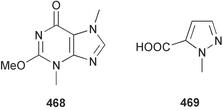
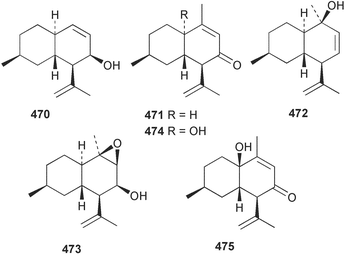
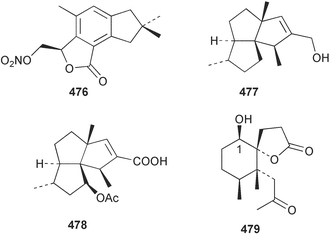
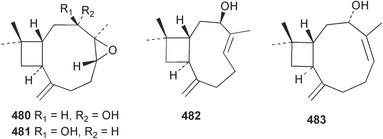
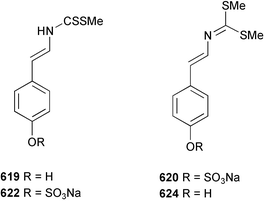
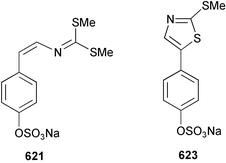
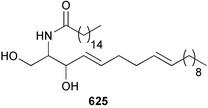
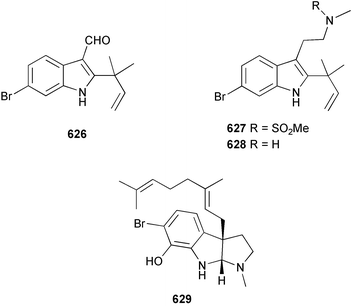
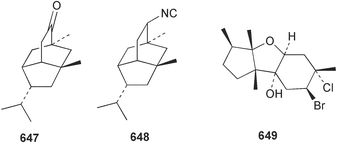
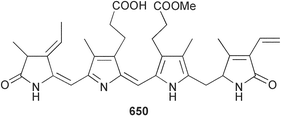
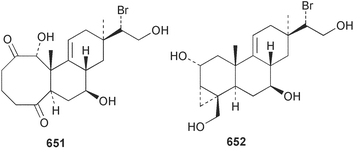
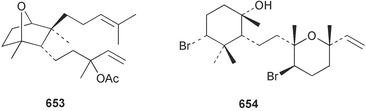
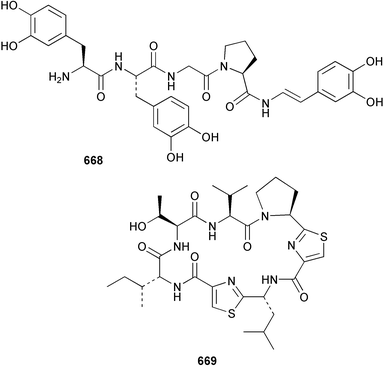
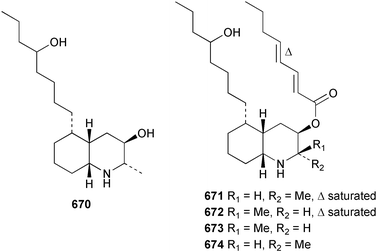
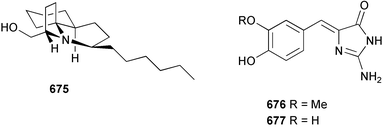
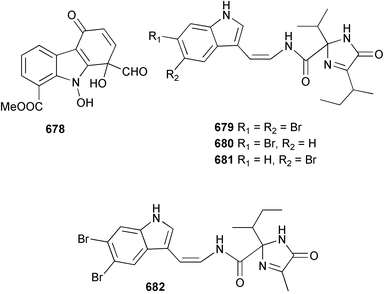
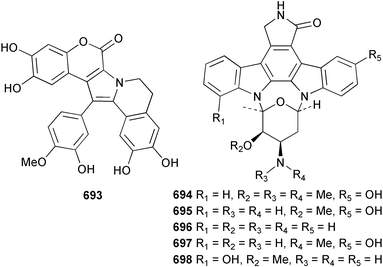
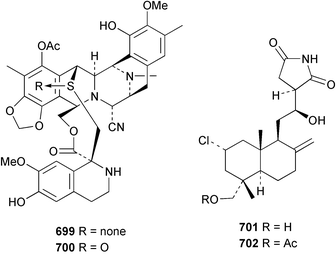
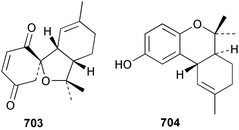
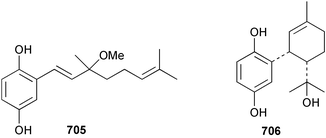
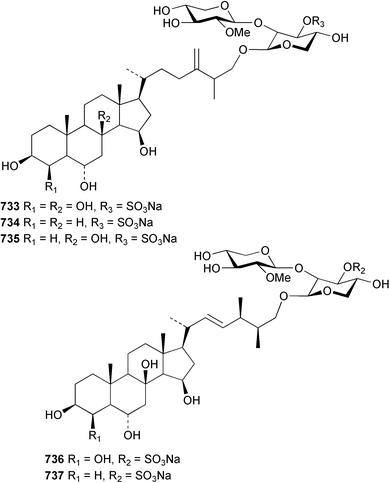
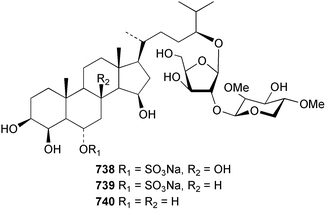
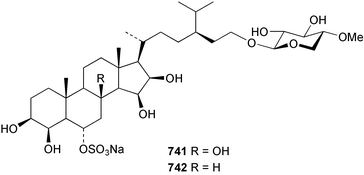
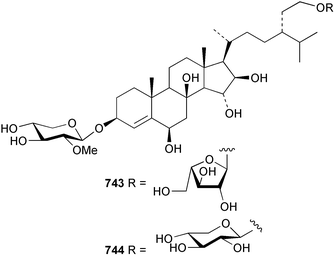
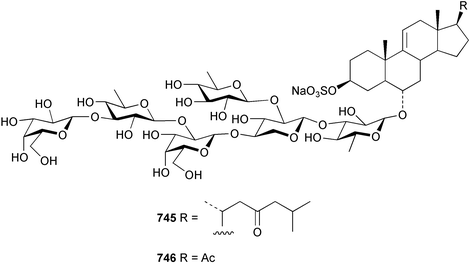
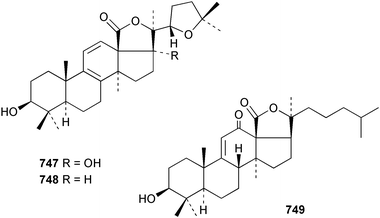
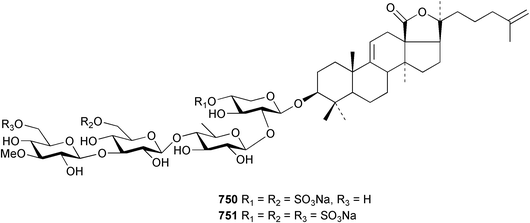
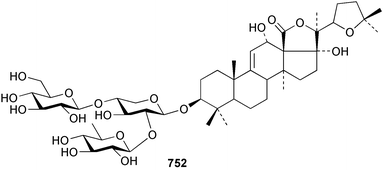
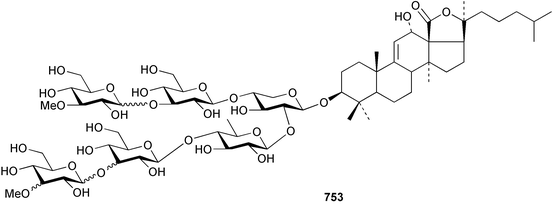
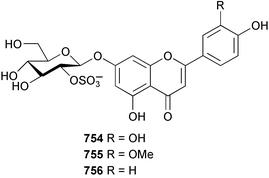
Marine natural products
John W.
Blunt
*a,
Brent R.
Copp
b,
Murray H. G.
Munro
a,
Peter T.
Northcote
c and
Michèle R.
Prinsep
d
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: j.blunt@chem.canterbury.ac.nz
bDepartment of Chemistry, University of Auckland, Auckland, New Zealand
cSchool of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dDepartment of Chemistry, University of Waikato, Hamilton, New Zealand
First published on 14th January 2004
Abstract
Covering: 2002. Previous review: Nat. Prod. Rep., 2003, 20, 1
This review covers the literature published in 2002 for marine natural products, with 579 citations (413 for the period January to December 2002) referring to compounds isolated from marine microorganisms and phytoplankton, green algae, brown algae, red algae, sponges, coelenterates, bryozoans, molluscs, tunicates and echinoderms. The emphasis is on new compounds (677 for 2002), together with their relevant biological activities, source organisms and country of origin. Syntheses that lead to the revision of structures or stereochemistries have been included (114), including any first total syntheses of a marine natural product.
 John W. Blunt | John Blunt obtained his BSc (Hons) and PhD degrees from the University of Canterbury, followed by postdoctoral appointments in Biochemistry at the University of Wisconsin-Madison, and with Sir Ewart Jones at Oxford University. He took up a lectureship at the University of Canterbury in 1970, where he is now a Professor. His research interests are with natural products, and the application of NMR techniques to structural problems. |
 Brent R. Copp | Brent Copp received his BSc (Hons) and PhD degrees from the University of Canterbury, where he studied the isolation, structure elucidation and structure–activity relationships of biologically active marine natural products under the guidance of Professors Blunt and Munro. He undertook postdoctoral research with Jon Clardy at Cornell and Chris Ireland at the University of Utah. 1992-93 was spent working in industry as an isolation chemist with Xenova Plc, before returning to New Zealand to take a lectureship at the University of Auckland, where he is currently a Senior Lecturer. |
 Murray H. G. Munro | Murray Munro, a Professor in Chemistry at the University of Canterbury, Christchurch, New Zealand, has worked on New Zealand natural products all of his professional career. His interest in marine natural products was stimulated by a study-leave period spent with Professor Kenneth Rinehart, University of Illinois, in 1973. A marine chemistry group at Canterbury followed soon after and has been active since. In more recent years his research interests have widened to include marine fungi and actinomycetes and drug delivery systems based on polymer therapeutics. |
 Peter Northcote | Peter Northcote received his BSc and PhD degrees from the University of British Columbia, Canada where he was a member of R. J. Andersen's marine natural products research group. He carried out postdoctoral research with Professors Blunt and Munro at the University of Canterbury before taking a position as a senior research scientist at Lederle Laboratories, American Cyanamid Co. He joined the faculty of the Victoria University of Wellington in 1994 where he is currently a Senior Lecturer in organic chemistry. |
 Michèle Prinsep | Michèle Prinsep received her BSc (Hons) and PhD degrees from the University of Canterbury, where she studied the isolation and structural elucidation of biologically active secondary metabolites from sponges and bryozoans under the supervision of Professors Blunt and Munro. She undertook postdoctoral research on cyanobacteria with Richard Moore at the University of Hawaii before returning to New Zealand to take up a lectureship at the University of Waikato, where she is currently a Senior Lecturer. |
1 Introduction
In the introduction to the previous review1 in this series, we paid tribute to the late Professor D John Faulkner for his contributions not only to the preparation of all the prior reviews in this series, but also for his enormous contribution to research in marine natural products. We are very pleased to be able to continue this series of reviews, especially for inclusion in this issue dedicated to John Faulkner.Unfortunately, we now have to pay a further tribute – this time, to the late Professor Paul J Scheuer who died in January 2003. Paul Scheuer has been widely regarded as the ‘grandfather’ of marine natural products. There are many working in the field today who can track their academic genealogy back to Professor Scheuer. Like John Faulkner, he was a true leader in the field. His vision and personality have marked the last forty plus years of research into marine natural products and his contributions too have been of enormous impact. Scheuer would have been cited over 150 times in the ‘Faulkner’ review series. The earliest reference describing his interests with marine natural products was his paper in 1960 on “Observations on ciguatera-type toxin in fish”.2
Despite health problems over the past few years both Paul Scheuer and John Faulkner continued their vital interest in research. An indication of their continued involvement until the times of their death is the inclusion in this review of four publications from Professor Scheuer describing 18 new compounds, and seven publications from Professor Faulkner describing 16 new compounds. Over the next year much will be written about the contributions each has made, but one thing is certain: between them Scheuer and Faulkner set the foundations for ‘marine natural products’, and then were instrumental in the evolution of the general field into more niche areas such as marine ecology and marine pharmacology.
This review is of the literature for 2002 and describes 677 new compounds from 257 articles. We show structures only for new compounds, or for previously reported compounds where there has been a structural revision or a newly established stereochemistry. Previously reported compounds for which first syntheses or new bioactivities are described, are referenced, but structures are not given.
2 Reviews
Several reviews have dealt with particular marine-derived compounds. The microcystins and nodularins are the focus of “Peptide toxins of cyanobacteria”.3 Didemnins are comprehensively reviewed in “Natural products as probes of cell biology: 20 years of didemnin research”.4 “Okadaic acid: the archetypal serine/threonine protein phosphatase inhibitor”5 focuses on the role that okadaic acid has played in stimulating a broad spectrum of modern scientific research. Dolastatins and related compounds are reviewed in two articles, “Symbiotic and dietary marine microalgae as a source of bioactive molecules”6 and “The cyanobacterial origin of potent anticancer agents originally isolated from sea hares”.7 A personal perspective on research programs which have been inspired by palytoxin has been given by Kishi.8 Callystatins are included in “The chemistry and biology of the leptomycin family”.9 Ecteinascidins feature in two reviews, “Ecteinascidin 743: a novel anticancer drug with a unique mechanism of action”10 and “Chemistry and biology of the tetrahydroisoquinoline antitumour antibiotics”.11 Bryostatins are reviewed in “The chemistry and biology of the bryostatin antitumour alkaloids”12 and “The clinical development of the bryostatins”.13A series of reviews have dealt with broad compound classes. “Survey of briarane-type diterpenoids of marine origin”14 contains a compilation of 299 briarane-type metabolites. Marine-derived compounds are included in “Natural guanidine derivatives”,15 “Muscarine, imidazole, oxazole, thiazole, Amaryllidaceae and Sceletium alkaloids”,16 “Natural halogenated fatty acids: their analogues and derivatives”,17 “Simple indole alkaloids and those with a nonrearranged monoterpenoid unit”,18 “Indolizidine and quinolizidine alkaloids”,19 “Bromo- and iodo-containing alkaloids from marine microorganisms and sponges”,20 “Natural occurrence of boron-containing compounds in plants, algae and microorganisms”,21 “Sterols in marine invertebrates”,22 and “Structural diversity of marine oxylipins”.23 An unusual approach to looking at possible biosynthetic relationships is presented in “The pyridoacridine family tree: a useful scheme for designing synthesis and predicting undiscovered natural products”.24 Synthesis is the focus of “Ladder-extension in the synthesis of marine polyether toxins”.25
Reviews of the chemistry of particular types of marine organism include “The oxylipin chemistry of attraction and defense in brown algae and diatoms”,26 “Bioactive compounds from bryozoans”,27 “Neuropeptides in cnidarians”,28 “Toxins and bioactive compounds from cyanobacteria and their implications on human health”,29 “The heterocyclic natural products of gorgonian corals of genus Briareum exclusive of briarane-type diterpenoids”,30 “Secondary metabolites from marine fungi”,31 “Secondary metabolites from marine microorganisms”,32 “Pore-forming proteins from sea anemones and the construction of immunotoxins for selective killing of harmful cells”,33 “A survey of the sterol composition of the marine dinoflagellates Karenia brevis, Karenia mikimotoi and Karlodinium micrum”,34 “The chemistry of lithistid sponge: a spectacular source of new metabolites”,35 and “Chemical defense of early life stages of benthic marine invertebrates”.36
More general reviews have been “Drugs from the seas – current status and microbiological implications”,37 “Marine pharmacology in 1999: compounds with antibacterial, anticoagulant, antifungal, anthelmintic, anti-inflammatory, antiplatelet, antiprotozoal and antiviral activities affecting the cardiovascular, endocrine, immune and nervous systems, and other miscellaneous mechanisms of action”,38 “Australian biodiversity via its plants and marine organisms. A high-throughput screening approach to drug discovery”,39 and “Secondary metabolites with antinematodal activity”.40 While not specifically focusing on marine natural products, the article on “Application of a new expert system for the structure elucidation of natural products from their 1D and 2D NMR data”41 should be of significant interest to those investigating new compounds. There has been a further report on the Chinese database “A marine natural product database”42 while the Marinlit database43 continues to be updated and has again been used as the basis for the preparation of this present review.
