Diamagnetic and paramagnetic ring currents in expanded porphyrins
Erich
Steiner
and
Patrick W.
Fowler
Department of Chemistry, School of Biological and Chemical Sciences, University of Exeter, Stocker Road, Exeter, UK EX4 4QD. E-mail: P.W.Fowler@exeter.ac.uk; Fax: +44 1392 263434
First published on 6th November 2003
Abstract
Ipsocentric current density maps are computed at the coupled Hartree–Fock level in the 6-31G** basis set for the planar C2v B3LYP geometries of the expanded porphyrins, sapphyrin and orangarin. Both give clearly dominant global macrocyclic ring currents, but with opposite senses of circulation: in 22π sapphyrin, a diatropic current runs, with some bifurcation, around the conventional 22-centre delocalisation pathway; in 20π orangarin, a paratropic current runs around the inner 17-atom pathway. In agreement with the annulene analogy for these macrocycles, analysis of orbital contributions shows that in each case topology, energy and symmetry of the frontier orbitals together determine the macrocyclic ring current. In sapphryrin, 4-electron diamagnetism (aromaticity) arises from translationally allowed HOMO–LUMO excitations as in benzene itself; in orangarin, 2-electron paramagnetism (antiaromaticity) arises from rotationally allowed HOMO–LUMO excitations as in planarised cyclooctatetraene. The active orbitals invoked in the explanation of ring currents are those involved in the longstanding four-orbital model of porphyrin electronic spectra.
1. Introduction
Expansion of the polypyrrole macrocycle offers porphyrins with spectroscopic, chemical and biological activity tailored for applications ranging from medicine to materials science.1 Chemists have found it natural to account for the planarity, stability, redox flexibility and optical response of these expanded systems by the formal analogy between conjugation pathways in the macrocycle and simple annulenes. Conventional electron counting rationalises, for example, the aromaticity of sapphyrin2,3 (I) and putative antiaromaticity of orangarin4 (II) (Scheme 1) in terms of the venerable Hückel 4n + 2 and 4n rules, but the analogy goes further. It has detailed implications for the property that is taken to define aromaticity,5,6 the global ring current induced by a perpendicular magnetic field.Here, we use ipsocentric7–9 electronic-structure calculations to make a direct visualisation of the induced currents in I and II. We show that in both cases the origin, sense and magnitude of the global ring current obey precisely the annulene rules. A predictive model invoking only orbital topology and energy differences attributes the ring current to the mobility of a small subset of frontier π electrons, just as in the corresponding annulenes.10
 | ||
| Scheme 1 | ||
2. Current density maps
Ipsocentric current density maps are computed here for the first time for sapphyrin (I) the core of the first recorded expanded porphyrin,2,3 and orangarin (II) the smallest. All computations were performed with the Gaussian 6-31G** basis set. Density Functional theory (DFT) with a B3LYP functional in this basis gives a planar ground state with C2v symmetry for both systems.11 The calculations of current densities were performed at the DFT optimum geometry by means of coupled Hartree–Fock theory12 within the DZ (diamagnetic-zero) variant of the CTOCD (continuous transformation of origin for current density) method. In this approach, the induced current density at a given point is calculated with that point as its own origin of vector potential, hence the alternative name ‘ipsocentric’9 for the method. Calculations of nuclear shieldings were performed within the PZ2 (paramagnetic-zero) variant of CTOCD.13 The maps are plotted in a plane at 1 bohr from that of the nuclei, close to planes of maximum π current and charge densities. The arrows represent in-plane projections of current density vectors and the intensity of colour represents relative magnitude. Diamagnetic circulation is shown anticlockwise, paramagnetic clockwise.The maps (Fig. 1) show the total π-electron current density distributions induced in the free-base unsubstituted expanded porphyrins I and II by a magnetic field at right angles to the molecular plane. Both molecules give clearly seen dominant global ring currents, but running in opposite directions; the different colours, blue and red, highlight this essential difference between the diamagnetic (diatropic) current in I and the paramagnetic (paratropic) current in II.
