagita, K. Okumura and A. Takashima, Dendritic cells undergo rapid apoptosis in vitro during antigen-specific interaction with CD4+ T cells, J. Immunol., 1999, 162, 5287–5298 Search PubMed.
- M. L. Kripke and M. S. Fisher, Immunologic parameters of ultraviolet carcinogenesis, J. Natl. Cancer Inst., 1976, 57, 211–215 CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2004 |