3 Marine microorganisms and phytoplankton
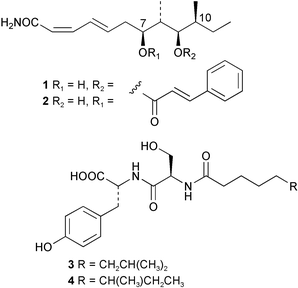
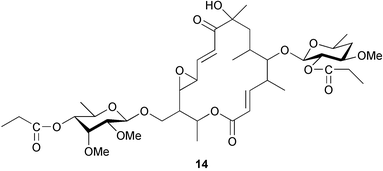
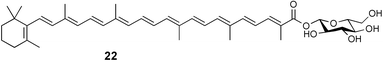
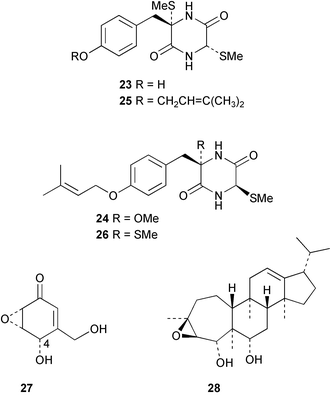
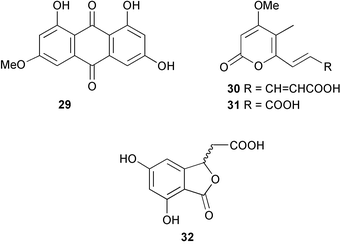
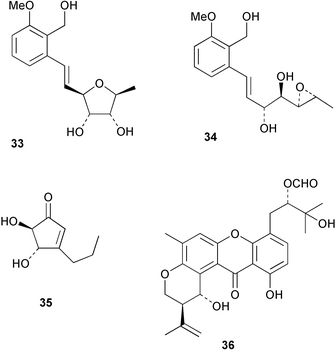
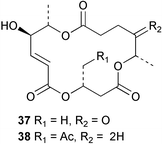
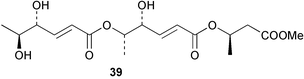
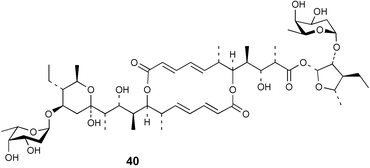
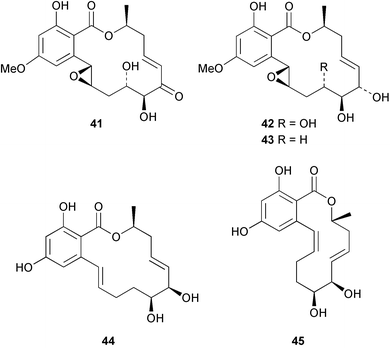
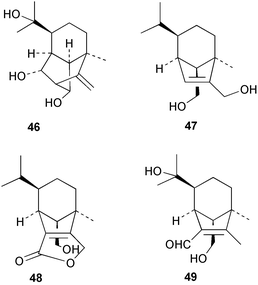
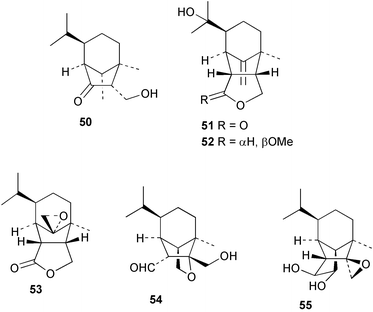
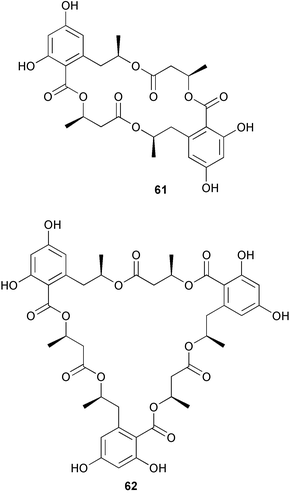
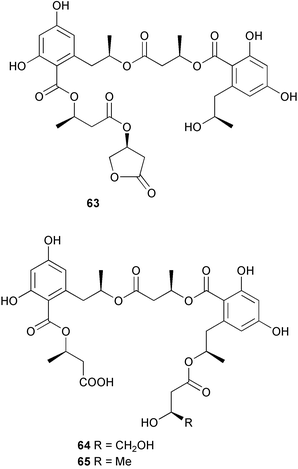
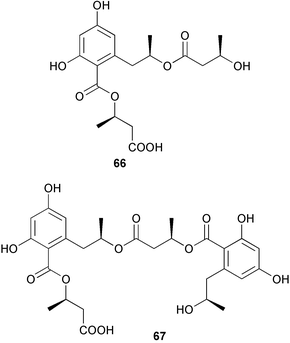
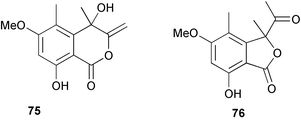
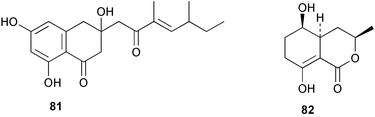
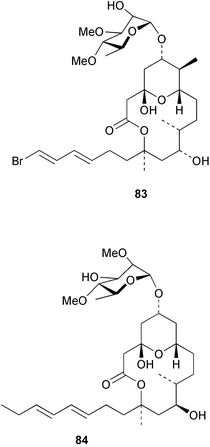
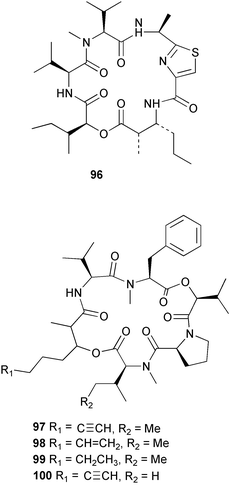
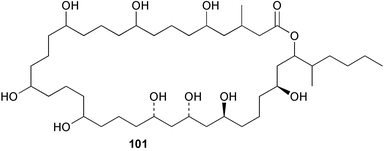
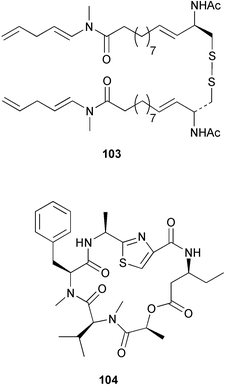
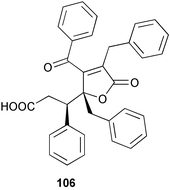
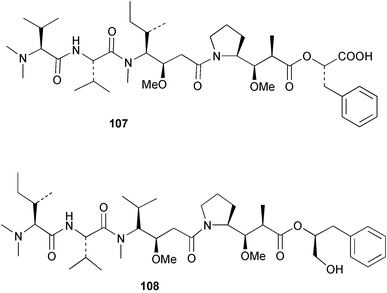
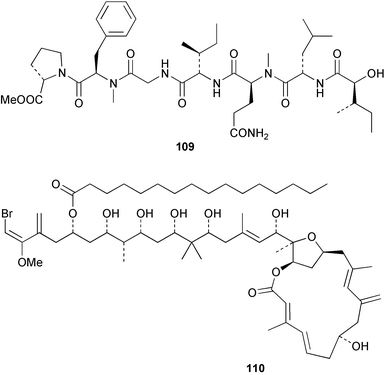
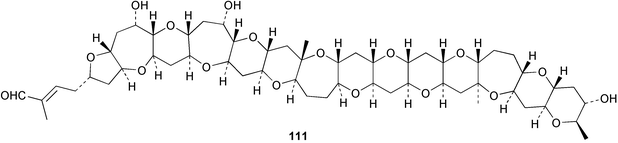
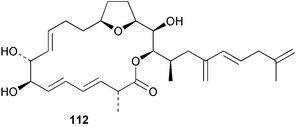
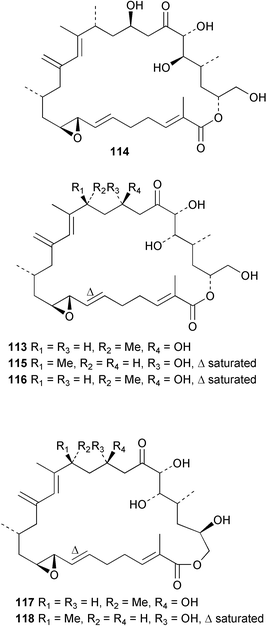
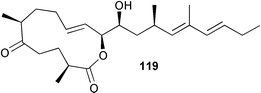
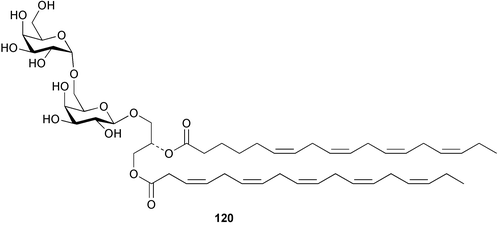
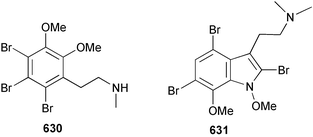
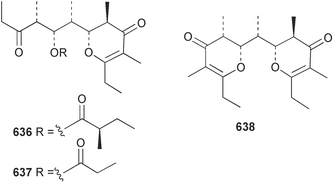
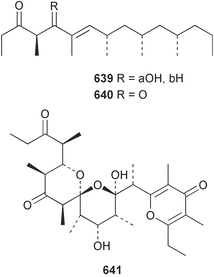
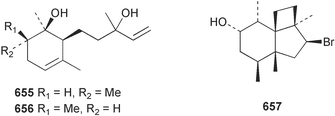
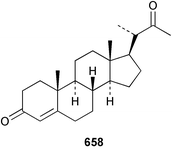
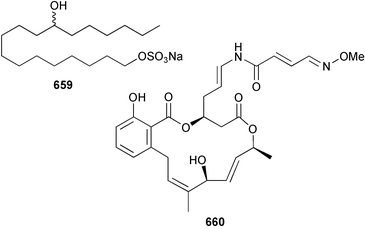
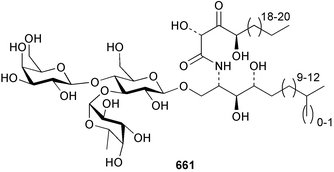
Extracts of marine microorganisms, whether obtained from culture or directly from a collected sample, continue to yield an array of novel compounds. A culture of Bacillus laterosporus, isolated from the tissues of an unidentified tube worm from Loloata Island, Papua New Guinea, was the source of the novel antifungal polyketide metabolites basiliskamides A 1 and B 2 and of two new acyldipeptides, tupuseleiamides A 3 and B 4.44 The (S) configuration of the secondary alcohol at C-7 in basiliskamide A 1 was determined by Ohtani's Mosher ester analysis method,45 but the configuration at C-10 was not determined and is assumed to be (S) as in a known homologue.46,47 The diagrams here for 1 and 2, showing (7S,8S,9R,10S), are corrections from those given in the original paper.44 The (R) configuration of the tyrosine and serine residues in tupuseleiamides A 3 and B 4 was determined by chiral GC analysis but the configuration at C-18 was not determined. Basiliskamides A 1 and B 2 were both converted to the same diol by DIBAL reduction, indicating identical configurations in both molecules. Basiliskamides A and B showed potent in vitro activity against Candida albicans while basiliskamide A 1 exhibited activity comparable to amphotericin B, but was at least four-fold less cytotoxic to human fibroblast cells.44 Cultured Bacillus pumilus, isolated from the surface of the ascidian Halocynthia aurantium from Troitza Bay in Russian waters, yielded a mixture of surfactin-like cyclic depsipeptides 5–9. These lipopeptides differed from surfactin by substitution of the 4-valine by leucine and were isolated as two carboxy-terminal variants with either valine or isoleucine in the 7-position.48 The bis-catechol α-hydroxy acid siderophore, petrobactin 10, was isolated from the cultured oil-degrading marine bacterium Marinobacter hydrocarbonoclasticus.49 The cyclic hexapeptide halolitoralin A 11 and tetrapeptides halolitoralins B 12 and C 13 were isolated from the fermentation broth of Halobacillus litoralis, which had originated from a high-salt sediment from the Huanghai Sea, China. All amino acid residues were established as (S) by hydrolysis and subsequent Marfey's analysis. The halolitoralins A–C 11–13 exhibited moderate antifungal activity against C. albicans, T. rubrum and four crop-threatening fungi, in addition to moderate activity against the human gastric tumour BGC cell line.50 The macrolide antibiotic chalcomycin B 14 was isolated from the culture broth of a Streptomyces sp. derived from mangrove sediment collected near Pohoiki, Hawaii.51 Chalcomycin B 14 exhibited activity against a variety of microorganisms and microalgae. Cultures of Humicola grisea, a filamentous fungus isolated from drift wood in New Caledonian waters, were the source of humicolone 15, a phenolic tetralone in acetal form that exhibited appreciable cytotoxicity towards KB cell lines. The absolute configuration of humicolone 15 was established by Mosher's method and molecular modelling.52 A cyclic tetrapeptide, designated JM47 16, was isolated from a marine Fusarium species isolated from the green alga Codium fragile subsp. atlanticum collected in Scottish waters and was determined to be cyclo(Ala-Ala-Aoh-Pro), where Aoh is (2S,9S)-2-amino-8-oxo-9-hydroxydecanoic acid.53 The absolute stereochemistry of the core was determined by acidic hydrolysis and chiral TLC analysis of the proline residue.53 A culture of a strain of the mangrove fungus Eutypa sp. isolated from wood in the South China Sea yielded eutypoid A 17 from the culture mycelium.54 Four new epipolysulfanyldioxopiperazines, leptosins M 18, M1 19, N 20 and N1 21 were isolated from a culture of the fungus Leptosphaeria sp. originating from the Japanese brown alga Sargassum tortile.55 Absolute stereochemistries were determined by chemical analyses and transformations. Each compound possessed significant cytotoxic activity against the P388 cell line while leptosin M 18 also exhibited appreciable cytotoxicity against a disease-oriented panel of 39 human cancer cell lines and specifically inhibited two protein kinases and topoisomerase II.55 A carotenoid glycosyl ester 22 was isolated from cultured cells of a Fusarium species isolated from the seawater surface at Tanegashima, Japan.56 Cultured Fusarium chlamydosporum, isolated from the Japanese marine red alga Carpopeltis affinis, was the source of fusaperazines A 23 and B 24, two new sulfur-containing dioxopiperazine derivatives, and two known compounds 25 and 26 which had been originally isolated from a fermentation of the fungus Tolypocladium sp.57 In the current report, the absolute configurations of 25 and 26 were determined by chemical transformations and the stereostructures of 23 and 24 established by comparison.58 A new gabosine derivative, parasitenone 27 was isolated from a culture of the fungus Aspergillus parasiticus from the Korean red alga Carpopeltis cornea. The absolute configuration of parasitenone 27 was determined to be (4S,5S,6S) on the basis of CD data and a chemical transformation.59 Aspergilloxide 28, a sesterterpene epoxy-diol, was isolated from an extract of an undescribed Aspergillus species from the Bahamas. The absolute configuration was assigned by application of the modified Mosher method.60 The fungus Curvularia lunata, isolated from the marine sponge Niphates olemda from Indonesian waters, was the source of lunatin 29, shown to be active against S. aureus, E. coli and B. subtilis but inactive against C. albicans.61 Two new α-pyrones, herbarin A 30 and herbarin B 31 along with a new phthalide herbaric acid 32 were isolated from two cultured strains of the fungus Cladosporium herbarum isolated from the sponges Aplysina aerophoba and Callyspongia aerizusa collected in the French Mediterranean and in Indonesian waters respectively.61 Herbarins A 30 and B 31 displayed activity in the brine shrimp assay.61 A culture of the fungus Emericella variecolor isolated from a sponge collected in the Caribbean Sea off Venezuela yielded varitriol 33, varioxirane 34, dihydroterrein 35 and varixanthone 36 which were characterised by spectroscopic methods and chemical transformations.62 Varitriol 33 displayed increased potency toward some renal, CNS and breast cancer cell lines in the NCI's 60-cell line panel while varixanthone 36 displayed antimicrobial activity against a range of bacteria.62 Macrosphelide L 37 was obtained from a strain of the fungus Periconia byssoides cultured from the sea hare Aplysia kurodai.63 The absolute stereostructure, along with that of macrosphelide H 38, previously isolated from the same fungal species,64,65 was determined by spectroscopic analyses and chemical transformations. Both compounds inhibited the adhesion of human-leukemia HL-60 cells to human-umbilical-vein endothelial cells (HUVEC).63 The triester seco-macrosphelide E 39 was isolated from a strain of the fungus P. byssoides separated from the sea hare Aplysia kurodai. The absolute stereostructure was elucidated by spectroscopic analyses and synthesis.66 The syntheses of macrosphelides H 38 and G,65 also from P. byssoides, have been published.67,68 The Japanese fish Halichoeres bleekeri69 was the source of a cultured strain of Streptomyces hygroscopicus from which halichoblelide 40, a macrolide with potent cytotoxicity against the murine P388 cell line and 39 human cancer cell lines, was isolated. The absolute configuration of halichoblelide 40 was established by spectroscopic analyses and chemical transformation.70 The source of the 14-membered resorcylic macrolides aigialomycins A–E 41–45 was a culture of the mangrove fungus Aigialus parvus. The structures, including absolute configurations, were determined by spectroscopic methods, chemical conversions and X-ray analysis of a derivative of aigialomycin C 43. Aigialomycin D 44 displayed antimalarial activity in vitro against Plasmodium falciparum in addition to moderate cytotoxicity against the KB, BC-1 and Vero cell lines.71 Ten new sesquiterpenoids, isosativenetriol 46, drechslerines A 47 and B 48, 9-hydroxyhelminthosporol 49, drechslerines C–G 50–54 and sativene epoxide 55 were isolated from a culture of the fungus Drechslera dematioidea from the red alga Liagora viscida. Drechslerines E 52 and G 54 exhibited antiplasmodial activity against two strains of P. falciparum.72 Two γ-pyrone derivatives, microsphaerones A 56 and B 57, were isolated from a culture of the fungus Microsphaeropsis sp. from the marine sponge Aplysina aerophoba collected in French waters.73 The presence of an (S)-2-methylsuccinic acid moiety in microsphaerone A 56 was established by GC-MS analysis of a (−)-menthylated hydrolysis product.73 Three diterpene glycosides, virescenosides O–Q 58–60 have been isolated from a cultured strain of Acremonium striatisporum associated with the holothurian Eupentacta fraudatrix.74 Virescenosides O–Q 58–60 were cytotoxic against Ehrlich carcinoma cells in vitro while virescenoside P 59 was also cytotoxic to developing eggs of the sea urchin Strongylocentrotus intermedius.74 Cultures of the marine fungus Hypoxylon oceanicum75 from mangrove wood from Shenzen, China, yielded the macrocyclic polyesters 61 and 62 and the linear polyesters 63–67.76 The absolute configurations of the polyesters were deduced from CD spectral studies. Compounds 61 and 62 exhibited modest activity against the phytopathogenic fungus Neurospora crassa.76 The marine sponge Xestospongia exigua collected from the Bali Sea, Indonesia, was the source of fungal isolates of Penicillium cf. montanense. Cultures of these isolates gave the xestodecalactones A–C 68–70, 10-membered macrolides with a fused 1,3-dihydroxybenzene ring. Xestodecalactones 69 and 70 are diastereoisomeric but only 69 was active against C. albicans.77 A culture of the facultative marine ascomycete Zopfiella latipes, originally isolated from Indian Ocean soil, was the source of zopfiellamides A 71 and B 72 which were moderately active against Arthrobacter citreus, B. brevis, B. subtilis, B. licheniformis, Corynebacterium insidiosum, Micrococcus luteus, Mycobacterium phlei, Streptomyces sp. and Acinetobacter calcoaceticus.78 Halorosellins A 73 and B 74 were isolated from the culture broth of the marine fungus Halorosellinia oceanica of Thai origin. In addition, 75–77 were isolated. The isobenzofuran-1-one 76 exhibited moderate antimycobacterial activity.79 A culture of an unidentified fungus from the South China Sea yielded the cyclic tetrapeptides 78–80,80 which are very similar to the bacterial metabolites, the halolitoralins B 12 and C 13 (vide supra). The filamentous marine fungus Keissleriella sp. isolated from a Yellow Sea sediment source gave a dihydronaphthalen-1(4H)-one 81 which was antifungal in vitro against C. albicans, T. rubrum and A. niger.81 5-Hydroxyramulosin 82 originated from the fungus Phoma tropica, isolated from the brown alga Fucus spiralis. The structure was secured by an X-ray analysis.82 A Palauan collection of the marine cyanobacterium Lyngbya sp. was the source of the brominated glycoside macrolide, lyngbyaloside B 83. The relative stereochemistry of the 12 stereocentres has been proposed on the basis of coupling constant and ROESY NMR data. Lyngbyaloside B 83 exhibited weak cytotoxicity against KB cells.83 A related glycosidic macrolide, lyngbouilloside 84, was isolated from L. bouillonii collected from Papua New Guinea. Lyngbouilloside 84 exhibited modest cytotoxicity to neuro-2a neuroblastoma cells.84 A Guam collection of Lyngbya sp. yielded apratoxin B 85 while a collection of the same species from Palau afforded apratoxin C 86. The chirality of the constituent amino acids was determined as (S) by chiral HPLC analysis of the hydrolysis products. Apratoxin C 86 exhibited appreciable cytotoxicity against KB and LoVo cells, while apratoxin B 85 and the semisynthetic (E)-dehydroapratoxin A were considerably less active.85Lyngbya sp. from Palau also yielded the dichlorinated thiazole hydroxy acid-containing cytotoxic macrolide lyngbyabellin C 87, the modified tetrapeptides lyngbyapeptins B 88 and C 89 and a cytotoxic N-acylpyrrolinone, palau'imide 90.86 The absolute stereochemistry of lyngbyabellin C 87 could not be determined as only minute amounts were available but cytotoxicity to both KB and LoVo cell lines was noted. One ester linkage of lyngbyabellin C was particularly prone to methanolysis. Regioselective ester cleavage at C-16 caused conversion to the methyl ester homohydroxydolabellin and led to the speculation that the sea hare metabolite dolabellin87 is likely to be an artifact rather than a natural product. Homohydroxydolabellin had virtually identical activity against KB and LoVo cell lines to lyngbyabellin C 87. The absolute stereochemistries of lyngbyapeptins B 88 and C 89 were found to be all (S) by chiral HPLC analysis of hydrolysis products and the (E) stereochemistry for the olefin in lyngbyapeptin C 89 was established by a ROESY NMR experiment. The configurations of C-4 and C-15 of palau'imide 90 were deduced by analysis of degradation products but that at C-20 was not determined due to lack of material. Palau'imide was cytotoxic to KB and LoVo cells.86 The first total syntheses of the lipopeptides lyngbyabellin A, originally isolated from collections of L. majuscula from Guam,88 and lyngbyabellin B isolated from collections of Lyngbya sp. from Guam89 and Florida90 respectively, have been described. The functionalised thiazole carboxylic acids were prepared by oxidative dehydrogenation of the corresponding thiazolidines with manganese dioxide.91 Collections of Lyngbya sp. from various Palauan dive sites were the source of six new β-amino acid-containing cyclic depsipeptides, the ulongamides A–F 91–96. The absolute stereochemistries of the hydroxy acid and all α-amino acid-derived units were elucidated as (S) by chiral HPLC analysis of hydrolysis products. Advanced Marfey's analysis of the acid hydrolysates determined the stereochemistry of 3-amino-2-methylhexanoic acid as (2R,3R) in ulongamides A–C 91–93 and (2S,3R) in ulongamides D–F 94–96. Ulongamides A–E 91–95 were weakly cytotoxic against KB and LoVo cells in vitro, while ulongamide F 96 was inactive.92 A Madagascan collection of L. majuscula was the source of further depsipeptides, the antanapeptins A–D 97–100. Structures were deduced by 2D NMR and mass spectral analysis.93 A 36-membered macrolactone (25S,27S,29S,33S)-caylobolide A 101 was isolated from L. majuscula from the Bahamas. The relative stereochemistry of the 1,3,5-triol was determined using Kishi's Universal NMR database while the absolute stereochemistry at C-25, C-27, C-29 and C-33 was determined by Mosher's analysis. Caylobolide A 101 displayed cytotoxicity against human colon tumour cells (HCT-116) in vitro.94 Hectochlorin 102 was isolated from L. majuscula collected from Hector Bay, Jamaica, and Boca del Drago Beach, Panama. The absolute stereochemistry of 102 was determined as (2S,3S,14S,22S) by X-ray analysis. Hectochlorin 102, a potent stimulator of actin polymerisation, shows a unique profile of cytotoxicity by the COMPARE algorithm in the NCI panel and is strongly inhibitory towards C. albicans.95 A total synthesis of hectochlorin 102 has now been accomplished.96 A mixed assemblage of L. majuscula and a Schizothrix sp. from Fijian waters yielded the cytotoxic disulfide dimer somocystinamide A 103. The absolute stereochemistry was established as (2R,2′R) by HPLC analysis of hydrolysis products.97 A collection of L. confervoides from Saipan in the Commonwealth of the Northern Mariana Islands was the source of a cytotoxic cyclic depsipeptide, obyanamide 104. The absolute stereochemistry was determined by chiral chromatography of hydrolysis products and comparison with authentic and synthetic standards. Obyanamide 104 was cytotoxic to KB cells.98 An antifungal cyclododecapeptide, lobocyclamide B 105 