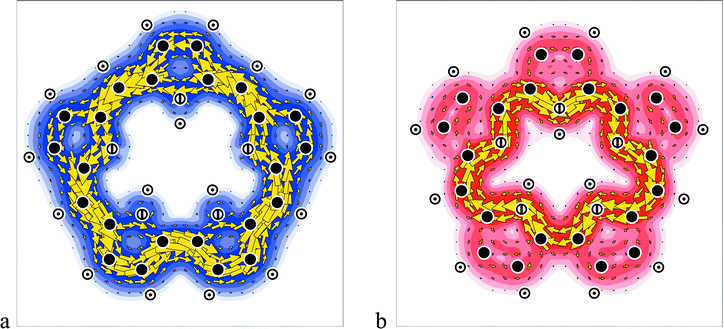 | ||
| Fig. 1 Total π current density maps of free-base expanded porphyrins; (a) sapphyrin and (b) orangarin. The colours distinguish the two types of global circulation: blue for a diamagnetic ring current, red for a paramagnetic ring current. The intensity of colour represents relative magnitude of current density. | ||
Natural porphyrins have four pyrrole units linked by methine bridges, and are normally considered as heteroatom-bridged [18]-annulenes,14 taking the conjugation pathway to exclude two fixed double bonds and the two NH π lone pairs, and thereby satisfying the Hückel (4n + 2) rule for aromaticity of a monocycle (the actual number of electrons in orbitals of π symmetry is 26). The expanded porphyrins discussed here contain five pyrrole rings linked by direct and methine bridges. In the same convention, sapphyrin is an aromatic [22]-annulene (32 electrons in π orbitals) and, like porphin, it has characteristic Soret and visible bands in the electronic spectrum. Its aromatic character is confirmed by the large downfield shifts of the methine protons in the 1H NMR spectrum (δ ≈ 11–12 for a protonated alkyl-substituted analogue;3 calc. 9.9, 10.3), corresponding to strong deshielding by a diamagnetic ring current, and large upfield shifts (expt. δ ≈ −5;3 calc. −8.4, −6.0) of the internal NH protons. The global π ring current of Fig. 1a shows that the conventional 22-centre pathway is indeed dominant although, as in the porphyrins,15 this diamagnetic circulation in fact spans all main-group atoms, with bifurcation across the pyrrole units. The maximum strength of the π current is twice that in benzene16 and about equal to that in porphin.
In contrast to sapphyrin, orangarin, with two methine bridges replaced by direct links, is conventionally regarded as an antiaromatic [20]-annulene4 (30 electrons in π orbitals). Although II exhibits macrocycle Soret and visible bands, the antiaromaticity is indicated by the upfield shifts of the methine protons (expt. δ ≈ 4;4 calc. 4.2) and large downfield shifts of the NH protons (expt. ca. 30 for a protonated form;4 calc. δ 20.0, 21.5), both attributed to the effects of global paramagnetic circulation. The map of total π current in Fig. 1b shows that this paramagnetic circulation follows, not the conventional 20-atom pathway, but the innermost, shortest possible, 17-atom pathway around the macrocycle, with only weak current density on peripheral CC bonds (cf. calc. δ = 4.6–6.2 for β protons). The strength of the paramagnetic π current is ∼1.5 times that of the (diamagnetic) π current in benzene.
Thus, the maps of total π currents in I and II already distinguish between alternative models of the conjugation pathway and change our view of II. Another key strength of the ipsocentric method is that it allows a partitioning of total current density into orbital contributions9,10 that goes much further in providing insight than simple electron counting or tabulation of aromaticity indices.17 This partitioning is applied in the following section to deduce the origins of the opposed global currents.
3. Orbital analysis
In the ipsocentric treatment of magnetic properties, the current induced in the electronic structure of a molecule is ascribed wholly to first-order perturbation of the wavefunction, with none of the complications generated by traditional formulations in terms of competing formal diamagnetic and paramagnetic contributions. The current density and the magnetic response properties that depend on it, such as magnetic susceptibility and nuclear shieldings, are then determined wholly by the accessibility of excited electronic states of the system, just as are the electronic spectrum and electric response properties such as electric polarisability. In molecular orbital theory, it is the allowed transitions between occupied and unoccupied orbitals that determine these properties and the translation–rotation selection rules that determine the sense of the current.10In common with other chemical and physical properties, therefore, the π ring current in the ipsocentric formulation is dominated by the electrons in the frontier orbitals and by the HOMO–LUMO virtual excitations. For annulenes,10 the 4n + 2 Hückel rule for aromaticity based on π-electron count has its counterpart in a 4-electron rule for diamagnetic (aromatic) circulation of induced current that is attributed to virtual translational excitations from the doubly degenerate HOMO level to the LUMO-level. In the same way, the 4n Hückel rule for antiaromaticity of a closed-shell ground state corresponds to a 2-electron rule for paramagnetic (antiaromatic) circulation based on a rotational HOMO–LUMO excitation. These rules are exact only for delocalised planar annulenes in Hückel theory but, as with the Hückel rules themselves, they have been found to be remarkably resilient for understanding the magnetic response of a much wider range of organic molecules.9 In particular, they can be used to rationalise the global π ring current in porphyrins and in expanded porphyrins, as is now shown.