has been isolated from a benthic mat of L. confervoides from the Bahamas. The absolute stereochemistry of 105 was established by a combination of chiral HPLC and Marfey's methods. Lobocyclamide B 105 displays antifungal activity against fluconazole-resistant C. albicans.99 Maculalactone M 106, a benzofuranone derivative, was isolated from the epilithic encrusting cyanobacterium Kyrtuthrix maculans collected in Hong Kong. The planar structure was established by spectral analysis but an attempt to determine the absolute stereochemistry by CD spectroscopy was unsuccessful.100 A new dolastatin 10101 analogue, symplostatin 3 107, has been isolated from a tumour-selective extract of an Hawaiian collection of Symploca sp. VP452 which taxonomically appears to be S. hydnoides. The absolute stereochemistry of 107 was established by chiral HPLC of acid hydrolysis products. Symplostatin 3 107 only differs from dolastatin 10 in the C-terminal unit, where the dolaphenine unit is substituted by a 3-phenyllactic acid residue. Symplostatin 3 also displays strong in vitro cytotoxicity towards a range of human tumour cell lines and disrupts microtubules but at a higher concentration than dolastatin 10.102 A cytotoxic peptide ester, malevamide D 108, closely related to isodolastatin H,103 was isolated from an Hawaiian collection of S. hydnoides. Partial stereochemical assignments were made by chiral HPLC analysis of hydrolysates. Malevamide D 108 displayed toxicity against P388, A-549, HT-29 and MEL-28 cell lines in the sub-nanomolar range.104 A Palauan collection of Symploca sp. was the source of the acyclic peptide tasiamide 109. The absolute stereochemistry of 109 was established by chiral HPLC of degradation products. Tasiamide 109 was cytotoxic against KB and LoVo cells.105 A laboratory culture of a Phormidium sp. from Sulawesi, Indonesia yielded phormidolide 110, a bromine-containing macrolide. The absolute stereochemistry of the 11 stereocentres was determined on a bis-acetonide derivative using the variable temperature Mosher ester method. Phormidolide 110 was a potent brine shrimp toxin.106 A new cytotoxic polyether, gymnocin-A 111 has been isolated from a culture of the red tide dinoflagellate Gymnodinium mikimotoi from Japanese waters. The structure, which consists of 14 contiguous ether rings and a 2-methyl-2-butenal sidechain, was determined by NMR and mass spectral analysis while the absolute stereochemistry of 111 was elucidated by the modified Mosher method. Gymnocin-A 111 was cytotoxic in a cell-based assay but only weakly toxic to fish.107 The first total synthesis of the polycyclic ether toxin (−)-gambierol, isolated from cultured cells of the ciguatera-causative dinoflagellate Gambierdiscus toxicus,108 has been achieved.109,110 The synthesis features a Stille coupling for the stereoselective construction of the triene sidechain.110 The absolute stereochemistry at eight chiral centres in amphidinolide E 112, a cytotoxic 19-membered macrolide isolated from the marine dinoflagellate Amphidinium sp.,111 has been determined as (2R,7R,8R,13S,16S,17R,18R,19R) by NMR spectroscopic data analysis, modified Mosher's method and the exciton chirality method.112 Meanwhile, six new macrolides, amphidinolides H2–H5 113–116, G2 117 and G3 118 were isolated from the dinoflagellate Amphidinium sp. obtained from an Okinawan acoel flatworm Amphiscolops sp.113 The absolute stereochemistries of compounds 113–117 were determined from coupling constant data, distance geometry calculations and chemical means, while that of amphidinolide G3 118 was established by comparison of NMR data with those of amphidinolide G.114 Compounds 113–117, in addition to amphidinolides G–H,114 B,115 D116 and five derivatives of amphidinolide H, exhibited varying levels of cytotoxicity against murine L1210 cells and human epidermoid carcinoma KB cells.113 A further cytotoxic 12-membered macrolide, amphidinolide W 119, has also been isolated from an Amphidinium sp. from the flatworm Amphiscolops sp.117 Spectroscopic means, including analysis of 13C-13C INADEQUATE correlations for a 13C-enriched sample, established the structure, while the absolute stereochemistry of 119 was assigned by J-based configuration analysis and the modified Mosher method. Amphidinolide W 119 is the first macrolide without an exomethylene unit among all amphidinolides obtained to date.118 Three groups more or less simultaneously succeeded in achieving total syntheses of the originally proposed structure of amphidinolide A,119via a ring-closing metathesis,120 ruthenium-catalysed alkene–alkyne coupling121 or employing extensive use of inter-and intramolecular Stille reactions.122 It was concluded that the structure of amphidinolide A needed revision. A total synthesis of amphidinolide T4123 has been accomplished.124 A digalactosyl diacylglycerol 120 was isolated from a culture of the Japanese marine dinoflagellate Heterocapsa circularisquama. Compound 120 exhibits cytolytic activity towards oyster heart cells.125 The first stereoselective synthesis of the cytotoxic peptide aspergillamide B from an Aspergillus sp.126 has been reported127 as well as the first total synthesis of somamide A, a 19-membered macrocyclic depsipeptide isolated from assemblages of the cyanobacteria L. majuscula and Schizothrix sp.128 The anti-inflammatory and anti-proliferative properties of scytonemin, an extracellular sheath pigment originally isolated from the cyanobacterium Stigonema sp.,129 have been reported.130,131 Goniodomin A, an antifungal polyether macrolide from the dinoflagellate Goniodoma pseudogoniaulax132 has been shown to inhibit angiogenesis by inhibition of endothelial cell migration and basic fibroblast growth factor (bFGF) induced tube formation and is active in vivo.1334 Green algae
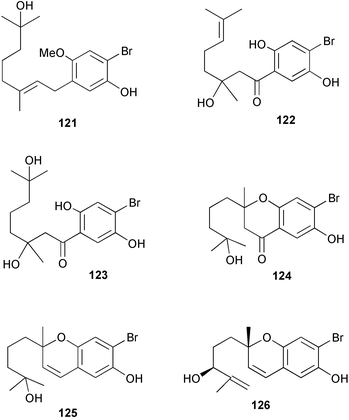
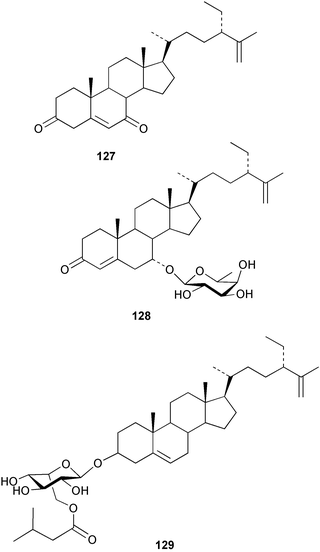
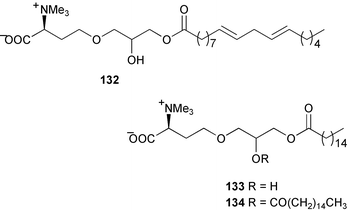
Relatively few compounds have been reported from green algae recently. From the Cuban green alga Cymopolia barbata six new prenylated bromohydroquinones 121–126 were isolated.134 The NMR data for five known but related cymopol compounds were also assigned, as literature data135,136 were either incomplete or unassigned.134 The green alga Codium iyengarii from the Karachi coast of the Arabian Sea was the source of a new steroid, iyengadione 127 and two new steroidal glycosides, iyengarosides A 128 and B 129. Iyengaroside-A 128 displayed moderate activity against a range of bacteria.137 Two novel carotenoid C14:1 trans-Δ2 esters, siphonaxanthin C14:1 trans-Δ2 ester 130 and 6′-hydroxy siphonaxanthin C14:1 trans-Δ2 ester 131 were isolated from the green alga Pterosperma cristatum collected in Japanese waters.138 An inseparable mixture of nitrogenous glycerolipids 132–134 was isolated from the green alga Ulva fasciata collected from the Indian Coast. 133 was the major component of the mixture.139 Some glycoglycerolipids from the green alga Ulvella lens have been identified as chemical inducers to settlement and metamorphosis of planktonic larvae of the sea urchin Strongylocentrotus intermedius.1405 Brown algae
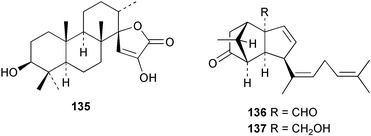
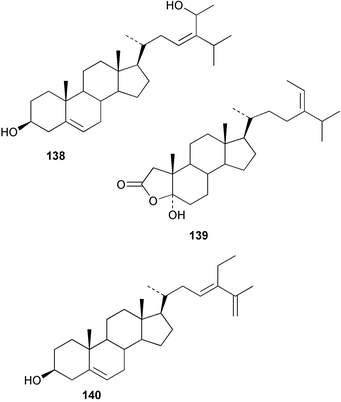
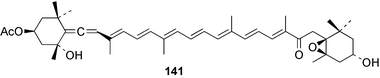
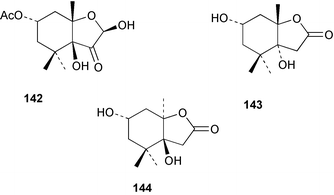
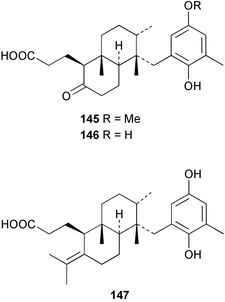
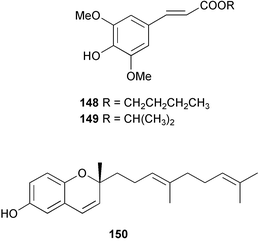
Terpenoids and steroids dominate the compounds reported from brown algae. In the course of examination of the sterol composition of the brown alga Cystoseira crinita collected in the Mediterranean off Turkey, a new sterol 24-norchola-5,22-dien-3β-ol, was identified by GC-MS analysis.141 Stypolactone 135, a diterpenoid of mixed biogenesis has been isolated from the brown alga Stypopodium zonale. The structure and relative stereochemistry were determined from spectroscopic evidence and biogenetic considerations. Stypolactone 135 displayed weak cytotoxicity in vitro against the A-549 and H-116 cell lines.142 Two diterpenoids with a novel skeleton, dictyterpenoids A 136 and B 137, were isolated from the brown alga Dilophus okamurae collected from the Japan Sea Coast and displayed antifeedant activity against young abalone.143S. carpophyllum from the South China Sea was the source of two new bioactive sterols 138 and 139. These sterols induced morphological abnormality in the plant pathogenic fungus Pyricularia oryzae and 138 also exhibited cytotoxic activity against several cultured cancer cell lines.144S. polycystum collected in the North China Sea yielded a new sterol, stigmast-5,23,25-triene 140.145 The absolute configuration of the secondary alcohol in fucoxanthin 141, isolated in this case from the brown alga Turbinaria triquatra but otherwise ubiquitous in brown algae,146 was determined by the Mosher method. The T. triquatra extract displayed activity against NINH3T3 fibroblast and KA3IT murine cancer cell lines.147 Three loliolide derivatives, 142–144 have been isolated from the brown alga Undaria pinnatifida from Japanese waters. Containing a hemiacetal group, 142 is a unique loliolide derivative. The relative stereochemistry of 142 was determined by NOE measurements while 143 and 144 were determined to be diastereomers.148 The tropical brown alga Stypopodium zonale collected off the coast of Tenerife was the source of three terpenoids 145–147. Structures and relative stereochemistries were determined from the derived methyl esters. The methyl ester of 147 exhibited in vitro cytotoxic activity against HT-29 H-116 and A-549.149 Two shikimate derivatives 148 and 149 were isolated from the brown alga Spatoglossum variabile collected from the coast off Karachi.150 Total syntheses of sporochnols B and C, fish feeding deterrents originally isolated from the brown alga Sporochnus bolleanus,151 have been reported, using the C-H insertion reaction of alkylidenecarbene as the key step.152 Total syntheses of dictyochromenol 150 from the brown alga Dictyopteris undulata from Japan153 and the (Z)-stereoisomer have been reported and it was established that the natural enantiomer has an (R) configuration.154 A total synthesis of yahazunol, originally isolated from the brown alga Dictyopteris undulata,155 has been achieved, starting from (+)-albicanic acid.1566 Red algae
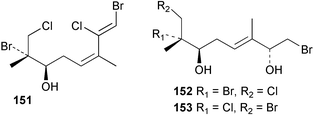
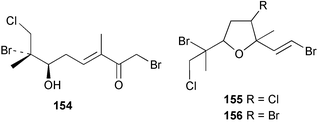
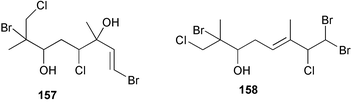
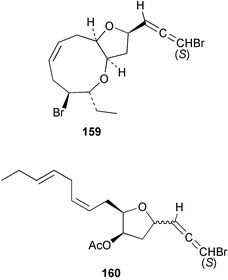
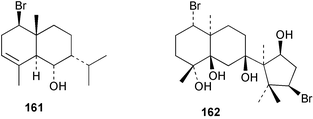
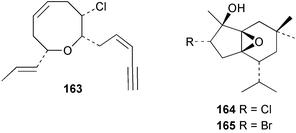
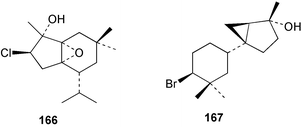
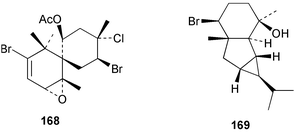
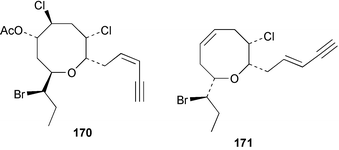
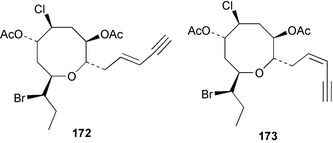
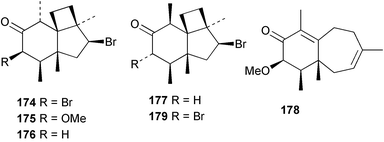
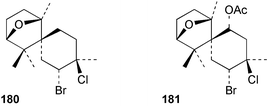
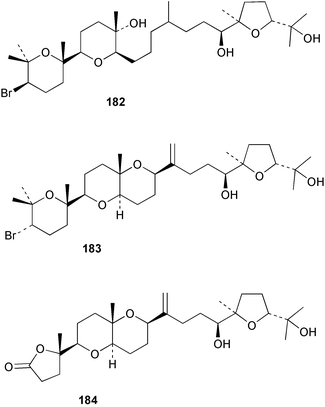
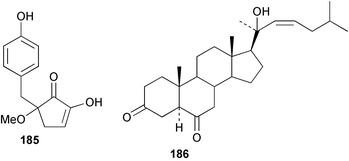
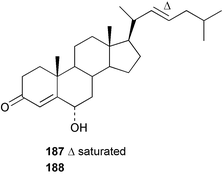
As for the brown algae, terpenoids and steroids are the dominant metabolite classes reported from red algae. A monoterpene, prefuroplocamioid 151, has been isolated from a Chilean sample of Plocamium cartilagineum. The (Z)-stereochemistries of the double bonds along with the C-6 relative stereochemistry were determined from NMR analyses but instability problems prevented assignment of absolute stereochemistry.157 This P. cartilagineum was also the source of the plocamenols A–C 152–154, linear polyhalohydroxylated monoterpenes,158 and three other halogenated monoterpenes 155–157.159 The structures and relative stereochemistries of these compounds were elucidated by spectroscopic means and led to assignment of the relative stereochemistry at C-7 as (S*) for furoplocamioids A–C, also from P. cartilagineum.160P. cartilagineum was the source of yet another new halogenated monoterpene 158.161 The antifeedant effects of a range of halogenated monoterpenes, originally isolated from P. cartilagineum160,162–166 and Pantoneura plocamioides,167 were tested against several divergent insect species including the Colorado potato beetle Lepinotarsa decemlineata, the aphids Myzus persicae and Ropalosiphum padi, and Spodoptera frugiperda-derived Sf9 cells.161 Two C15 acetogenins containing a terminal bromoallene moiety, itomanallenes A 159 and B 160, and a brominated sesquiterpene itomanol 161, have been isolated from Laurencia intricata from Okinawa.168 Another Okinawan red alga, L. yonaguniensi, was the source of neoirietetraol 162, a brominated diterpene based on the rare neoirieane skeleton. Also isolated was a chlorinated C15 acetogenin, (3Z)-laurenyne 163. The relative stereochemistry of 162 was determined from spectral data but an attempt to determine the absolute configuration was unsuccessful. Both neoirietetraol 162 and (3Z)-laurenyne 163 were toxic to brine shrimp and 162 was active against the marine bacteria Alcaligenes aquamarinus and E. coli.169 Three halogenated rearranged sesquiterpenes 164–166 containing the brasilane skeleton and a 1,6-epoxy moiety have been isolated from L. obtusa collected off Symi Island in the Aegean Sea, Greece. The structures and relative stereochemistries were established by spectral data analyses and molecular modelling.170 Bromocyclococanol 167, a sesquiterpene possessing a novel skeleton, was isolated from L. obtusa from Cayo Coco, Cuba. The structure and stereochemistry were established from spectroscopic data and biogenetic considerations.171 The 5-acetate derivative 168 of prepacifenol was isolated from both L. filiformis and the sea hare predator Aplysia parvula collected from Taroona, Tasmania. Also isolated was the known prepacifenol, originally found in a Laurencia sp. seaweed.172 An X-ray analysis was reported for 168 and the NMR spectra of prepacifenol were fully assigned for the first time. Both compounds exhibited moderate activity in the brine shrimp bioassay.173 The first 6,8-cycloeudesmane sesquiterpene of marine origin 169, has been isolated from L. microcladia from the Baia di Calenzana, Elba Island.174L. obtusa, collected from off Symi Island in the Aegean Sea, Greece was the source of four C15 acetogenins, 13-epilaurencienyne (3Z) 170, 13-epipinnatifidenyne (3E) 171 and two diacetoxypentadec-3-en-1-yne derivatives 172–173. 170 and 173 exhibited strong toxicity to ants with considerable knockdown effect on the first day, while compounds 171 and 172 exhibited gradual toxicity that escalated at the fourth day with > 70% mortality.175 The structures and relative stereochemistries of four undecane-3-one sesquiterpenes 174–177 and perforenone D 178 have been reported from L. obtusa collected at Milos Island in the Aegean Sea, Greece. The relative stereochemistry of the known compound perforatone 179 was also revised.176 Two sesquiterpenes with an oxacyclic chamigrene skeleton, oxachamigrene 180 and 5-acetoxyoxachamigrene 181, have been isolated from L. obtusa from Cayo Coco, Cuba.177L. viridis from Tenerife, Canary Islands was the source of three polyethers, clavidol 182, 3-epi-dehydrothyrsiferol 183 and lactodehydrothyrsiferol 184. The relative stereochemistries were proposed on the basis of ROESY and NOEDIFF data and biogenetic considerations.178 The Indonesian red alga Vidalia sp. was the source of the phenolic metabolite vidalenolone 185,179 while a new 3,6-diketosteroid 186 was isolated from the red alga Hypnea musciformis collected on the Atlantic Coast of Morocco. Compound 186 exhibited anti-elastase activity against porcine pancreas elastase (PPE). Two novel steroids 187 and 188 were also isolated as an inseparable mixture.180 A sulfoglycolipidic fraction from the red microalga Porphyridium cruentum, shown to contain large amounts of palmitic acid, arachidonic acid and eicosapentaenoic acid, strongly inhibited production of the superoxide anion and growth of DLD-1, MCF-7, PC-3 and M4 Beu cell lines.181Several syntheses of red algal metabolites were reported in 2002. 2,3,6-Tribromo-1-methylindole, originally isolated from L. brongniartii182 has been synthesised from 2,3-dibromo-1-methylindole,183 and the first asymmetric total synthesis of (+)-laurenyne, a metabolite of Laurencia obtusa,184 has been achieved.185
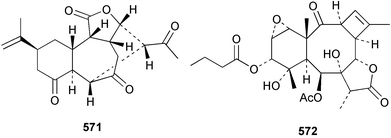
7 Sponges
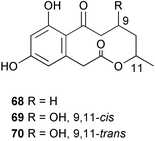
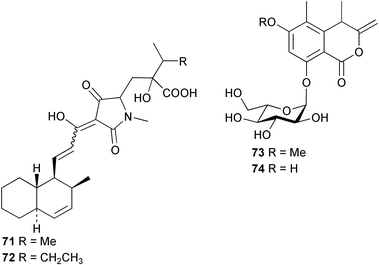
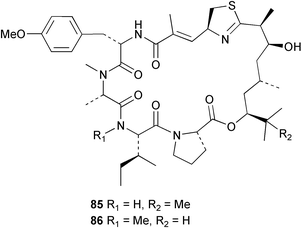
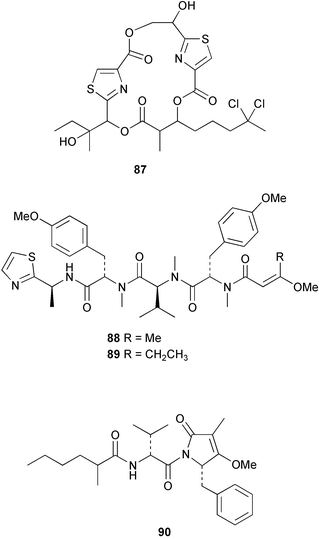
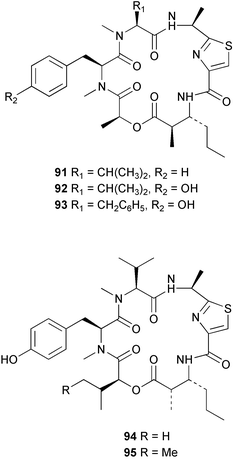
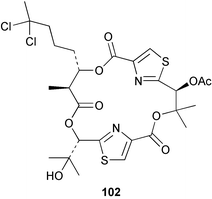
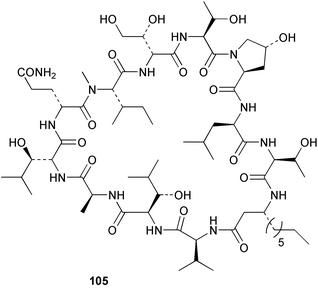
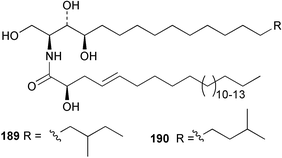
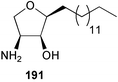
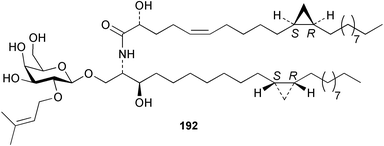
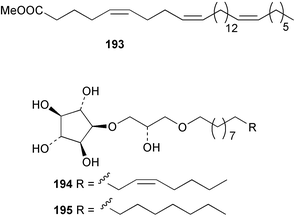
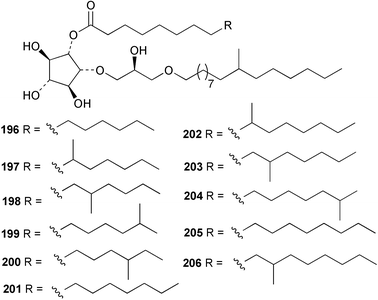
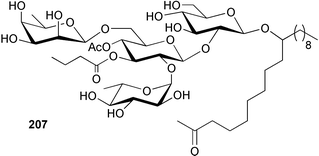
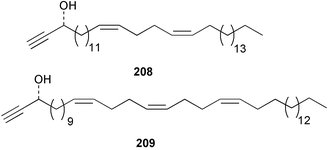
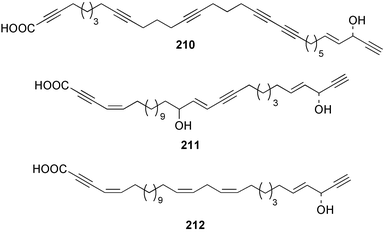
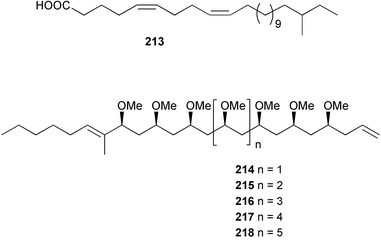
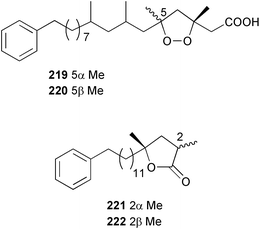
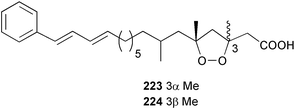
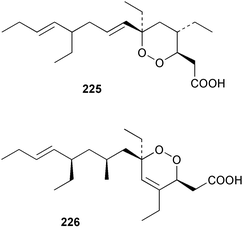
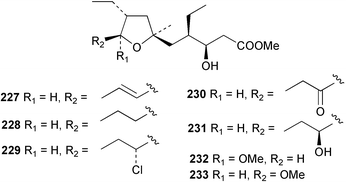
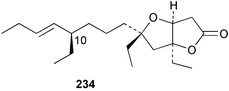
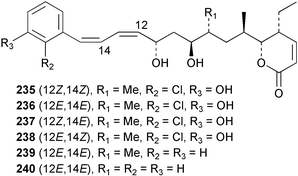
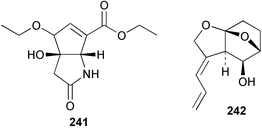
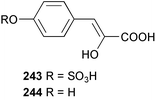
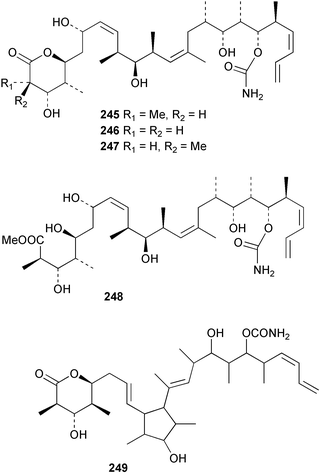
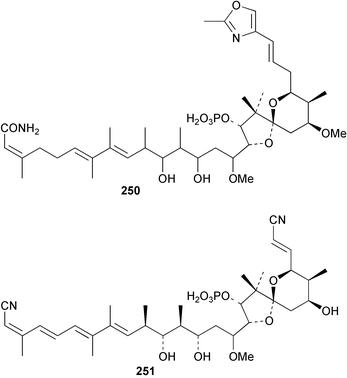
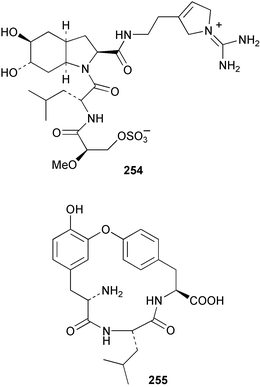
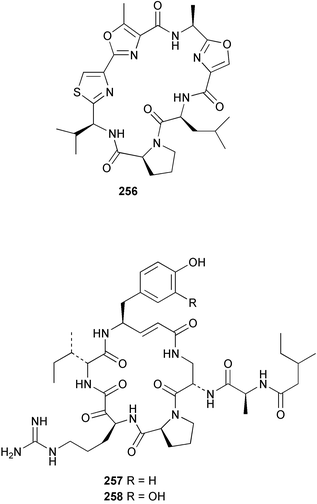
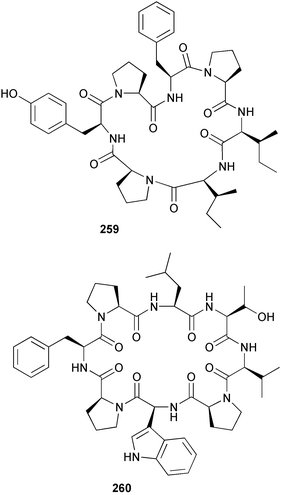
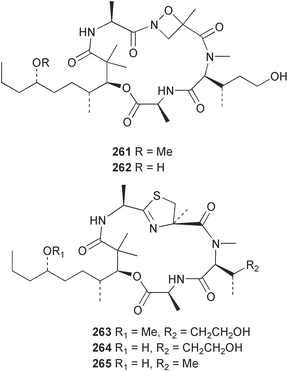
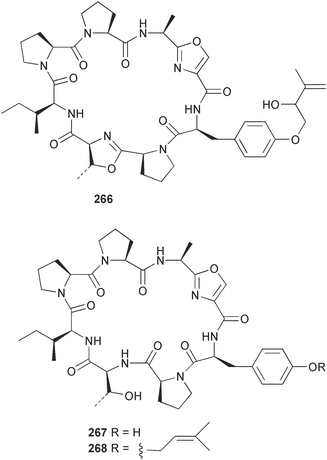
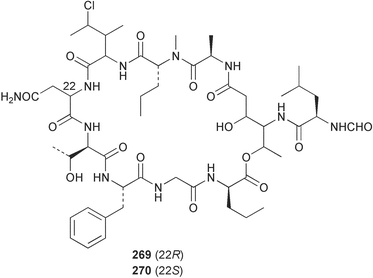
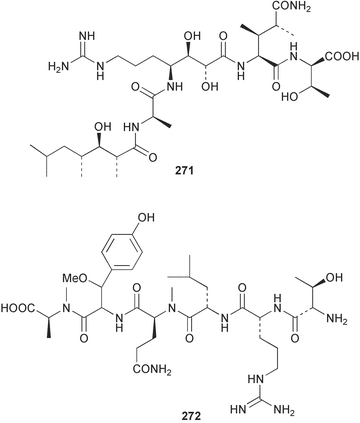
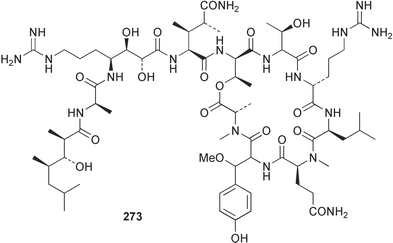
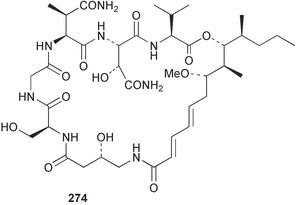
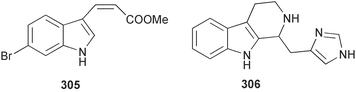
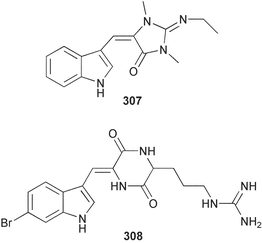
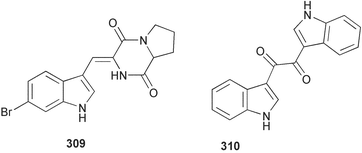
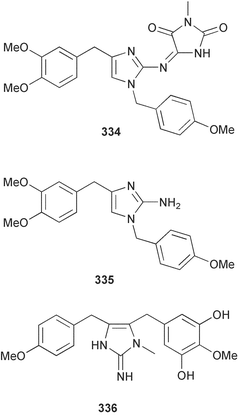
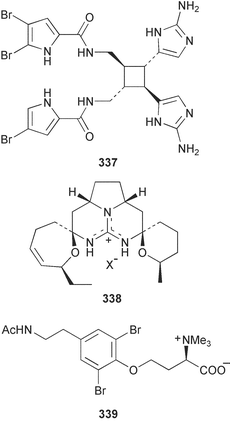
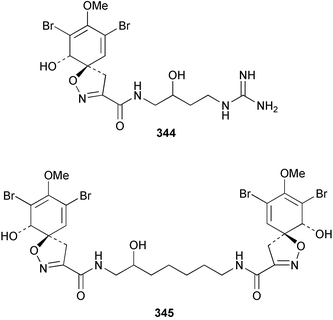
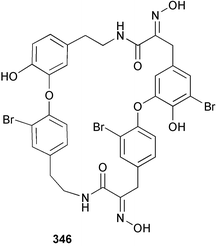
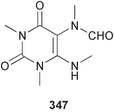
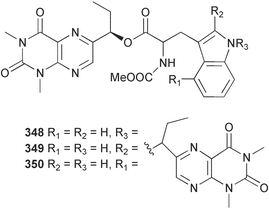
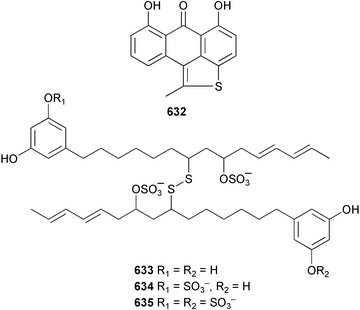
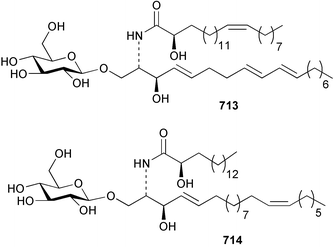
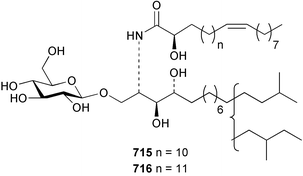
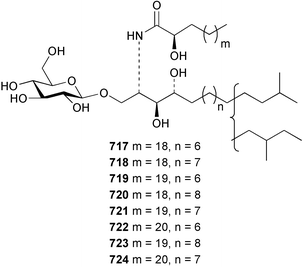
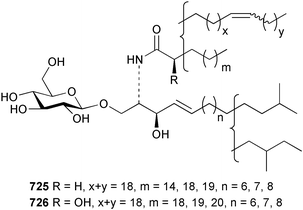
The phylum Porifera (sponges) continues to be a rich source of novel secondary metabolites, with a diversity of biological activities that continue to inspire the efforts of synthetic organic chemists. A pair of ceramides with variable alkyl chain lengths 189 and 190 were isolated from Clathria fasciculata collected in southern China.186 Pachastrissamine 191, a sphingosine derivative, was reported from the Okinawan sponge Pachastrissa sp. and the absolute stereochemistry determined from analysis of the MTPA amides.187 The relative and absolute stereochemistry of the immunosuppressive sphingolipid, plakoside A 192, isolated from the Caribbean sponge Plakortis simplex,188 was finally determined by degradation and derivatisation.189,190 The cyclopropyl-containing side chains were cleaved off as carboxylic acids from the natural product. These acids were synthesised with known absolute stereochemistry and derivatives with (1S,2S)- and (1R,2R)-2-(2,3-anthracenedicarboximido)-cyclohexanol were prepared and compared by HPLC. Previous syntheses of plakoside with the correct,191 and incorrect192 relative stereochemistry had resulted in compounds with the same optical rotation. The methyl ester of 5,9,23-triacontatrienoic acid 193, isolated from Chondrilla nucula collected from the French Mediterranean coast, was found to inhibit elastase.193 Two further new cytotoxic cyclitol derivatives, sarcotrides B 194 and C 195 were isolated from a Korean Sarcotragus sp.194 The isocrasserides 196–206 were isolated from the Caribbean sponge Plakortis simplex.195 The authors report that the isocrasserides 196–206 and the related, previously described crasserides,196 were found in all sponges examined from a wide variety of genera within the phylum and propose that they are a characteristic, distinguishing metabolite of the phylum Porifera. The antimicrobial glycolipid caminoside A 207, isolated from Dominican specimens of Caminus sphaeroconia, was found to be a potent inhibitor of the bacterial type III secretion system.197 The lembehynes B 208 and C 209, isolated from an Indonesian species of Haliclona, were found to possess neuritogenic activity against neuroblastoma cells.198 Strongylodiol A, originally isolated from an Okinawan Strongylophora species,199 has been synthesised confirming the (R) stereochemistry.200 Japanese specimens of Callyspongia truncata yielded the α-glucosidase inhibitor callyspongynic acid 210201 while corticatic acids D 211 and E 212 along with the previously reported corticatic acid A,202 were isolated from a Japanese Petrosia corticata and found to be geranylgeranyltransferase type I inhibitors.203 A cytotoxic fatty acid, (5Z,9Z)-22-methyl-5,9-tetracosadienoic acid 213 was isolated from Geodinella robusta collected from the Sea of Okhotsk, Russia.204 A series of polymethoxydienes 214–218, similar to the alkenes isolated from the cyanophyte Tolypothrix conglutinata,205 were isolated from a Philippine specimen of Myriastra clavosa, and found to be moderately cytotoxic.206Plakortis nigra, collected from a depth of 115 m in Palau, was found to contain epiplakinic acids G 219 and H 220 and the γ-lactones 221 and 222 along with several β-carbolines (vide infra). All compounds were found to inhibit the growth of HCT-116 cells.207 A peroxylactone, originally isolated from a Plakinastrella species,208 has been synthesised as a racemic mixture.209 Two new 1,2-dioxolane peroxide acids 223 and 224, isolated from P. onkodes collected in Florida, possessed moderate antifungal activity.210 A 1,2-dioxane peroxide acid 225, also with moderate antifungal activity, was isolated from a Jamaican specimen of Plakortis halichondrioides.210 The relative and absolute stereochemistry of the cyclic peroxide 226, originally isolated from P. angulospiculatus,211 has been proposed by comparison to the optical rotation and NMR spectral data of synthesised diastereomers.212 Seven methyl esters, plakortethers A–G 227–233 with selective toxicity towards murine macrophage cells (RAW 264–7), were obtained from a Bahaman specimen of P. simplex.213 Plakortone D 234, originally isolated from P. halichondrioides,214 has been synthesised thereby establishing the relative stereochemistry of C-10 and its absolute configuration as illustrated.215 The plakortides named I and J, recently isolated from the Jamaican sponge Plakortis sp.,216 have been renamed plakortides M and N as the names plakortide I and J had been used previously for related metabolites isolated from P. simplex collected in the Bahamas.217Theonella cf. swinhoei from Indonesia was found to contain the bitungolides A–F 235–240 which were shown to weakly inhibit dual-specificity phosphatase.218 Subereatensin 241 was isolated from a Thailand specimen of Suberea aff. praetensa.219 An Okinawan collection of Terpios hoshinota contained the cytotoxic alcohol terpiodiene 242.220 The symbiotic complex of the sponge Haliclona cymaeformis and the red alga Ceratodictyon spongiosum collected in the Philippines was found to contain p-sulfooxyphenylpyruvic acid 243 and its phenol congener 244.221 Five new cytotoxic discodermolide analogues 245–249 were isolated from several different Discodermia sp. specimens collected in the Bahamas.222 The highly cytotoxic metabolite swinhoeiamide A 250, related to calyculinamide A, was obtained from Theonella swinhoei collected in Papua New Guinea.223 The same compound with similar optical activity was isolated from an Australian collection of Luffariella geometrica and named geometricin A.224 Another calyculin A congener, hemicalyculin A 251 with potent cytotoxicity, has been obtained by bioassay-guided fractionation of Japanese specimens of Discodermia calyx, the original organism from which calyculin was isolated.225 An enantioselective synthesis of bengamide Z, originally isolated from a Jaspis sp.,226 has been reported.227,228 Two potently cytotoxic amides, theopederins K 252 and L 253, were obtained from a Discodermia species collected from Honduras.229Sponges continue to be a rich source of novel and biologically active peptides. The inhibitor of factor VIIa and thrombin, dysinosin A 254, was isolated from a new genus of dictyoceratid sponge of the family Dysideidae from Australia.230 The structure of dysinosin A has been confirmed by stereoselective synthesis.231 The cyclic tripeptide renieramide 255, which was isolated from a new species of Reniera collected in Vanuatu, showed immunomodulatory activity.232 Interestingly, the structure of 255 proved to be identical to a patented synthetic analogue of the microbial product OF4949.233 A Northern Australian collection of Leucetta microraphis yielded the cytotoxic heptapeptide leucamide A 256.234 Cyclotheonamides E2 and E3, originally isolated from a Theonella species,235 have been synthesised.236,237 Two new cyclotheonamides E4 257 and E5 258 were obtained from an Ircinia sp. from Japan and were found to be potent tryptase inhibitors.238 The trans, trans rotamer of ceratospongamide has been synthesised along with the previously reported cis, cis isomer.239,240 Both forms of ceratospongamide were originally isolated from the symbiotic complex of Sigmadocia symbiotica and its host red alga Ceratodictyon spongiosum.241 The two rotamers were remarkably stable at temperatures up to 95 °C, but could be interconverted at 175 °C in DMSO. The trans, trans rotamer was found to be a potent inhibitor of the expression of secreted phospholipase A2 (sPLA2) while the cis, cis form was inactive.241 Similarly, two distinct conformers of the previously reported peptide phakellistatin 2 259 were isolated and characterised from the Fijian specimen of Stylotella aurantum.242 The less polar conformer was found to contain an intramolecular hydrogen bond. Phakellistatin 2 was originally isolated from Phakellia carteri and reported to be potently cytotoxic.243 A subsequent reisolation and a total synthesis failed to reproduce the biological activity. The less polar conformer isolated from Stylotella was found to have a similar activity to that originally reported, and was found to lose activity at room temperature on standing for several weeks.242 Isolated from the same sponge was the weakly cytotoxic octapeptide axinellin C 260.244 The structures proposed for halipeptins A 261 and B 262, originally isolated from a Haliclona species from Vanuatu,245 have been revised.246 The molecular formulae have been revised to include sulfur, replacing the proposed oxazetidine ring with a thiazoline moiety 263 and 264. A new compound, halipeptin C 265 was obtained from the same sponge.246 A Palauan specimen of the genus Haliclona was found to contain the new haliclonamides C–E 266–268 which were found to repel the settlement of Mytilus edulis adults.247 Two depsipeptides with nematocidal activity, phoriospongins A 269 and B 270, were isolated from both a Phoriospongia species and Callyspongia bilamellata from southern Australia.248 Callipeltins D 271 and E 272 were isolated from a Latrunculia species collected from Vanuatu.249 The determination of the presence of (R)-allo-threonine and (R)-alanine in their structures by use of Marfey's reagent led to the re-examination of the stereochemistry of callipeltin A 273, originally isolated from a Callipelta species.250 The stereochemistry has been revised as illustrated. A Japanese collection of Theonella swinhoei was found to contain an antibacterial depsipeptide nagahamide A 274.251
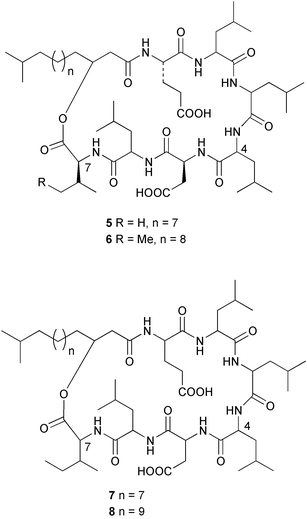
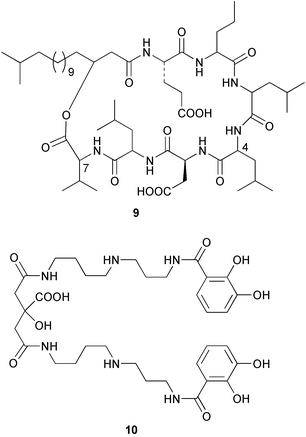
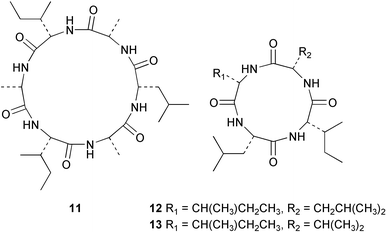
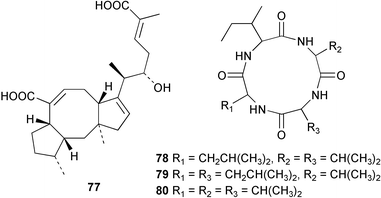
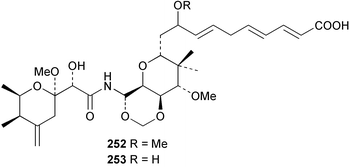
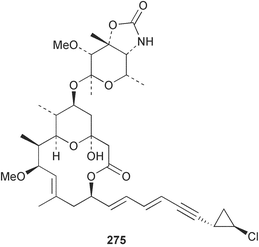
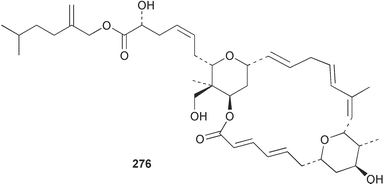
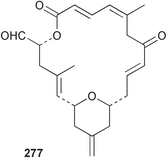
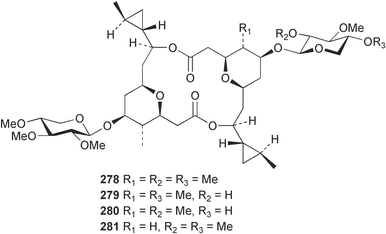
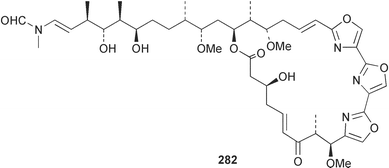
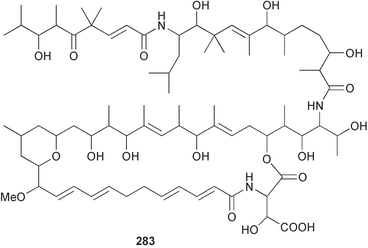
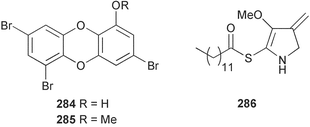
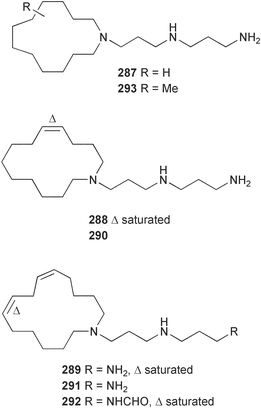
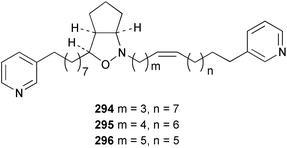
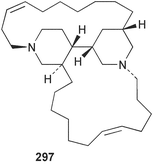
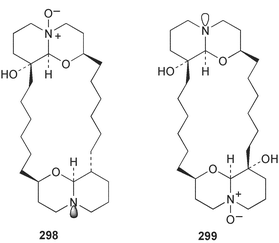
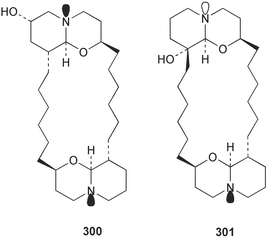
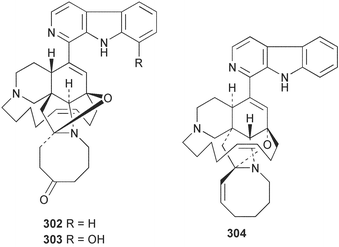
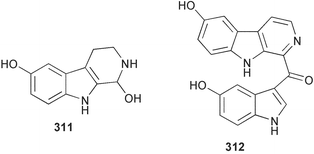
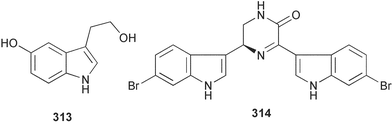
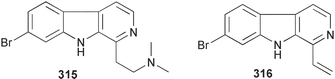
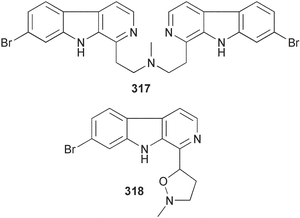
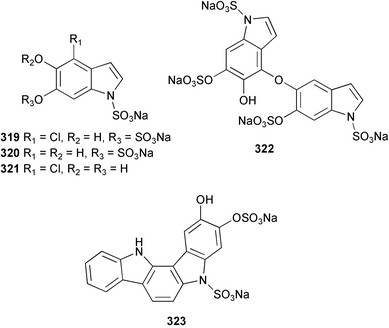
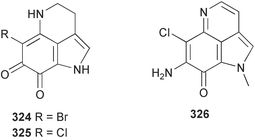
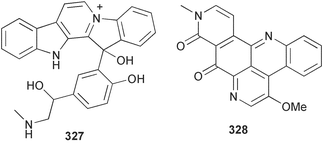
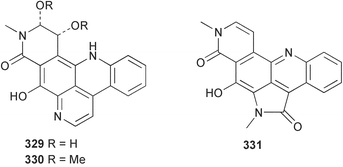
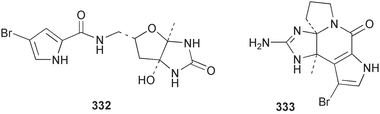
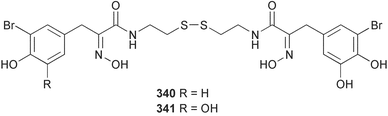
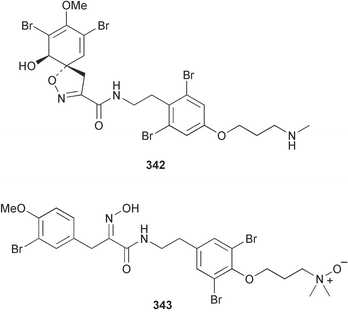
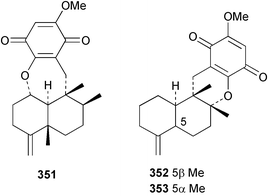
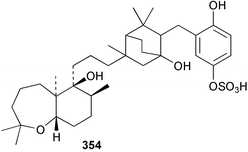
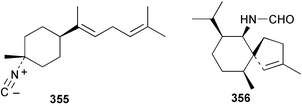
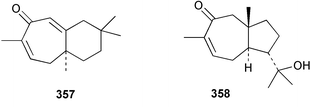
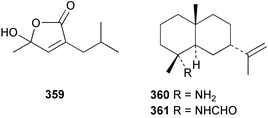
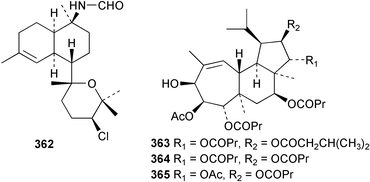
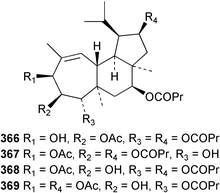
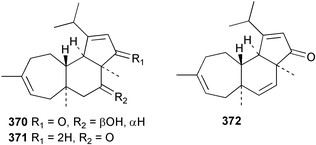
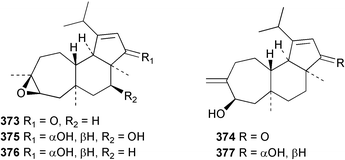
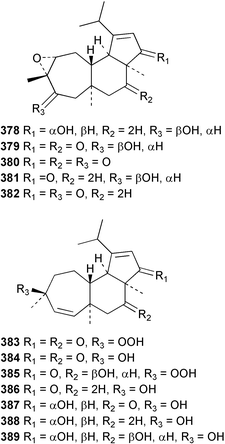
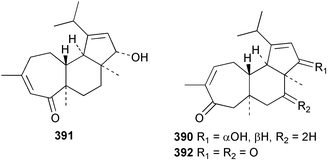
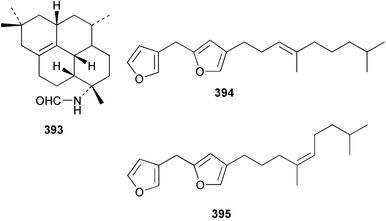
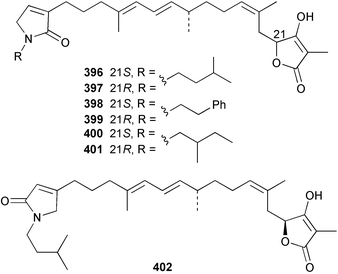
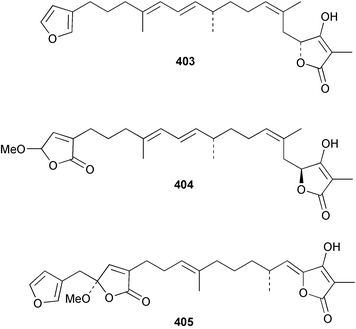
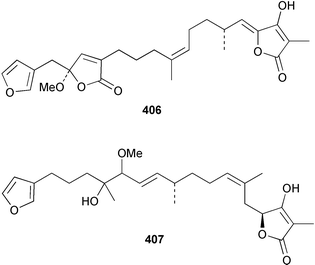
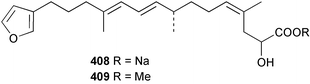
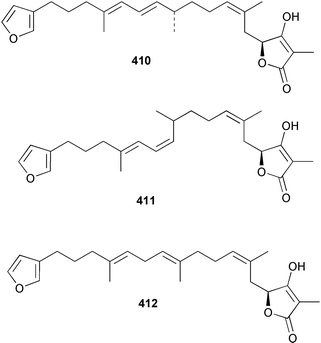
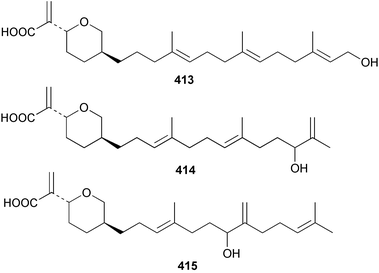
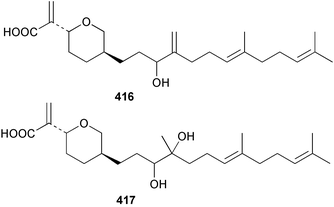
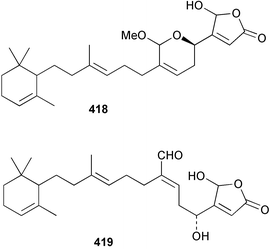
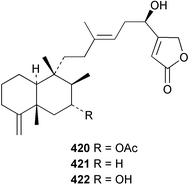
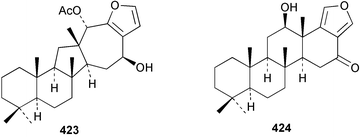
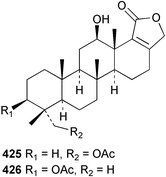
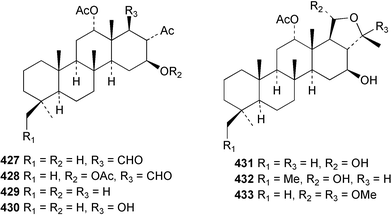
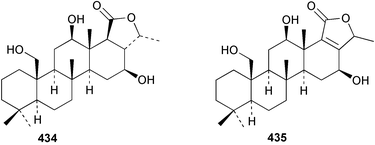
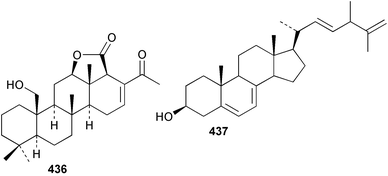
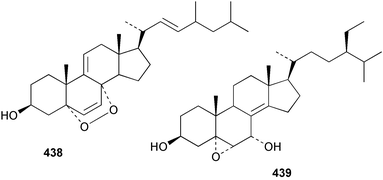
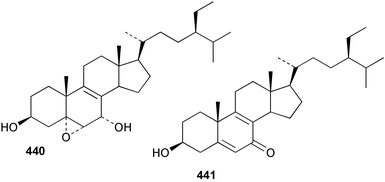
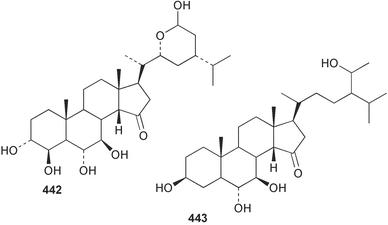
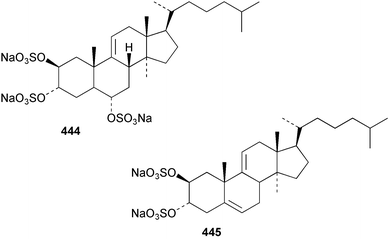
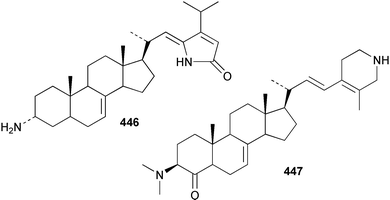
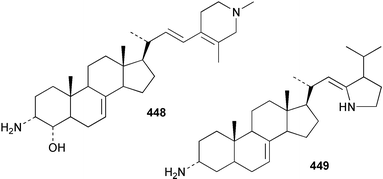
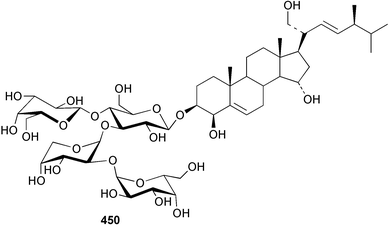
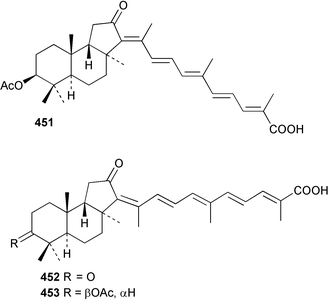
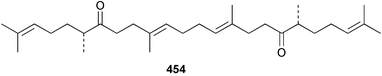
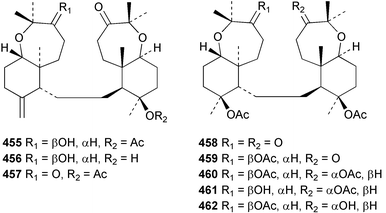
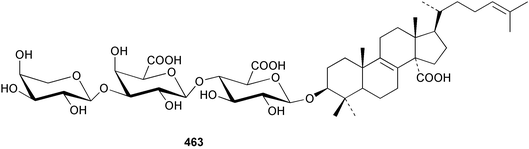
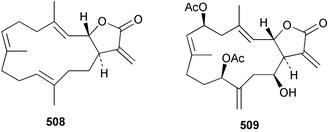
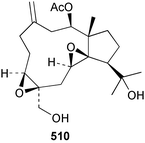
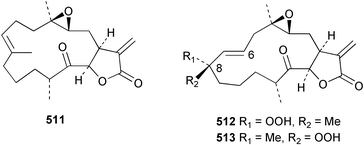
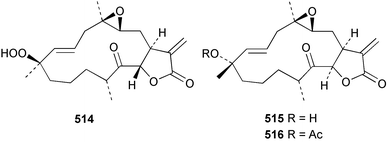
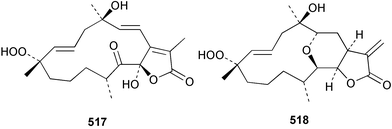
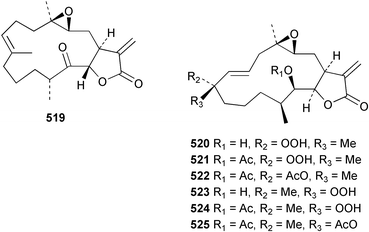
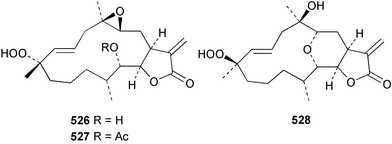
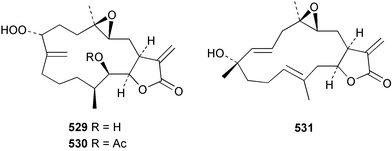
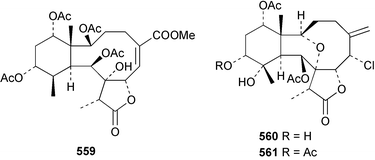
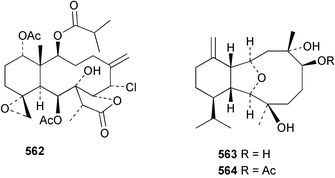
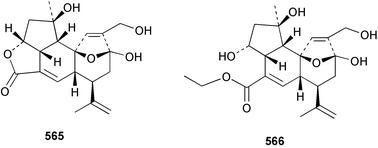
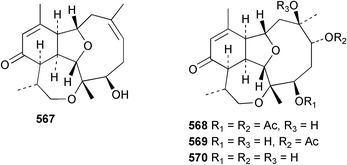
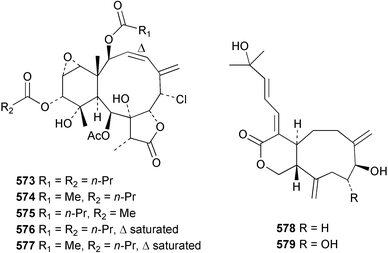
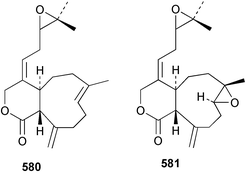
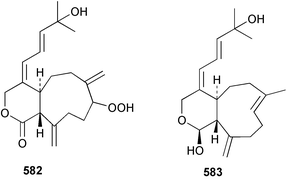
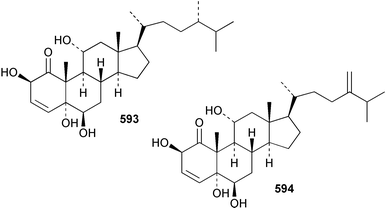
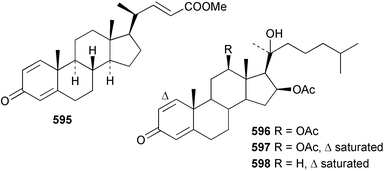
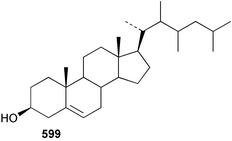
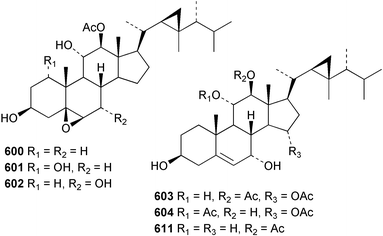
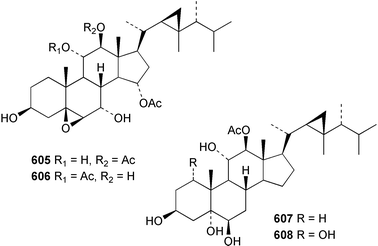
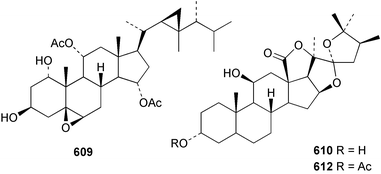
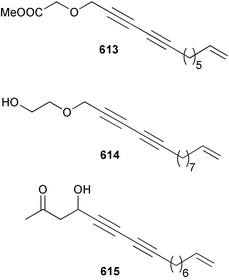
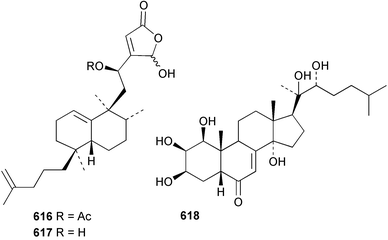
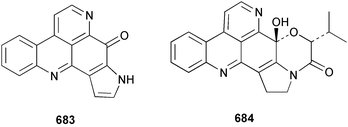
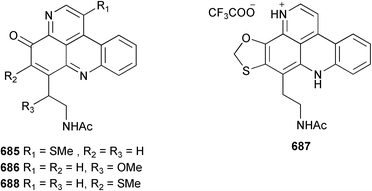
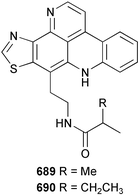
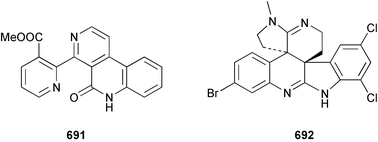
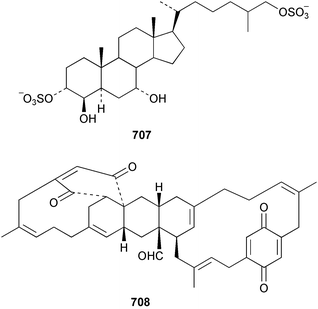
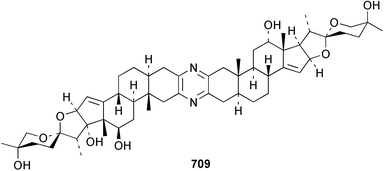
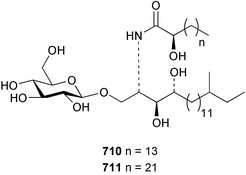
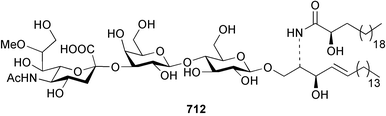
Sponge derived macrolides have proven to be of considerable interest to both synthetic and natural product research groups. Callipeltoside A 275, originally isolated from a Lithistid sponge of the genus Callipelta,252 has been synthesised stereoselectively independently by two groups, establishing the relative and absolute stereochemistry of the chlorocyclopropyl side chain.253–255 The structure of lasonolide A, isolated from a Caribbean Forcepia species,256 has been revised to 276 by enantioselective synthesis of its antipode.257,258 (+)-Dactylolide 277, isolated from a Dactylospongia species,259 has been synthesised, establishing both the relative stereochemistry of the acyloxymethine and overall absolute stereochemistry.260,261 Interestingly, it appears to be a pseudo-enantiomer of the closely related sponge metabolite (−)-zampanolide.262 Clavosides A 278 and B 279 were isolated from the Philippine sponge Myriastra clavosa.263 The same compounds were described independently from M. clavosa, along with clavosides C 280 and D 281.264 A new mycalolide, 30,32-dihydroxymycalolide 282, with potent cytotoxicity, was obtained from Mycale izuensis from Japan.265 A macrolide lactam, poecillastrin A 283, related in structure to the chondropsins,266 was isolated from a Bahamas collection of a deep water Poecillastra species.267 Poecillastrin A was found to have cytotoxicity and antiproliferative properties similar to those of the chondropsins. Two brominated dibenzo-p-dioxins, spongiadioxin C 284 and the methyl ether 285, found to inhibit the division of fertilised sea urchin eggs, were isolated from an Australian collection of Dysidea dendyi.268 The moderately cytotoxic thioester irciniamine 286 was isolated from an Ircinia species collected in Japan.269 The previously reported motuporins A–C 287–289270 along with the new congeners D–F 290–292 were found to inhibit the invasion of breast carcinoma cells into new tissues. These compounds were isolated from Xestospongia exigua collected in Papua New Guinea along with an unresolved mixture of three isomers 293.271 Xestamine D, originally isolated from Calyx podatypa,272 has been synthesised employing a palladium coupling strategy.273 The syntheses of hachijodines F and G, originally isolated from an Amphimedon species,274 have also been reported.275 The structure 294, originally proposed for pyrinodemin A isolated from an Amphimedon species,276 was synthesised independently by two groups but did not have spectral characteristics identical to the natural compound.277,278 The alternative structure 295 was proposed and synthesised by both groups. Recently, a third structure 296 has been synthesised which matches the spectral data reported for the natural compound more closely. The researchers note “that it would be hasty to conclude that 296 is the correct structure of natural pyrinodemin A”.279 A Halichondria species collected from Eritrea was found to contain the bispiperidine alkaloid halichondramine 297.280 The relative stereochemistry of each piperidine ring was determined but their stereochemical relationship remains unresolved due to severe spectral overlap. Two novel N-oxide containing