Fig. 2 gives schematic energy diagrams for the frontier orbitals of I and II, with their nodal patterns and indications of the transitions that are largely responsible for the ring currents. The π structure of sapphyrin is a closed shell in which the near-degenerate (7a2, 9b2) HOMO pair has a nodal symmetry corresponding to angular momentum quantum number λ = 5. The near-degenerate (8a2, 10b2) LUMO pair has one more nodal plane, and quantum number λ = 6. The HOMO–LUMO transitions for Δλ = 1 in an annulene are translationally allowed and give rise to a diamagnetic global ring current.
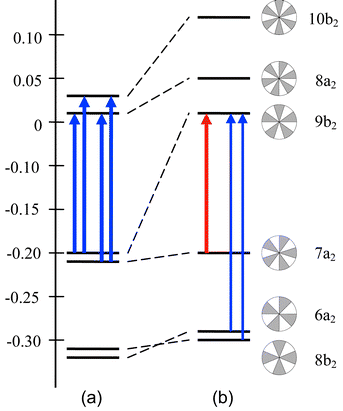 | ||
| Fig. 2 Orbital energy diagrams of (a) sapphyrin and (b) orangarin. Transitions shown in blue are translationally allowed and so give rise to a diamagnetic global ring current, dominant in sapphyrin but secondary in orangarin; the transition shown in red is rotationally allowed and is responsible for the paramagnetic ring current in orangarin. The schematic nodal representations used here underline the fact that strong rotational transitions are associated with simple rotation of the nodal structure, whereas translational transitions produce unit change in nodal line count. | ||
The contribution to the sapphyrin ring current of the four electrons in the HOMO pair is shown in Fig. 3a. Comparison with Fig. 3b, the map of the current density arising from all the electrons except these four, and with the map of total π current in Fig. 1a, makes it clear that the 28 π electrons outside the HOMO play a comparatively insignificant role in π currents. Sapphyrin, like benzene itself, therefore displays 4-electron diamagnetism and, on the magnetic criterion, is aromatic.
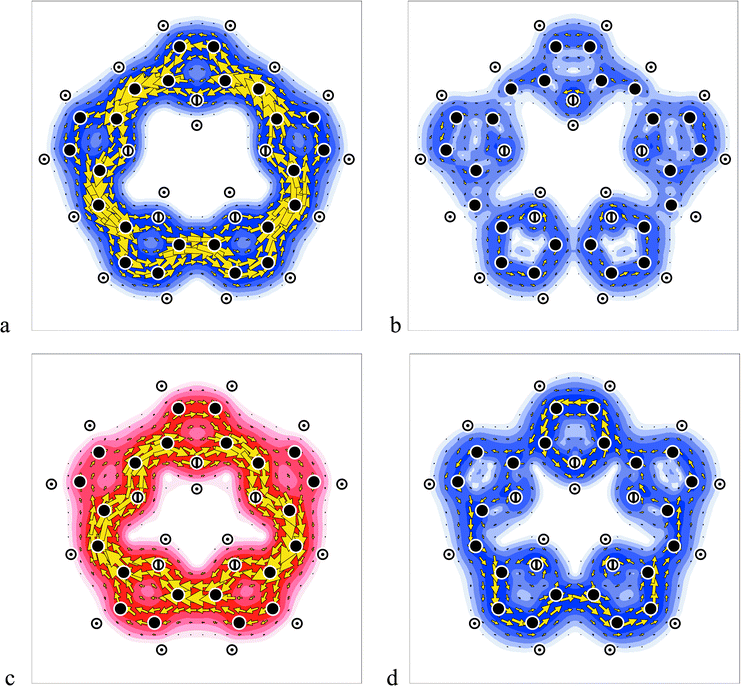 | ||
| Fig. 3 Current density maps of free-base expanded porphyrins: (a) sapphyrin; (7a2, 9b2) HOMO pair, (b) sapphyrin; all π except the (7a2, 9b2) HOMO pair, (c) orangarin; 7a2 HOMO, (d) orangarin; all π except the 7a2 HOMO. Colour conventions as in Fig. 1. | ||
In contrast, the π structure of orangarin, with two fewer electrons, has one member of the λ = 5 pair, 7a2, acting as HOMO, whilst the other, 9b2, is now the LUMO. Transitions with Δλ = 0 are rotationally allowed, so that the dominant contribution to the global ring current in orangarin can be ascribed to the HOMO–LUMO transition between the members of the split pair. This gives rise to the paramagnetic ring current mapped in Fig. 3c. The global ring current in orangarin is an exact analogue of the paramagnetic current in plane forms of cyclooctatetraene (COT).19 In COT the λ = 2 open shell of the regular octagon is closed by Jahn–Teller distortion, yielding a rotationally related HOMO and LUMO pair separated by a small energy gap. Orangarin, like COT, displays 2-electron paramagnetism and, in the magnetic criterion, is therefore antiaromatic.