alkaloids araguspongines K 298 and L 299 were isolated from Saudi Arabian specimens of Xestospongia exigua.281 A Western Australian Xestospongia species yielded (7S)-hydroxyxestospongin A 300; its absolute stereochemistry, and that of the previously reported282 (+)-xestospongin D 301, was determined by X-ray analysis.283 Three novel manzamine type alkaloids, ent-12,34-oxamanzamines E 302, F 303 and 12,34-oxamanzamine A 304 were isolated from three closely related species of an undescribed genus of the family Petrosiidae (Order Haplosclerida) from Indonesia.284 An intramolecular Stille/Diels–Alder reaction has been employed in the enantioselective synthesis of ircinal.285 The bromoindole 305, isolated from a South Australian Hymeniacidon species was found to have nematocidal activity.286 A Micronesian sponge belonging to the order Haplosclerida, and probably representing a new genus, contained a cathepsin inhibitor haploscleridamine 306.287N-3′-Ethylaplysinopsin 307, isolated from the Jamaican sponge Smenospongia aurea, has a high affinity for the human serotonin 5-HT2 receptor.288 A diketopiperazine 308, isolated from Geodia barretti collected at 300 m from Norwegian waters,289 was found to have spectral data identical to that of barettin previously isolated from the same sponge and originally assigned as structure 309.290 A subsequent synthesis of 309 disproved this structure for barettin.291 A Smenospongia species from Queensland, Australia, yielded the bisindole 310.292Hyrtios reticulata from Indonesia was found to contain 1,6-dihydroxy-1,2,3,4-tetrahydro-β-carboline 311, while Hyrtios erectus, also from Indonesia, yielded hyrtiosulawesine 312 and 5-hydroxy-3-(2-hydroxyethyl)-indole 313.293 Piperazine-based bisindole metabolites have received considerable synthetic attention. Hamacanthin B 314 from a deep water Hamacantha species294 has been synthesised and the absolute stereochemistry determined as (S).295 The cis and trans diastereomers of 6′-debromo-3,4-dihydrohamacanthin A, originally isolated from Rhaphisia lacazei,296 have been synthesised as a diastereoisomeric and a racemic mixture; similarly, 6′-debromo-cis-3,4-dihydrohamacanthin B was also prepared.297 The trans isomer of dihydrohamacanthin A from the same sponge was also synthesised along with the cis isomer of dragmacidin C.298 Dragmacidin D, isolated from a Spongosorites species,299,300 has been synthesised as a racemic mixture.301 The plakortamines A–D 315–318 were isolated from the same deepwater Plakortis nigra specimens that yielded the epiplakinic acids (vide supra).207 A series of sulfamate indoles 319–322 and an indolocarbazole 323 were isolated from a New Zealand Ancorina species.302 Makaluvamine O 324 was isolated from the same Jamaican collection of Smenospongia aurea that was found to contain N-3′-ethylaplysinopsin 307.288 The Indopacific sponge Zyzzya fuliginosa yielded batzelline D 325 and isobatzelline E 326 which was found to inhibit the cell fusion of HIV-1.303 A synthesis of discorhabdin A, originally isolated from a Latrunculia species,304 has been reported.305 Thorectandamine 327, a β-carboline alkaloid with weak cytotoxicity, was obtained from a Palauan Thorectandra species.306 Four new cytotoxic bisannulated acridines 5-methoxyneoamphimedine 328, neoamphimedine Y 329, neoamphimedine Z 330, and alpkinidine 331 were isolated from Xestospongia cf. exigua (328) and X. cf. carbonaria (328–331).307 Slagenins B and C, originally isolated from Agelas nakamurai,308 were synthesised with the correct absolute stereochemistry.309 The stereochemistry of slagenin A 332, also from Agelas nakamurai, was established by an independent stereoselective approach that also produced slagenins B and C.310 Racemic syntheses of both dibromophakellstatin from Phakellia mauritiana311 and dibromophakellin from Acanthella carteri312 have been achieved.313 An Agelas species collected in the Bahamas was found to contain monobromoisophakellin 333 which along with related bromopyrroles was found to inhibit feeding by the reef fish Thalassoma bifasciatum.314 (E)-Debromoaxinohydantoin isolated from a Hymeniacidon species,315 and (Z)- debromoaxinohydantoin, also known as spongiacidin C, isolated from Stylotella aurantium316 and a Hymeniacidon species,317 have both been synthesised.318 Stereoselective syntheses of agelastatins A and B from Agelas dendromorpha319 have been reported.320,321 The cytotoxic imidazole alkaloids naamine C from Leucetta chagosensis,322 and pyronaamidine from a Leucetta species323 have been synthesised.324 Two related imidazole alkaloids 334 and 335 have been isolated from an Australian specimen of L. chagosensis while a third compound 336 was obtained from a Fijian specimen of a closely related sponge.325 Bromosceptrin 337, a dimeric pyrrole alkaloid, was obtained from a Florida Keys specimen of Agelas conifera.326 Crambescidin 359 338, isolated from Monanchoraarbuscula,327 has been synthesised stereoselectively establishing the absolute stereochemistry.328 A Verongid sponge of the family Aplysinellidae collected in Okinawa yielded nakirodin A 339 and the absolute stereochemistry was established by the hydrolytic release of N,N,N-trimethyl-(R)-homoserine.329 Psammaplins K 340 and L 341 were obtained from the Fijian sponge Aplysinella rhax.330 Psammaplin A, previously described from a Psammaplysilla species,331 was found to be a chitinase inhibitor. A Druinella species, also from Fiji, was found to contain purealidin S 342 and purpuramine J 343, which were both moderately cytotoxic.332 The endemic Brazilian sponge Aplysina caissara yielded caissarines A 344 and B 345.333 Bastadin 21 346 was isolated from Ianthella quadrangulata from Queensland, Australia.334 The formamide-containing seco-xanthine hymeniacidin 347 was obtained from the same specimen of an Australian Hymeniacidon species from which bromoindole 305 was reported (vide supra).286 Pseudoanchynazines A–C 348–350, containing a methylcarbamate moiety, were isolated from an Argentinian Clathria species.335 Stereoselective syntheses of 18-methoxyavarone isolated from Dysidea cinerea336 and D. avara,337 and 19-methoxyavarone from a Dysidea species338 and D. avara337 have been accomplished.339 Aureol, originally isolated from Smenospongia aurea,340 has been synthesised with the correct absolute stereochemistry via a rearrangement of (+)-arenarol.341 The Okinawan sponge Dactylospongia elegans yielded dactyloquinones C–E 351–353.342 A sulfated hydroquinone, phuklona sulfate 354 that displayed modest cytoprotection against HIV-1, was obtained from a Haliclona species collected in Thailand.343 An Okinawan Axinyssa species contained the monocyclic sesquiterpene (E)-3-isocyanobisabolane-7,10-diene 355 that is toxic to brine shrimp,344 while exiguamide 356 from a Japanese collection of Geodia exigua inhibited sea urchin embryogenesis.345 A Jamaican specimen of Myrmekioderma styx yielded styxones A 357 and B 358 as well as the degraded terpenoid styxlactone 359.346Axinyssa ambrosia, collected from the Caribbean coast of Columbia, was the source of two nitrogenous eudesmane-type sesquiterpenes, 360 and 361. Compound 360 was cytotoxic to P388, HT-29 and A-549 cell lines and the polyps of the scleractinian coral Madracis mirabilis.347 A total synthesis of the spirocyclic sesquiterpene (+)-axenol, originally isolated from an Eurypon sp. of sponge from New Zealand,348 has been achieved via a “one-pot” reaction.349 Kalihinene X 362, originally isolated from Acanthella cavernosa,350 was synthesised stereoselectively establishing the absolute stereochemistry.351 Seven tricyclic diterpenes, the gagunins A–G 363–369, with widely varying cytotoxicity were obtained from a Korean Phorbas species.352 A quite remarkable number of new diterpenes, the cyanthiwigins E to AA 370–392, with cytotoxic and anti-HIV–1 activity were obtained from the Jamaican sponge Myrmekioderma styx.353 A Vanuatuan collection of an Axinella species yielded the tetracyclic diterpene N-formyl-7-amino-11-cycloamphilectene 393.354 A Korean collection of a sponge of the genus Sarcotragus was found to contain two bisfuranoditerpenoids, sarcotins K 394 and L 395 which displayed weak cytotoxicity.355 In the same report are also described the isolation of an additional seven pyrrolosesterterpenoids 396–402, five furanosesterterpenoids 403–407, and two trinorsesterterpenoids 408 and 409 which had varying levels of cytotoxicity. The stereochemistries of the previously reported 410–412356 were revised on the basis of a re-interpretation of CD spectral data.355 Compound 411 was inadvertently drawn with (8Z) geometry in this and the previous report.355,356 A series of five sesterterpenoids, barangcadoic acid 413 and rhopaloic acids D–G 414–417, were isolated from an Indonesian Hippospongia species.357 All compounds were found to inhibit the protease activity of human RAS converting enzyme (hRCE1) and are the first natural products reported with this activity. An Okinawan Luffariella species yielded two new luffariolides H 418 and J 419 that were found to be cytotoxic and antimicrobial.358 Three further cytotoxic sesterterpenes, thorectandrols C–E 420–422 were isolated from a Thorectandra species collected in Palau.359 Both the natural (+) and unnatural (−) enantiomers of cacospongionolide B, originally isolated from Fasciospongia cavernosa,360 have been synthesised; the natural enantiomer is more than twice as active as an inhibitor of sPLA2.361Hyrtios erecta collected from the Egyptian Red Sea was found to contain salmahyrtisol A 423 and B 424 and 3-acetyl- and 19-acetyl-sesterstatin 425 and 426, all of which showed significant cytotoxicity in human cancer cell lines.362 A species of Phyllospongia collected in Indonesia yielded the scalarane sesterterpenoids 427–433.363 Three further scalarane-type sesterterpenoids 434–436 were obtained from a Papua New Guinean specimen of Ledenfeldia frondosa.364 A Jamaican sample of Agelas sceptrum was found to contain the C29 steroid 437.365 A peroxy steroid, 9(11)-dehydroxyaxinysterol 438, from an Okinawan species of the genus Axinyssa, was found to inhibit the growth of several human cancer cell lines.344 Three oxygenated sterols 439–441 were obtained from a collection of Polymastia tenax obtained from the Caribbean Coast of Columbia. Compounds 439 and 440 were found to have significant cytotoxicity to a range of human and murine cancer cell lines.366 The absolute stereochemistry of contignasterol 442 was established by the preparation of MPA and MTPA esters as well as CD curve analysis, confirming the customary steroid configuration.367 A polyoxygenated steroid, clathriol 443, also with anti-inflammatory activity, was obtained from a New Zealand collection of Clathria lissosclera.368 The abeo-sterol orostanal, recently isolated from Stelletta hiwasaensis,369 has been synthesised from hyodeoxycholic acid.370 An Indonesian specimen of Petrosia strongylata yielded two thymidine phosphorylase inhibiting sulfated sterols, lembehsterols A 444 and B 445.371 Four new plakinamine type steroidal alkaloids 446–449 have been isolated from a Vanuatuan Corticium species.372 The steroidal tetraglycoside, mycaloside A 450, was obtained from a Cuban specimen of Mycale laxissima.373 A Philippine specimen of Rhabdastrella globostellata yielded the isomalabaricane type triterpenoids stellettins H 451 and I 452 along with the antipode 453 of (+)-stellettin E374 originally isolated from a Stelletta species.375 (−)-Stellettin E and the previously reported stellettin B were found to be selectively cytotoxic towards a p21-deficient HCT cell line.375 Both enantiomers (S,S) and (R,R) of the acyclic diketotriterpene 454, originally isolated from Hyrtios erectus,376 have been synthesised asymmetrically, establishing the absolute stereochemistry of the natural product as (R,R).377 Eight further raspacionins 455–462 were isolated from a Sicilian collection of Raspaciona aculeata; all were found to be both ichthyotoxic and cytotoxic.378 The triterpenoid trisaccharide nobiloside 463, obtained from the Japanese sponge Erylus nobilis, is a neuraminidase inhibitor.379
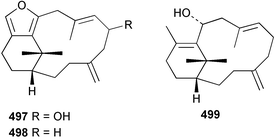
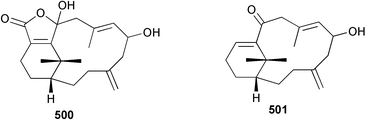
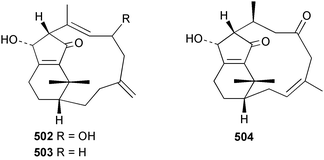
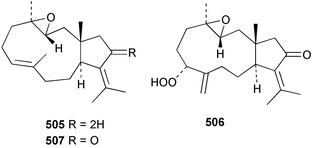
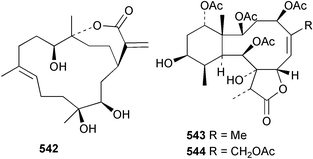
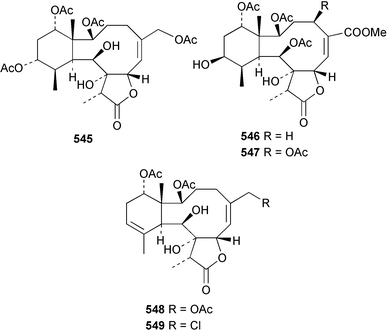
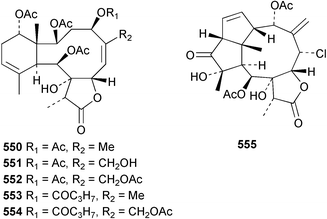
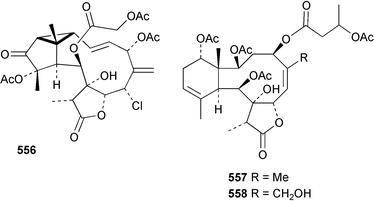
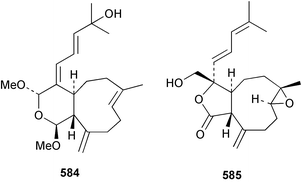
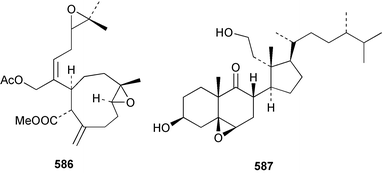
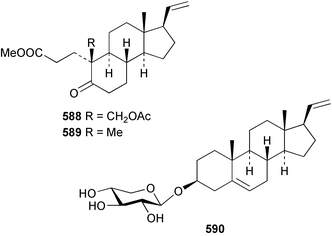
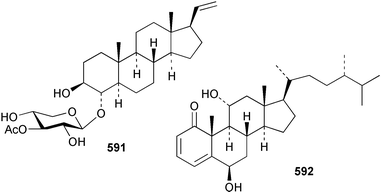
8 Coelenterates
There was a slight increase in the number of metabolites reported from coelenterates in 2002. The examples reported continue to be dominantly of terpenoid biogenesis. Bioassay guided fractionation of extracts obtained from an Indian Ocean collection of the soft coral Lobophytum crassum afforded ceramide 464 as a moderately antibacterial component.380 Specimens of the soft coral Sinularia sp. collected from the Andaman and Nicobar Islands contained the ceramides 465 and 466.381 Cervicoside 467, a new glycoside, was isolated from a Chinese collection of Sinularia cervicornis.382 In a study of soft corals from the Karwar region of India, xanthine 468 and pyrazole 469 were isolated from Echinomuraceae splendens.383 New examples of cadinene-skeleton sesquiterpenes, xenitorins A–F 470–475, were isolated from a Taiwanese collection of Xenia puerto-galerae.384 The relative stereochemistries of 470–475 were secured by NOESY NMR experiments. Xentorins A 470 and E 474 exhibited cytotoxicity towards the A-549 and P388 tumour cell lines. The structure and absolute stereochemistry of alcyopterosin E 476, a nitrate ester-containing sesquiterpene isolated from a sub-Antarctic collection of Alcyonium paessleri,385 was secured by total synthesis.386 Subergorgiol 477 and 2β-acetoxysubergorgic acid 478 were isolated from Taiwanese collections of Subergorgia suberosa.387 Relative stereochemistry was determined by NOESY NMR experiments. Both 477 and 478 failed to exhibit cytotoxicity towards either KB or HeLa tumour cells, while subergorgic acid methyl ester, also isolated from the extract, exhibited mild cytotoxicity towards the HeLa cell line. A racemic synthesis of pathylactone A 479, isolated from the soft coral Paralemnalia thyrsoides,388 and 1-epi-pathylactone A has raised doubts about the spectral assignments of the norsesquiterpene natural product.389 A further study of a Taiwanese collection of Subergorgia suberosa yielded the sesquiterpenes suberosols A–D 480–483.390 Relative stereochemistries were determined by NOESY NMR experiments. All four metabolites exhibited cytotoxicity towards the P388 murine leukaemia cell line while suberosols C 482 and D 483 also exhibited cytotoxicity towards the A-549 and HT-29 tumour cell lines. The first chemical study of the soft coral Lemnalia flava, collected off Mombasa, Kenya, has yielded lemnaflavoside 484 and three monoacetate derivatives 485–487.391seco-Sethukarailin 488 was isolated from a collection of Sinularia dissecta from the Mandapam Coast, South India.392 However, the compound may be an artifact arising from the use of methanol in the extraction process. The Caribbean sea whip Pseudopterogorgia elisabethae was the source of ileabethin 489, a diterpene representing the first example of the ileabethane skeleton.393 Relative stereochemistry was ascertained through the interpretation of NOESY NMR data. The absolute configuration of elisabethin C 490, a bisnorditerpenoid also isolated from P. elisabethae,394 has been secured by total synthesis.395 Three cytotoxic prostanoids, claviridenones E–G 491–493, were isolated from a Taiwanese collection of Clavularia viridis.396 Claviridenone B exhibited pronounced cytotoxicity towards the A-549, HT-29 and P388 cell lines. The same publication also reported the isolation of cembranoid claviolide 494 from a Taiwanese collection of C. violacea. An Okinawan collection of C. viridis yielded the prostanoid-related oxylipins tricycloclavulone 495 and clavubicyclone 496.397 Relative stereochemistries of both compounds were principally determined by interpretation of NOESY NMR data, with the exception being a biogenetic argument that was used to assign the configuration of the sidechain acetoxy stereogenic centre. Clavubicyclone 496 exhibited mild cytotoxicity towards MCF-7 and OVCAR-3 tumour cell lines. Bioassay-directed fractionation of a Taiwanese collection of the soft coral Cespitularia hypotentaculata yielded diterpenes cespitularins A–D 497–500, a norditerpene cespitularin E 501 and three further diterpenes, cespitularins F–H 502–504, with a novel skeleton.398 Variable potency and selectivity was observed for the eight compounds towards tumour cell lines A-549, HT-29 and P388. Two new dolabellane-type diterpenoids 505 and 506, as well as the known diterpene clavenone 507,399 were isolated from an Okinawan collection of Clavularia species.400 The relative stereochemistries of 505 and 506 were secured by X-ray analysis, with absolute stereochemistry in both cases being inferred by the co-occurrence of 507 which has defined absolute configuration.401 The facile chemical conversion of 507 to 506 suggested that 506 was an artifact of isolation. Moderate in vitro cytotoxicity towards tumour cell lines was observed for 506. Clavularia koellikeri collected in Okinawa yielded two cembrane diterpenoids 508 and 509 and a dolabellane diterpene 510.402 The absolute configuration of 508 was secured by comparison with the known enantiomer,403 the relative stereochemistry of 509 was determined by interpretation of NOESY NMR data while the absolute configuration of 510 was obtained by NOESY NMR experiments, preparation of Mosher esters and comparison with the known absolute stereochemistry of the related metabolite stolonidiol. Diterpene 508 was found to exhibit cytotoxicity to a wide range of tumour cell lines. Investigation of the chemistry of Eunicea pinta collected from San Andrés Island, Colombia led to the report of eight γ-cembranolide-type diterpenes, 12-epieupalmerone 511 and uprolides H–K 512–515, K acetate 516, L 517 and M 518.404 The structure and stereochemistry of 511 was established by X-ray analysis, which in turn led to a correction of the stereochemistry of the co-occurring diterpene succinolide 519.405 The structure and relative stereochemistry of uprolide H 512 was also secured by X-ray analysis, allowing the stereochemistries of 513–518 to be determined by NOESY NMR experiments. The presence of (6E) geometry and a hydroperoxide group at C-8 in 512 also precipitated a revision of the structures of uprolide B 520,406 uprolide B acetate 521,406 uprolide B diacetate 522,406 8-epi-uprolide B 523,406 8-epi-uprolide B acetate 524,406 8-epi-uprolide B diacetate 525,406 12,13-bisepiuprolide B 526,407 12,13-bisepiuprolide B acetate 527,407 uproeunicin 528,407 uprolide C 529,406 uprolide C acetate 530406 and uproeuniolide 531.407 A Madagascan collection of Sarcophyton sp. was the source of cembrane diterpenes 532 and 533.408 The relative stereochemistry of 533 was deduced by interpretation of NOESY NMR data. An Australian collection of Sarcophyton sp. yielded cembranes 534–537 as two pairs of stereoisomers.409 Cembrane 534 is a previously reported semi-synthetic compound with defined absolute stereochemistry which in turn allowed the determination of absolute configuration for 535. The absolute stereochemistries of 536 and 537 were established by the preparation of Mosher ester derivatives of 536. Both 534 and 535 inhibited ligand binding to rat-brain adenosine A1 receptors. Scabrolides A–D 538–541 were isolated from a Taiwanese collection of Sinularia scabra.410 Relative stereochemistries were determined by the use of NOESY NMR experiments. Norditerpene 540 exhibited mild cytotoxicity towards KB and Hepa59T/VGH tumour cell lines. The structure and relative stereochemistry of cembrane 542, isolated from the soft coral Sinularia tenella, was established by X-ray analysis.411 Milolides G 543, 16-acetoxymilolide G 544, H–M 545–550, 16-hydroxymilolide M 551, 16-acetoxymilolide M 552, milolide N 553 and 16-acetoxymilolide N 554 were isolated from a collection of Briareum stechei from Yap, Micronesia.412 Relative stereochemistries were deduced by interpretation of NOESY NMR data. Artificial cultures of Erythropodium caribaeorum were found to produce a range of diterpenes including the antimitotic agent eleutherobin and aquariolide A 555.413 Inspection of ROESY NMR data and comparison with known related compounds led to the proposed absolute stereochemistry of 555. A Caribbean collection of the same gorgonian species led to the isolation of six new briarane diterpenes, erythrolides L–Q 556–561.414 Relative stereochemistries were established by interpretation of NOESY NMR data and by an X-ray analysis of 560. The structure and absolute stereochemistry of juncenolide A 562, a mildly cytotoxic briarane diterpene isolated from a Taiwanese collection of Junceella juncea, was also established by X-ray analysis.415 Two full accounts have been given of the structural and stereochemical reassessment of sclerophytins A 563 and B 564. Strong circumstantial evidence is also provided for suggested structural revisions of several other sclerophytin-type diterpenes.416,417 A Far-Eastern collection of the gorgonian Plumarella sp. was the source of diterpenes plumarellide 565 and a possible artifact, the ethyl ester of plumarellic acid 566.418 Relative stereochemistry was deduced from NOESY NMR data and both compounds exhibited mild haemolytic activity towards mice blood erythrocytes. Pachyclavulariaenones D–G 567–570 were isolated from a Taiwanese collection of Pachyclavularia violacea.419 The relative configurations of the compounds were secured by NOESY NMR experiments and an X-ray analysis of 569. Compound 570 exhibited mild cytotoxicity. An Indian Ocean collection of Sinularia sp. was the source of horiolide 571.420 Cyclobutenbriarein A 572 and five new examples of briarane skeleton diterpenes 11-hydroxybrianthein V 573, 11-hydroxybrianthein U 574, 11-hydroxybrianthein Y 575, 3,4-dihydro-11-hydroxybrianthein V 576 and 3,4-dihydro-11-hydroxybrianthein U 577 were isolated from a Bahamian collection of Briareum asbestinum.421 The absolute configuration of 573 was secured by an X-ray analysis while the relative stereochemistries of the other compounds were established by interpretation of NOESY NMR data. An Indonesian collection of Xenia sp. yielded xeniolide F 578 and 9-hydroxyxeniolide F 579. The relative stereochemistries were established by NOESY NMR experiments.422 Seven mildly cytotoxic xenicane-skeletoned diterpenes 580–586 were isolated from Xenia umbellata collected off Taiwan.423 Secosterol 587 was reported from Pachyclavularia violacea collected near Sulawesi Island, Indonesia.424 The degraded pregnanes muricenones A 588 and B 589, were isolated from Muricea sp. collected in the Bay of Mazatlán, Mexico.425 The collection of Eunicea pinta from San Andrés Island, Colombia that afforded diterpenes 511–518 (vide supra) also yielded the saponin 590 which was characterised by X-ray analysis of the peracetate derivative.404 Saponin 591 was isolated from Lobophytum sp. collected from Sanya Bay, Hainan Island.426 The Taiwanese collection of Clavularia viridis that yielded prostanoids 491–493 (vide supra) also afforded three cytotoxic steroids, stoloniferones E–G 592–594.396 Both the (20R) and (20S) stereoisomers of 595, isolated from the octocoral Dendronephthya sp.,427 have been prepared leading to the conclusion that the natural product has the (20S) configuration.428Nephthea bayeri, collected off Nanji Island China, yielded nanjiols A–C 596–598.429 The structure of 22,23-dimethylcholest-5-en-3β-ol 599, isolated as the major sterol component of an Andaman and Nicobar Island collection of Sinularia species, must remain tentative as it was derived solely from mass fragmentation analysis of the monoacetate derivative.381 A study of Japanese collections of Isis hippuris led to the isolation of steroids 600–610430 in addition to the previously reported 611.431 X-Ray analysis established the stereochemistries of 611 and 610, the latter result also establishing the stereochemistry of 612, previously reported from the same species.432 Most of the compounds showed moderate cytotoxicity towards drug-resistant cells expressing P-gp but not against cells expressing multidrug resistance protein-1 (MRP-1). Further investigation of a Korean collection of the stony coral Montipora sp. yielded diacetylenes 613–615 of which 615 was the most potent cytotoxin towards a range of tumour cell lines.433 The structures proposed for cladocorans A 616 and B 617, isolated from the Mediterranean anthozoan Cladocora cespitosa,434 are in question as spectroscopic data of synthetic material were not identical to data reported for the natural products.435 Ecdysteroid zoanthusterone 618 was isolated from a Thai collection of Zoanthus species.436 Tridentatols D–H 619–623 were isolated from the marine hydroid Tridentata marginata collected off North Carolina.437 Enzyme-mediated hydrolysis of the sulfate esters leads to the corresponding phenols, which include the potent feeding deterrent 624. The sea anemone Anthopleura pacifica yielded ceramide (4E,8E)-spingol-n-hexadecamide 625.438 The solution structure of equinatoxin II, a 19.8 kDa cytolysin isolated from the Mediterranean anemone Actinia equina,439 has been determined by NMR analysis.440 The structure provides some clues as to how the peptide may bind to cell membranes and form pores, while a separate study has utilised lipid monolayers and surface plasmon resonance to further examine the events leading to pore formation.441 A polypeptide toxin, PsTX-20A, of molecular mass 20 kDa was purified from an Okinawan collection of the anemone Phyllodiscus semoni.442Radianthus macrodactylus, collected in the Seychelles, yielded RTX-A, RTX-S and RTX-G, three high molecular weight (20 kDa) cytolysins, two low molecular weight cytolysins, RmI (5100 Da) and RmII (6100 Da) and InI, a 7100 Da trypsin inhibitor.443 The first total syntheses of montipyridine444 and montiporynes A and B,445 metabolites of the stony coral Montipora species,446,447 have been reported. Further investigation of the diterpene glycoside lemnabourside, originally isolated from the soft coral Lemnalia bournei,448 has shown it to be an inhibitor of 5α-reductase and to exhibit antiproliferative activity via the caspase-3 apoptotic pathway.449 The sodium channel toxins Bg II and Bg III, isolated from the sea anemone Bunodosoma granulifera,450 have been found to be especially potent towards insect sodium channels.4519 Bryozoans
Despite their promise as excellent sources of novel, bioactive metabolites, only a handful of new compounds have been reported from bryozoans. Most of these are alkaloids. Four new bromotryptamine derivatives 626–629 have been isolated from the North Sea bryozoan Flustra foliacea collected in German waters, and the complete 13C NMR spectral data for the known compound flustrabromine452 have been reported for the first time.453 A sample of F. foliacea collected in the southern North Sea also yielded deformylflustrabromine 628 which displayed moderate cytotoxicity against the HCT-116 cell line.454 The complete 1H and 13C NMR assignments for the F. foliacea alkaloids dihydroflustramine C,455 flustramine A,456 flustramine E,457 debromoflustramine B,457 and flustramine B456 have been published, reconciling deficiencies and ambiguities from earlier literature assignments.458 The marine bryozoan Amathia convoluta collected from the East coast of Tasmania, was the source of the tribrominated alkaloids, convolutamine H 630 and convolutindole A 631. Compounds 630 and 631 displayed potent and selective activity against Haemonchus contortus, a parasitic nematode of ruminants.459Watersipora subtorquata from Tsutsumi Island, Japan was the source of bryoanthrathiophene 632. This compound 632 exhibited potent antiangiogenic activity on bovine aorta endothelial cell (BAEC) proliferation.460 Three disulfides, pentaporins A–C 633–635, have been isolated from the Mediterranean bryozoan Pentapora fascialis. Energy dispersive X-ray analysis assisted in the determination of the existence of sulfur atoms. The pentaporins 633–635 displayed anthelmintic activity against Trichinella spiralis.461 Several synthetic firsts have also been reported for bryozoan metabolites: the total syntheses of dihydroflustramine C455 and flustramine E457 have been achieved,462 as has the total synthesis of amathaspiramide F,463 an alkaloid from a New Zealand collection of Amathia wilsoni.464 Asymmetric syntheses of amathamides A and B, alkaloids from the bryozoan A. wilsoni collected in Tasmania,465 have been accomplished starting from 3-hydroxybenzaldehyde.46610 Molluscs
Fewer examples of new metabolites were reported from molluscs in 2002 than during the time period of the previous review. The absolute stereochemistries of membrenones A–C 636–638, γ-dihydropyrone-containing polypropionates isolated from the skin of the Mediterranean mollusc Pleurobranchus membranaceus,467 have been determined by stereocontrolled syntheses of the enantiomers.468 The stereochemical assignment of 638 is a correction of an earlier synthetic effort469 necessitated by the conclusion that the sign of the optical rotations of 637 and 638 were misreported in the original isolation publication.467 Stereoselective syntheses have led to correction of the relative stereochemistry and established the absolute stereochemistry of siphonarienolone 639 and siphonarienedione 640,470 polypropionates originally isolated from the mollusc Siphonaria grisea.471 The first synthesis of siphonarin B 641 has confirmed the absolute stereochemistry of the metabolite,472 isolated from the molluscs Siphonaria zelandica and S. atra.473 The structure and stereochemistry of a new polychlorinated sulfolipid 642 was reported from collections of the mussel Mytilus galloprovincialis made in the Adriatic Sea.474 The relative stereochemistry of 642 was established by 1H-13C and 1H-1H coupling constant analysis while absolute stereochemistry was established by the preparation and analysis of Mosher MTPA esters. Mild cytotoxicity to murine fibrosarcoma and monocyte/macrophage cell lines was observed for 642. In a separate study of the same mollusc species, LC-MS/MS was used to identify a new yessotoxin analogue 643 designated noroxoYTX.475 The novel chlorinated pyrrolidone 644 was isolated from extracts of a Philippino collection of the dorid nudibranch Asteronotus cespitosus.476 As similar metabolites have been reported previously from the sponge Dysidea herbacea, the study concluded that the carnivorous mollusc in question acquired the metabolite as part of its diet as opposed to de novo synthesis. A new member to the malyngamide series of metabolites, malyngamide S 645, was reported from a New Zealand collection of the sea hare Bursatella leachii.477 The compound also exhibited mild cytotoxicity and anti-inflammatory activities. Kulokekahilide-1 646 is a moderately cytotoxic depsipeptide isolated from the mollusc Philinopsis speciosa collected off Pupukea, O'ahu.478 The absolute stereochemistry of 646 was determined by degradation combined with Marfey analysis as well as the synthesis of all stereoisomers of the two unusual amino acids, 4-phenylvaline and 3-amino-2-methylhexanoic acid. Bursatellanin-P, a 60 kDa protein was purified from the purple ink of the sea hare Bursatella leachii.479 The protein exhibited anti-HIV activity. In an intriguing twist to the usual natural product isolation paradigm, PCR amplification, using primers of the α-conotoxin gene sequence, of genomic DNA from the predatory marine snail Conus geographus yielded a single specific α-conopeptide gene product.480 Subsequent cloning and sequencing identified the α-conotoxin GIC sequence. The predicted mature toxin, a 16-amino acid peptide, was then synthesised and found to act as a potent antagonist of the neuronal nicotinic receptor. The biosynthesis of 2,6-dimethyl-5-heptenal, a volatile component of skin extracts of the dendronotid nudibranch Melibe leonina,481 has been investigated using stable isotope incorporation experiments.482 Successful incorporation of 13C demonstrated that the metabolite was the product of de novo terpenoid biosynthesis by the nudibranch. The first enantiospecific synthesis of (−)-9-pupukeanone 647, a degradation product of the volatile defensive isonitrile 9-isocyanopupukeanane 648 isolated from the nudibranch Phyllidia varicosa,483 has been reported.484 The synthesis confirms the absolute stereochemistry of the pupukeanane skeleton. The absolute stereochemistry of ibhayinol 649, a sesquiterpenoid metabolite isolated from a South African collection of the sea hare Aplysia dactylomela,485 was established by X-ray analysis.486 MS-MS studies have firmly established the position of the methyl ester moiety in the purple pigment aplysiaviolin 650, isolated from a Tasmanian collection of the sea hare Aplysia parvula.173 This study included an investigation of the sea hare's algal diet Laurencia filiformis which revealed the presence of the 5-acetate derivative 168 of prepacifenol, as well as prepacifenol and a range of other known metabolites (vide supra). Sardinian collections of the sea hare Aplysia punctata afforded a range of metabolites including four new diterpenes, 651–654, and three new sesquiterpenes 655–657.487 Relative stereochemistries of 651–657 were secured by NOESY NMR experiments – the absolute configurations were not established. A progesterone homologue 658 was isolated from the skin of the dorid nudibranch Aldisa smaragdina collected off Cabo Cope, Spain.488 The absolute stereochemistry was secured by synthesis from stigmasterol.The first total syntheses of aplyolides B–E, ichthyotoxic macrolides isolated from the skin of sea hare Aplysia depilans,489 have been reported confirming the absolute stereochemistry reported for the metabolites.490,491 A new synthesis of 3-isocyanotheonellin, a nitrogenous bisabolene sesquiterpene isolated from a Sri Lankan collection of the nudibranch Phyllidia sp.,492 was also amenable to the synthesis of related compounds all of which exhibited potent in vitro antifouling activity towards barnacle larvae.493 (−)-Doliculide, a cytotoxic depsipeptide isolated from a Japanese collection of the sea hare Dolabella auricularia,494,495 exhibits cytotoxicity by enhancing actin assembly.496
11 Tunicates (ascidians)