The map Fig. 3d, for all except the two HOMO electrons, shows, in this case, that weaker contributions from lower-lying orbitals lead to damping of the paramagnetic current in the outer regions of the molecule. Further analysis shows that the damping can be ascribed to the translationally allowed transition to the LUMO from the (6a2, 8b2) HOMO-1 pair. As Fig. 1b shows, addition of this diamagnetic contribution induces localisation of current in the peripheral CC bonds. This too has its parallels in COT.20
4. Conclusion
In the expanded porphyrins, as in many macrocyclic and polycyclic π systems, currents obtained from ipsocentric calculations are informative about frontier electronic structure, depend in a clear way on HOMO–LUMO symmetry and nodal topology, and show the power of simple orbital-based rationalisations. It is interesting to note that the active orbitals invoked in the explanation of ring currents are those involved in the longstanding four-orbital model18 of porphyrin electronic spectra.5. References
- J. L. Sessler and S. J. Weghorn, Expanded, contracted and isomeric porphyrins, Pergamon Tetrahedron Organic Chemistry Series 15, Elsevier, Amsterdam, 1997 Search PubMed.
- M. J. Broadhurst, R. Grigg and A. W. Johnson, J. Chem. Soc., Perkin Trans. 1, 1972, 2111–2116 RSC.
- V. J. Bauer, D. L. J. Clive, D. Dolphin, J. B. Paine, F. L. Harris, M. M. King, J. Loder, S.-W. C. Wang and R. B. Woodward, J. Am. Chem. Soc., 1983, 105, 6429–6436 CrossRef CAS.
- J. L. Sessler, S. J. Weghorn, Y. Hiseada and V. Lynch, Chem. Eur. J., 1995, 1, 56–67 CrossRef CAS.
- V. I. Minkin, M. N. Y. Glukhovtsev and B. Y. Simkin, Aromaticity and antiaromaticity. Electronic and structural aspects,Wiley, New York, 1994 Search PubMed.
- P. von R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. van Eikema Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS.
- T. Keith and R. F. W. Bader, Chem. Phys. Lett., 1993, 210, 223–231 CrossRef CAS.
- S. Coriani, P. Lazzeretti, M. Malagoli and R. Zanasi, Theoret. Chim. Acta, 1994, 89, 181–192 CAS.
- E. Steiner and P. W. Fowler, J. Phys. Chem. A, 2001, 105, 9553–9562 CrossRef CAS.
- E. Steiner and P. W. Fowler, Chem. Commun., 2001, 2220–2221 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle and J. A. Pople, GAUSSIAN 98 (Revision A.6), Gaussian, Inc., Pittsburgh, PA, 1998 Search PubMed.
- Sysmo Package P, Lazzeretti and R. Zanasi, University of Modena, 1980, with additional routines for evaluation and plotting of current density byE. Steiner, P. W. Fowler and R. W. A. Havenith.
- R. Zanasi, P. Lazzeretti, M. Malagoli and F. Piccinini, J. Chem. Phys., 1995, 102, 7150–7157 CrossRef CAS.
- P. George, Chem. Rev., 1995, 25, 85–111.
- E. Steiner and P. W. Fowler, ChemPhysChem., 2002, 3, 114–116 CrossRef CAS.
- E. Steiner and P. W. Fowler, Int. J. Quantum Chem., 1996, 60, 609–616 CrossRef CAS.
- P. von R. Schleyer and H. Jiao, Pure Appl. Chem., 1996, 68, 209–218 CAS.
- M. Gouterman, J. Chem. Phys., 1959, 30, 1139–1161 CrossRef CAS.
- P. W. Fowler, R. W. A. Havenith, L. W. Jenneskens, A. Soncini and E. Steiner, Angew. Chem., Int. Ed. Engl., 2002, 41, 1558–1560 CrossRef CAS.
- R. W. A. Havenith, L. W. Jenneskens and P. W. Fowler, Chem. Phys. Lett., 2003, 367, 468–474 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2004 |