A similar number of new metabolites were reported in 2002 compared to 2001 and they continue to be dominantly amino acid-derived. The inhibitor of matrix metalloproteinase 2 (MMP2) from an ascidian of the family Polyclinidae collected off Kii Peninsula, Western Japan was identified as sodium 1-(12-hydroxy)octadecanyl sulfate 659.497 MTPA ester analysis indicated that 659 occurs naturally as a 55 : 45 mixture of the (12R) and (12S) enantiomers. Synthesis from (R)-12-hydroxystearic acid confirmed the structure of 659. Both natural and synthetic material inhibited MMP2 with equal potency. The absolute stereochemistry of lobatamide C 660, a cytotoxic macrolide isolated from Aplidium lobatum collected off the southwestern coast of Australia,498 has been defined by stereospecific synthesis.499 A novel triglycosylceramide derivative, sulcaceramide 661, was reported from a Mediterranean collection of the ascidian Microcosmus sulcatus.500 The structure was solved by a combination of spectroscopic techniques and degradation/derivatisation studies. Two unusual 1,2,3-trithiocane derivatives, 662 and 663 were isolated from the ascidian Perophora viridis collected off the Atlantic coast of North Carolina.501 Relative stereochemistries were deduced from NOESY NMR experiments while MTPA derivatisation of the hydroxyl at C-7′ helped secure the absolute configuration of 662. Both compounds exhibited mild antibacterial activity as well as toxicity towards brine shrimp. The total synthesis of a stereoisomer of bistramide C, a cytotoxic polyether isolated from the ascidian Lissoclinum bistratum,502 combined with chiroptical analysis led to the proposal of 664 as the predicted relative and absolute configuration of the natural product.503 The relative and absolute stereochemistries of didemnaketals B 665 and C 666, reported from an undescribed Palauan ascidian of the genus Didemnum,504,505 were established by a combination of degradation and derivatisation experiments.506 The structure of (+)-didemniserinolipid B 667, a serinolipid isolated from an Indonesian collection of Didemnum sp.,507 has been revised to the 31-sulfate and the relative and absolute configuration of the natural product established by synthesis.508 A new member of the tunichrome family of modified peptides, tunichrome Sp-1 668 was isolated from the hemocytes of Styela plicata, collected in Mission Bay, California.509 The sequence of 668 was determined by Edman degradation while stereochemistry was determined by acid hydrolysis followed by derivatisation and GC-MS and HPLC analysis. The ascidian Didemnum molle, collected at Ibo Island north of Mozambique, was the source of the cycloheptapeptide cyclodidemnamide B 669.510 Hydrolysis followed by Marfey's derivatisation and HPLC analysis allowed determination of configuration at many of the stereogenic centres with final confirmation being achieved by total synthesis. Localisation studies of the related cyclic peptides patellamides A–C suggested that the natural products are distributed throughout the tunic of the ascidian Lissoclinum patella and not located in the Prochloron sp. symbiotic cyanobacteria.511 Halocidin was isolated as an antimicrobial peptide (3443 Da) from the hemocytes of the solitary ascidian Halocynthia aurantium.512 Cloning of a peptide precursor from a cDNA library prepared from pharyngeal tissues of the tunicate Styela clava identified clavaspirin as a 23-residue antibacterial peptide.513 Synthetic clavaspirin inhibited the growth of Gram-positive and negative bacteria, permeabilised E. coli membranes and was potently haemolytic towards human and bovine erythrocytes. Polyclonal antibodies, raised against clavanin A, have been used to locate the clavanin family of antibacterial peptides in the eosinophilic granulocytes and macrophages of the ascidian Styela clava.514 Lepadins D 670, a salt of 670 with an unidentified counterion, E 671 and F 672 were isolated as antiplasmodial and antitrypanosomal alkaloid constituents of a Didemnum sp. ascidian collected from Stanley Reef, the Great Barrier Reef.515 The relative stereochemistries of substitution about the decahydroquinoline ring system in 670–672 were determined by NOESY NMR experiments and are illustrated here with the defined ring junction absolute stereochemistry of lepadin A.516 Lepadins F–H 672–674 were reported from extracts of the ascidian Aplidium tabascum collected from Swains Reef, Great Barrier Reef.517 In two separate accounts, the absolute stereochemistry of lepadiformine 675, a biologically active alkaloid isolated from the ascidians Clavelina lepadiformis and C. moluccensis,518,519 has been defined by stereoselective total synthesis.520,521 Two new 2-aminoimidazolone alkaloids, polyandrocarpamines A 676 and B 677, were isolated from a Fijian collection of the ascidian Polyandrocarpa sp. and the structures confirmed by synthesis.522 Coproverdine 678 is a cytotoxic alkaloid isolated by bioassay-directed fractionation of an unidentified ascidian collected at the Three Kings Islands, New Zealand.523 Cytotoxicity towards a variety of murine and human tumour cell lines was observed. Rubrolide M, recently isolated from a Spanish collection of the ascidian Synoicum blochmanni,524 was synthesised using palladium-catalysed coupling methodology.525 The compound and related congeners were found to exhibit cytotoxicity towards human tumour cell lines. The first syntheses of rhopaladins A–C and a new route to rhopaladin D have been reported,526 confirming the structures of the alkaloids isolated from a Rhopalaea sp. ascidian collected in Okinawa.527 Bioassay-directed fractionation of extracts of the New Zealand endemic ascidian Pycnoclavella kottae afforded the cytotoxic and anti-inflammatory alkaloids kottamides A–D 679–682.528 The structures were solved by spectroscopic techniques, including the use of 1H-15N 2D NMR experiments. Sebastianines A 683 and B 684 were isolated as biologically active pyridoacridine metabolites from a Brazilian collection of the ascidian Cystodytes dellechiajei.529 The relative stereochemistry of 684 was determined by interpretation of NOESY NMR data. The cytotoxicities of both compounds towards human colon tumour cells were determined to be p53 dependent by use of p53 and p21 knockout cell lines. The New Zealand ascidian Lissoclinum notti was found to contain the cytotoxic and antibacterial pyridoacridine alkaloids isodiplamine 685, cystodytin K 686 and lissoclinidine 687 in addition to several known related compounds including diplamine 688.530 Conversion of diplamine 688 to lissoclinidine 687 was achieved by photochemical-induced isomerisation, but the natural product status of lissoclinidine was confirmed by rapid analysis of extract that was collected underwater and kept away from light. Two new pyridoacridine alkaloids, kuanoniamines E 689 and F 690, and a putative biosynthetic precursor subarine 691 were isolated from a Singaporean collection of an unidentified ascidian.531 The mildly cytotoxic perophoramidine 692 was isolated from a Philippines collection of the ascidian Perophora namei.532 The carbon skeleton of 692 was established by analysis of HMBC and 2D INADEQUATE NMR data, while relative stereochemistry was determined by analysis of ROESY NMR data. The structures of the pyrrole alkaloids polycitones A and B, originally isolated from a South African collection of the ascidian Polycitor sp. and Madagascan collections of Polycitor africanus,533,534 have been confirmed by synthesis.535 A new member of the lamellarin class of alkaloids, lamellarin β 693 was reported from a collection of the ascidian Didemnum sp.536 Investigation of the ascidian Eudistoma toealensis and its predatory flatworm Pseudoceros sp., collected in Chuuk, Micronesia, yielded three new staurosporine derivatives 694–696.537 The absolute stereochemistries of 694–696 were established by comparison of CD data with those observed for staurosporine which has defined absolute stereochemistry.538 The study also led to revision of the absolute stereochemistries of derivatives 697 and 698, previously reported from the same organisms.539 A study of the Thai ascidian Ecteinascidia thurstoni, using a KCN-pretreatment isolation procedure, afforded the known alkaloid ecteinascidin 770 699 and the novel analogue ecteinascidin 786 700.540 Both 699 and 700 exhibited potent cytotoxicity towards tumour cell lines and growth inhibition of M. tuberculosis H37Ra. The structure of 699 was confirmed in a separate study that also reported the stereoselective synthesis of ecteinascidin 743.541 The mechanism of cytotoxic action of ecteinascidin 743 has been reviewed.10 A Lissoclinum species collected off Hateruma Island, Okinawa contained haterumaimides J 701 and K 702 with the relative stereochemistry being deduced from NOESY NMR experiments.542 Both of the diterpene alkaloids exhibited potent cytotoxicity towards the P388 cell line. A range of meroterpenoids including the new examples 703–706 were isolated from a Tarifa Island, Cádiz collection of the ascidian Aplidium conicum.543 The relative stereochemistry was determined by 1D and 2D NOE NMR experiments. The sulfated steroid 707 was found to be responsible for sperm activation and attraction in Japanese collections of the ascidians Ciona intestinalis and C. savignyi.544 The absolute stereochemistry of (−)-longithorone A 708, a dimeric prenylated quinone isolated from the ascidian Aplidium longithorax,545,546 has been deduced in an elegant study that utilised Diels–Alder reactions in a biomimetic fashion.547 The structure of ritterazine M 709, a cytotoxic steroidal alkaloid isolated from the Japanese collection of the ascidian Ritterella tokioka,548 has been corrected by total synthesis.549,550 The structure presented for 709 in the synthesis papers contains an inadvertent error in the depicted C-25′ stereochemistry of the isolated natural product – the correct structure of ritterazine M is shown in this review.Further investigation has shown that the mechanism of cytotoxic action of vitilevuamide, a bicyclic peptide isolated from Fijian collections of the ascidians Didemnum cuculliferum and Polysyncraton lithostrotum,551 involves the inhibition of tubulin polymerisation possibly via interaction at a unique site.552 The in vivo antitumour activity of the dimeric disulfide alkaloid polycarpine, isolated from the ascidians Polycarpa clavata553 and P. aurata,554 and related synthetic analogues has been investigated.555
12 Echinoderms
Glycosylated ceramides and saponins continue to be the major classes of metabolites identified in echinoderms. A full account of the isolation and characterisation of hedathiosulfonic acids A and B, isolated from a Japanese collection of the deep-sea urchin Echinocardium cordatum,556 has been reported.557 In addition, the compounds are the subject of a Japanese patent claiming the use of the compounds as antitumour, antibacterial and antifouling agents.558 Luidiacerebrosides A 710 and B 711 were isolated from the starfish Luidia maculata collected in Hakata Bay, Fukuoka, Japan.559 The stereochemistries of 710 and 711 were determined by degradation, fragment derivatisation and subsequent comparison with published data. In a separate study of L. maculata, the same research group also reported the new ganglioside molecular species LMG-3, of which 712 is the major component.560 A collection of the Patagonian starfish Allostichaster inaequalis afforded two new glucosylceramides 713 and 714.561 Ten glucocerebrosides, HPC-3-A to HPC-3-J 715–724 and two glucocerebroside molecular species HPC-1 725 and HPC-2 726 were isolated from the Japanese sea cucumber Holothuria pervicax (Torafunamako).562 While the ceramide portions of HPC-1 and HPC-2 were comprised of extensive heterogeneous mixtures of alkyl homologues, use of reversed-phase HPLC was effective in purifying 715–724, although regioisomer ambiguity still exists. All eight stereoisomers of pulcherrimine, a bitter principle isolated from the ovary of the sea urchin Hemicentrotus pulcherrimus,563 have been synthesised, leading to the revision of the structure for the (2′S,2R,4S) isomer 727.564 Investigation of the water-soluble components of crinoids collected at Goza, Japan afforded, in addition to a range of known quinones, the 6-O-sulfate of ptilometric acid 728 from Tropiometra afra macrodiscus and 729 from Oxycomanthus japonicus respectively.565 The new sulfated steroid 730 was isolated from both Leptasterias alaskensis asiatica and L. fisheri, starfish collected at the Kiril Islands.566 A study of the starfish Diplopteraster multipes, also collected in the Far East, afforded a range of sterol sulfates including the new example 731.567 Lysastroside A 732, a new steroidal glycoside was isolated from the starfish Lysastrosoma anthosticta, collected in the Sea of Japan.568 Ten new saponins, certonardosides A–J 733–742 were isolated from the starfish Certonardoa semiregularis, collected off the coast of Komun Island, Korea.569 The absolute configurations of the side chains were secured by the 1H NMR analysis of MTPA esters. All compounds were evaluated for a range of antiviral properties towards HIV, HSV, CoxB, EMCV and VSV, but only mild potency was observed for 741 and 742. Linckosides A 743 and B 744, neuritogenic steroidal glycosides, were reported from an Okinawan collection of the starfish Linckia laevigata.570 Both compounds induced neuronal differentiation in PC12 cells, with 744 being more potent. Significant synergistic effects on NGF-induced neuronal differentiation in PC12 cells were also observed for 743 and 744. Brine shrimp lethality-directed fractionation of the Patagonian starfish Anasterias minuta afforded anasterosides A 745 and B 746.571 Anasteroside A 745 exhibited antifungal activity towards the plant pathogen Cladosporium cucumerinum, while 746 was inactive at all tested concentrations. Hydrolysis of the crude triterpenoid glycosides purified from Andaman and Nicobar Islands, Indian Ocean collections of the sea cucumbers Holothuria nobilis and Bohadschia aff. tenuissima afforded the new genins 747–749.572 Hemoiedemosides A 750 and B 751 are cytotoxic and antifungal triterpene glycosides isolated from the Patagonian sea cucumber Hemoiedema spectabilis.573 Hemoiedemoside A 750 was more potent in the brine shrimp assay and more antifungal towards Cladosporium cucumerinum than 751, while the desulfated analogue of 750 was significantly less active in the same assays. In the search for antagonists of the chemokine receptor subtype 5 (CCR5) as possible anti-HIV agents, bioassay guided fractionation of an Andaman and Nicobar Island collection of the sea cucumber Telenata ananas afforded two triterpene glycosides, 752 and 753.574 Both compounds exhibited inhibitory activity in a CCR5 while no activity was observed towards the related receptor CXCR2.Two reports of new syntheses of echinoderm metabolites appeared in 2002. A new route for the synthesis of (2S,2′R,3S,4R)-2-(2′-hydroxy-21′-methyldocosanoylamino)-1,3,4-pentadecanetriol, a ceramide sex pheromone isolated from the female Hair Crab, Erimacrus isenbeckii,575,576 was reported,577 while squaric acid ester-based methodology was used in a new synthesis of echinochrome A, a polyhydroxylated napthoquinone pigment commonly isolated from sea urchin spines.578
13 Miscellaneous
A series of flavones, the thalassiolins A–C 754–756 has been isolated from the Caribbean sea grass Thalassia testudinum (turtle grass). Thalassiolins A–C 754–756 are HIV integrase inhibitors, of which thalassiolin A 754 is the most potent.57914 Conclusion
Despite the greater number of people working in more diverse areas on marine natural products the rate of discovery of new compounds during 2002 (677) was only comparable to that for any year in the last decade or so (∼718 per annum on average). This could perhaps be explained by a steady state being reached where the greater effort is balanced against the difficulties of finding new compounds. As in previous years new compounds from sponges and coelenterates dominate (37% and 21% respectively: see Fig. 1). Notable in 2002 was the decrease in compounds reported from molluscs (down from 7% to 2%).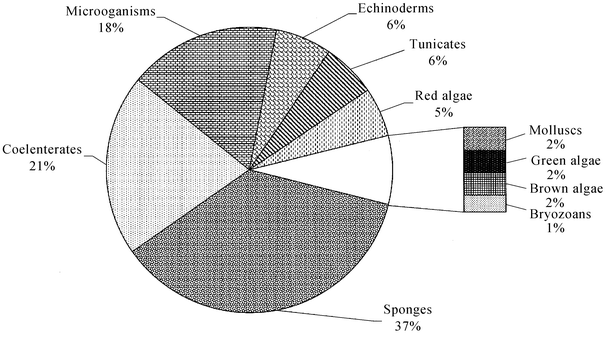 | ||
| Fig. 1 Distribution of marine natural products by phylum, 2002. | ||
The presumed biogenetic origins of the new compounds for 2002 have been systematically assigned. In assigning biogenetic origins divisions were made into peptide, terpenoid, alkaloid, polyketide and shikimate categories. Glycosides were classified by the origins of the aglycone; compounds of mixed biogenetic origins were classified as to the probable source of the majority of the carbon atoms; peptides, depsipeptides and peptide esters were grouped together separate from alkaloids, for which the criterion of a basic nitrogen was applied. Non-basic aromatic compounds of shikimate or tryptophan origin were classified as shikimate. Divisions were made within each category. The terpenoids were divided up into mono, sesqui etc., but steroids and triterpenoids and higher terpenoids were clustered and a category of meroterpenoids introduced. Alkaloids were sub-divided into five categories that included compounds of polyketide, 3-alkylpyridine, shikimate, tryptophan as well as other origins. Three subdivisions only were used for the polyketides – regular polyketides, compounds of fatty acid, ceramide or sphingolipid origin, and the macrolides.
The overall biogenetic distribution is shown in Fig. 2. The dominant pathway is that of terpenoid biogenesis (48%), which is perhaps not surprising as the chemistry of the two largest groups examined, the sponges and coelenterates, is dominated by terpenoid compounds. A more detailed breakdown of biogenetic origins is shown in Fig. 3. For clarity the algae are shown as just one grouping and compounds of direct shikimate origin have been omitted. It is emphasised that these distributions are for compounds reported in 2002, and should not be taken to necessarily reflect the distributions for all reported marine natural products.
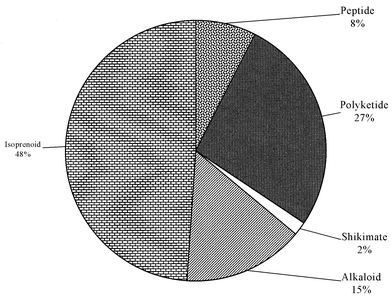 | ||
| Fig. 2 Biogenetic origins of marine natural products, 2002. | ||
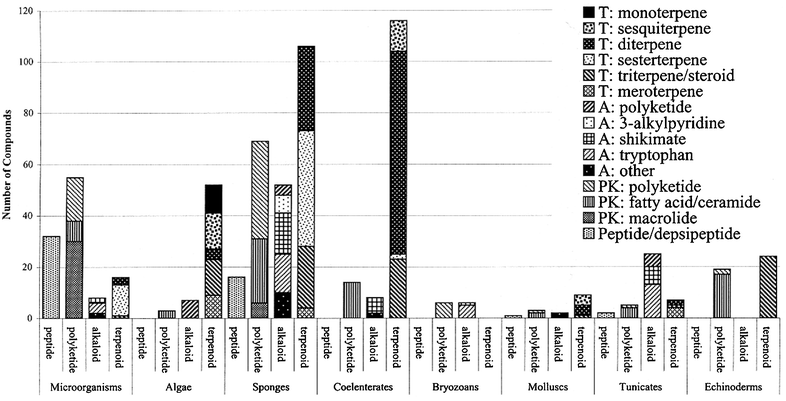 | ||
| Fig. 3 Detailed biogenetic origins of marine natural products, 2002. | ||
Acknowledgements
We thank Ekkehard Unger for assistance in the collection of data for this review.References
- J. W. Blunt, B. R. Copp, M. H. G. Munro, P. T. Northcote and M. R. Prinsep, Nat. Prod. Rep., 2003, 20, 1 RSC.
- A. H. Banner, P. J. Scheuer, S. Sasaki, P. Helfrich and C. B. Alender, Ann. N. Y. Acad. Sci., 1960, 90, 770 CAS.
- J. Lukomska, F. Kasprzykowski, L. Lankiewicz and Z. Grzonka, Wiad. Chem., 2002, 56, 57 Search PubMed.
- M. D. Vera and M. M. Joullié, Med. Res. Rev., 2002, 22, 102 CrossRef CAS.
- A. B. Dounay and C. J. Forsyth, Curr. Med. Chem., 2002, 9, 1939 Search PubMed.
- G. G. Harrigan and G. Goetz, J. Appl. Phycol., 2002, 14, 103 Search PubMed.
- H. Luesch, G. G. Harrigan, G. Goetz and F. D. Horgen, Curr. Med. Chem., 2002, 9, 1791 Search PubMed.
- Y. Kishi, Tetrahedron, 2002, 58, 6239 CrossRef CAS.
- M. Kalesse and M. Chirstmann, Synthesis, 2002, 8, 981 CrossRef.
- G. J. Aune, T. Furuta and Y. Pommier, Anti-Cancer Drugs, 2002, 13, 545 CrossRef CAS.
- J. D. Scott and R. M. Williams, Chem. Rev., 2002, 102, 1669 CrossRef CAS.
- K. J. Hale, M. G. Hummersone, S. Manaviazar and M. Frigerio, Nat. Prod. Rep., 2002, 19, 413 RSC.
- A. Clamp and G. C. Jayson, Anti-Cancer Drugs, 2002, 13, 673 CrossRef CAS.
- P. Sung, J. Sheu and J. Xu, Heterocycles, 2002, 57, 535 CAS.
- R. G. S. Berlinck, Nat. Prod. Rep., 2002, 19, 617 RSC.
- Z. Jin, Z. G. Li and R. Q. Huang, Nat. Prod. Rep., 2002, 19, 454 RSC.
- V. M. Dembitsky and M. Srebnik, Prog. Lipid Res., 2002, 41, 315 CrossRef CAS.
- S. Hibino and T. Choshi, Nat. Prod. Rep., 2002, 19, 148 RSC.
- J. P. Michael, Nat. Prod. Rep., 2002, 19, 719 RSC.
- V. M. Dembitsky, Russ. J. Bioorg. Chem., 2002, 28, 170 Search PubMed.
- V. M. Dembitsky, R. Smoum, A. A. Al-Quntar, H. A. Ali, I. Pergament and M. Srebnik, Plant Sci., 2002, 163, 931 CrossRef CAS.
- A. Kanazawa, Fish Sci., 2001, 67, 997 Search PubMed.
- W. H. Gerwick and I. P. Singh, Lipid Biotechnol., 2002, 249 Search PubMed.
- D. Skyler and C. H. Heathcock, J. Nat. Prod., 2002, 65, 1573 CrossRef CAS.
- F. P. Marmsäter and F. G. West, Chem. Eur. J., 2002, 8, 4346 CrossRef CAS.
- G. Pohnert and W. Boland, Nat. Prod. Rep., 2002, 19, 108 RSC.
- R. G. Kerr, A. C. Kohl and J. M. Boehnlein, Chem. Pharm. Bull., 2002, 50, 149.
- C. J. P. Grimmelikhuijzen, M. Williamson and G. N. Hansen, Can. J. Zool., 2002, 80, 1690 CrossRef CAS.
- P. V. L. Rao, N. Gupta, A. S. B. Bhaskar and R. Jayaraj, J. Environ. Biol., 2002, 23, 215 Search PubMed.
- P. J. Sung and M. C. Chen, Heterocycles, 2002, 57, 1705 CAS.
- P. R. Jensen and W. Fenical, Br. J. Pharmacol., 2002, 137, 293.
- A. Kelecom, An. Acad. Bras. Cienc., 2002, 74, 151 Search PubMed.
- G. Anderluh and G. Menestrina, Mar. Biotechnol., 2002, 8, 131.
- J. D. Leblond and P. J. Chapman, J. Phycol., 2002, 38, 670 CrossRef CAS.
- M. V. D'Auria, A. Zampella and F. Zollo, Stud. Nat. Prod. Chem., 2002, 26, 1175 Search PubMed.
- N. Lindquist, J. Chem. Ecol., 2002, 28, 1987 Search PubMed.
- P. Proksch, R. A. Edrada and R. Ebel, Appl. Microbiol. Biotechnol., 2002, 59, 125 Search PubMed.
- A. M. S. Mayer and M. T. Hamann, Comp. Biochem. Physiol., C: Toxicol. Pharm., 2002, 132, 315 Search PubMed.
- R. J. Quinn, P. de Almeida Leone, G. Guymer and J. N. A. Hooper, Pure Appl. Chem., 2002, 74, 519 Search PubMed.
- E. L. Ghisalberti, Stud. Nat. Prod. Chem., 2002, 26, 425 Search PubMed.
- M. E. Elyashberg, K. A. Blinov, A. J. Williams, E. R. Martirosian and S. G. Molodtsov, J. Nat. Prod., 2002, 65, 693 CrossRef CAS.
- J. Lei and J. Zhou, J. Chem. Inf. Comput. Sci., 2002, 42, 742 CrossRef CAS.
- MarinLit database, Department of Chemistry, University of Canterbury: http://www.chem.canterbury.ac.nz/research/marinlit.htm.
- T. Barsby, M. T. Kelly and R. J. Andersen, J. Nat. Prod., 2002, 65, 1447 CrossRef CAS.
- I. Ohtani, T. Kusuki, Y. Kashman and H. Kakisawa, J. Am. Chem. Soc., 1991, 113, 4092 CrossRef CAS.
- T. Sugawara, M. Shibazaki, H. Nakahara and K. Suzuki, J. Antibiot., 1996, 49, 345 CAS.
- M. Ermolenko, Tetrahedron Lett., 1996, 37, 6711 CrossRef CAS.
- N. I. Kalinovskaya, T. A. Kuznetsova, E. P. Ivanova, L. A. Romanenko, V. G. Voinov, F. Huth and H. Laatsch, Mar. Biotechnol., 2002, 4, 179 CrossRef CAS.
- K. Barbeau, G. Zhang, D. H. Live and A. Butler, J. Am. Chem. Soc., 2002, 124, 378 CrossRef CAS.
- L. Yang, R. Tan, Q. Wang, W. Huang and Y. Yin, Tetrahedron Lett., 2002, 43, 6545 CrossRef CAS.
- R. N. Asolkar, R. P. Maskey, E. Helmke and H. Laatsch, J. Antibiot., 2002, 55, 893 CAS.
- D. Laurent, G. Guella, I. Mancini, M.-F. Roquebert, F. Farinole and F. Pietra, Tetrahedron, 2002, 58, 9163 CrossRef CAS.
- Z. Jiang, M.-O. Barret, K. G. Boyd, D. R. Adams, A. S. F. Boyd and J. G. Burgess, Phytochemistry, 2002, 60, 33 CrossRef CAS.
- Y. Lin, H. Li, G. Jiang, S. Zhou, L. L. P. Vrijmoed and E. B. G. Jones, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2002, 41, 1542.
- T. Yamada, C. Iwamoto, N. Yamagaki, T. Yamanouchi, K. Minoura, T. Yamori, Y. Uehara, T. Andoh, K. Umemura and A. Numata, Tetrahedron, 2002, 58, 479 CrossRef CAS.
- H. Sakaki, H. Kaneno, Y. Sumiya, M. Tsushima, W. Miki, N. Kishimoto, T. Fujita, S. Matsumoto, S. Komemushi and A. Sawabe, J. Nat. Prod., 2002, 65, 1683 CrossRef CAS.
- M. Chu, R. Mierzwa, I. Trumees, F. Gentile, M. Petel, V. Gullo, T.-M. Chan and M. S. Puar, Tetrahedron Lett., 1993, 34, 7537 CrossRef CAS.
- Y. Usami, S. Aoki, T. Hara and A. Numata, J. Antibiot., 2002, 55, 655 CAS.
- B. W. Son, J. S. Choi, J. C. Kim, K. W. Nam, D. S. Kim, H. Y. Chung, J. S. Kang and H. D. Choi, J. Nat. Prod., 2002, 65, 794 CrossRef CAS.
- M. Cueto, P. R. Jensen and W. Fenical, Org. Lett., 2002, 4, 1583 CrossRef CAS.
- R. Jadulco, G. Brauers, R. A. Edrada, R. Ebel, V. Wray, Sudarsono and P. Proksch, J. Nat. Prod., 2002, 65, 730 CrossRef CAS.
- J. Malmstrøm, C. Christophersen, A. F. Barrero, J. E. Oltra, J. Justicia and A. Rosales, J. Nat. Prod., 2002, 65, 364 CrossRef.
- T. Yamada, M. Iritani, K. Minoura, A. Numata, Y. Kobayashi and Y.-G. Wang, J. Antibiot., 2002, 55, 147 CAS.
- A. Numata, M. Iritani, T. Yamada, K. Minoura, E. Matsumura, T. Yamori and T. Tsuruo, Tetrahedron Lett., 1997, 38, 8215 CrossRef CAS.
- T. Yamada, M. Iritani, M. Doi, K. Minoura, T. Ito and A. Numata, J. Chem. Soc., Perkin Trans. 1, 2001, 3046 RSC.
- H. Nakamura, M. Ono, T. Yamada, A. Numata and H. Akita, Chem. Pharm. Bull., 2002, 50, 303 CrossRef CAS.
- H. Nakamura, M. Ono, Y. Shida and H. Akita, Tetrahedron: Asymmetry, 2002, 13, 705 CrossRef CAS.
- Y. Kobayashi and Y. G. Wang, Tetrahedron Lett., 2002, 43, 4381 CrossRef CAS.
- C. Takahashi, T. Takada, T. Yamada, K. Minoura, K. Uchida, E. Matsumura and A. Numata, Tetrahedron Lett., 1994, 35, 5013 CrossRef CAS.
- T. Yamada, K. Minoura and A. Numata, Tetrahedron Lett., 2002, 43, 1721 CrossRef CAS.
- M. Isaka, C. Suyarnsestakorn, M. Tanticharoen, P. Kongsaeree and Y. Thebtaranonth, J. Org. Chem., 2002, 67, 1561 CrossRef CAS.
- C. Osterhage, G. M. König, U. Höller and A. D. Wright, J. Nat. Prod., 2002, 65, 306 CrossRef.
- C.-Y. Wang, B.-G. Wang, G. Brauers, H.-S. Guan, P. Proksch and R. Ebel, J. Nat. Prod., 2002, 65, 772 CrossRef CAS.
- S. S. Afiyatullov, A. I. Kalinovsky, T. A. Kuznetsova, V. V. Isakov, M. V. Pivkin, P. S. Dmitrenok and G. B. Elyakov, J. Nat. Prod., 2002, 65, 641 CrossRef CAS.
- D. Abbanat, M. Leighton, W. Maiese, E. B. G. Jones, C. J. Pearce and M. J. Greenstein, J. Antibiot., 1998, 51, 296 CAS.
- G. Schlingmann, L. Milne and G. T. Carter, Tetrahedron, 2002, 58, 6825 CrossRef CAS.
- R. A. Edrada, M. Heubes, G. Brauers, V. Wray, A. Berg, U. Gräfe, M. Wohlfarth, J. Mühlbacher, K. Schaumann, Sudarsono, G. Bringmann and P. Proksch, J. Nat. Prod., 2002, 65, 1598 CrossRef CAS.
- M. Daferner, T. Anke and O. Sterner, Tetrahedron, 2002, 58, 7781 CrossRef CAS.
- M. Chinworrungsee, P. Kittakoop, M. Isaka, R. Chanphen, M. Tanticharoen and Y. Thebtaranonth, J. Chem. Soc., Perkin Trans. 1, 2002, 2473 RSC.
- W. Yin, Y. Lin, S. Zhou and L. L. P. Vrijmoed, Zhongshan Daxue Xuebao, Ziran Kexueban, 2002, 41, 56 Search PubMed.
- C. H. Liu, J. C. Meng, W. X. Zou, L. L. Huang, H. Q. Tang and R. X. Tan, Planta Med., 2002, 68, 363 CrossRef CAS.
- C. Osterhage, G. M. König, P. G. Jones and A. D. Wright, Planta Med., 2002, 68, 1052 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, G. G. Harrigan, J. P. Doom, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 1945 CrossRef CAS.
- L. T. Tan, B. L. Márquez and W. H. Gerwick, J. Nat. Prod., 2002, 65, 925 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, Bioorg. Med. Chem., 2002, 10, 1973 Search PubMed.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, Tetrahedron, 2002, 58, 7959 CrossRef CAS.
- H. Sone, T. Kondo, M. Kiryu, H. Ishiwata, M. Ojika and K. Yamada, J. Org. Chem., 1995, 60, 4774 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore, V. J. Paul and S. L. Mooberry, J. Nat. Prod., 2000, 63, 611 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2000, 63, 1437 CrossRef CAS.
- K. Milligan, B. L. Marquez, R. T. Williamson and W. H. Gerwick, J. Nat. Prod., 2000, 63, 1440 CrossRef CAS.
- F. Yokokawa, H. Sameshima, D. Katagiri, T. Aoyama and T. Shioiri, Tetrahedron, 2002, 58, 9445 CrossRef CAS.
- H. Luesch, P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 996 CrossRef CAS.
- L. M. Nogle and W. H. Gerwick, J. Nat. Prod., 2002, 65, 21 CrossRef CAS.
- J. B. MacMillan and T. F. Molinski, Org. Lett., 2002, 4, 1535 CrossRef CAS.
- B. L. Marquez, K. S. Watts, A. Yokochi, M. A. Roberts, P. Verdier-Pinard, J. I. Jimenez, E. Hamel, P. J. Scheuer and W. H. Gerwick, J. Nat. Prod., 2002, 65, 866 CrossRef CAS.
- J. R. P. Cetusic, F. R. Green, P. R. Graupner and M. P. Oliver, Org. Lett., 2002, 4, 1307 CrossRef CAS.
- L. M. Nogle and W. H. Gerwick, Org. Lett., 2002, 4, 1095 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 29 CrossRef CAS.
- J. B. MacMillan and T. F. Molinski, Org. Lett., 2002, 4, 1883 CrossRef CAS.
- H.-F. Wong, G. A. Williams and G. D. Brown, Phytochemistry, 2002, 60, 425 CrossRef CAS.
- G. R. Pettit, Y. Kamano, C. L. Herald, A. A. Tuinman, F. E. Boettner, H. Kizu, J. M. Schmidt, L. Baczynskyj, K. B. Tomer and R. J. Bontems, J. Am. Chem. Soc., 1987, 109, 6883 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore, V. J. Paul, S. L. Mooberry and T. H. Corbett, J. Nat. Prod., 2002, 65, 16 CrossRef CAS.
- H. Sone, T. Shibata, T. Fujita, M. Ojika and K. Yamada, J. Am. Chem. Soc., 1996, 118, 1874 CrossRef CAS.
- F. D. Horgen, E. B. Kazmierski, H. E. Westenburg, W. Y. Yoshida and P. J. Scheuer, J. Nat. Prod., 2002, 65, 487 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 1336 CrossRef CAS.
- R. T. Williamson, A. Boulanger, A. Vulpanovici, M. A. Roberts and W. H. Gerwick, J. Org. Chem., 2002, 67, 7927 CrossRef CAS.
- M. Satake, M. Shoji, Y. Oshima, H. Naoki, T. Fujita and T. Yasumoto, Tetrahedron Lett., 2002, 43, 5829 CrossRef CAS.
- M. Satake, M. Murata and T. Yasumoto, J. Am. Chem Soc., 1993, 115, 361 CrossRef CAS.
- H. Fuwa, N. Kainuma, K. Tachibana and M. Sasaki, J. Am. Chem. Soc., 2002, 124, 14983 CrossRef CAS.
- H. Fuwa, M. Sasaki, M. Satake and K. Tachibana, Org. Lett., 2002, 4, 2981 CrossRef CAS.
- J. Kobayashi, M. Ishibashi, T. Murayama, M. Takamatsu, M. Iwamura, Y. Ohizumi and T. Sasaki, J. Org. Chem., 1990, 55, 3421 CrossRef CAS.
- T. Kubota, M. Tsuda and J. Kobayashi, J. Org. Chem., 2002, 67, 1651 CrossRef CAS.
- J. Kobayashi, K. Shimbo, M. Sato and M. Tsuda, J. Org. Chem., 2002, 67, 6585 CrossRef CAS.
- J. Kobayashi, H. Shigemori, M. Ishibashi, T. Yamasu, H. Hirota and T. Sasaki, J. Org. Chem., 1991, 56, 5221 CrossRef CAS.
- M. Ishibashi, Y. Ohizumi, M. Hamashima, H. Nakamura, Y. Hirata, T. Sasaki and J. Kobayashi, J. Chem. Soc., Chem. Commun., 1987, 1127 RSC.
- J. Kobayashi, M. Ishibashi, H. Nakamura, Y. Ohizumi, T. Yamasu, Y. Hirata, T. Sasaki, T. Ohta and S. Nozoe, J. Nat. Prod., 1989, 52, 1036 CAS.
- K. Shimbo, M. Tsuda, N. Izui and J. Kobayashi, J. Org. Chem., 2002, 67, 1020 CrossRef CAS.
- J. Kobayashi and M. Tsuda, Nat. Prod. Rep., 2004, 21, 77 RSC.
- J. Kobayashi, M. Ishibashi, H. Nakamura, Y. Ohizumi, T. Yamasu, T. Sasaki and Y. Hirata, Tetrahedron Lett., 1986, 27, 5755 CrossRef CAS.
- R. E. Maleczka, L. R. Terrell, F. Geng and J. S. Ward III, Org. Lett., 2002, 4, 2841 CrossRef CAS.
- B. Trost, J. D. Chisholm, S. J. Wrobleski and M. Jung, J. Am. Chem. Soc., 2002, 124, 12420 CrossRef CAS.
- H. W. Lam and G. Pattenden, Angew. Chem., Int. Ed., 2002, 41, 508 CrossRef CAS.
- J. Kobayashi, T. Kubota, T. Endo and M. Tsuda, J. Org. Chem., 2001, 66, 134 CrossRef CAS.
- A. Fürstner, C. Aïssa, R. Riveiros and J. Ragot, Angew. Chem., Int. Ed., 2002, 41, 4763 CrossRef CAS.
- Y. Hiraga, K. Kaku, D. Omoda, K. Sugihara, H. Hosoya and M. Hino, J. Nat. Prod., 2002, 65, 1494 CrossRef CAS.
- S. G. Toske, P. R. Jensen, C. A. Kauffman and W. Fenical, Tetrahedron, 1998, 54, 13459 CrossRef CAS.
- L. Rivas, L. Quintero, J.-L. Fourrey and R. Benhida, Tetrahedron Lett., 2002, 43, 7639 CrossRef CAS.
- F. Yokokawa and T. Shioiri, Tetrahedron Lett., 2002, 43, 8673 CrossRef CAS.
- P. J. Proteau, W. H. Gerwick, F. Garcia-Pichel and R. Castenholtz, Experientia, 1993, 49, 825 Search PubMed.
- C. S. Stevenson, E. A. Capper, A. K. Roshak, B. Marquez, K. Grace, W. H. Gerwick, R. S. Jacobs and L. A. Marshall, Inflammation Res., 2002, 51, 112 Search PubMed.
- C. S. Stevenson, E. A. Capper, A. K. Roshak, B. Marquez, C. Eichman, J. R. Jackson, M. Mattern, W. H. Gerwick, R. S. Jacobs and L. A. Marshall, J. Pharmacol. Exp. Ther., 2002, 303, 858 Search PubMed.
- M. Murakami, K. Makabe, S. Yamaguchi, S. Konosu and R. Walchi, Tetrahedron Lett., 1988, 29, 1149 CrossRef CAS.
- M. Abe, D. Inoue, K. Matsunaga, Y. Ohizumi, H. Ueda, T. Asano, M. Murakami and Y. Sato, J. Cell Physiol., 2002, 190, 109 CrossRef CAS.
- E. Dorta, J. Darias, A. San Martín and M. Cueto, J. Nat. Prod., 2002, 65, 329 CrossRef CAS.
- H.-E. Högberg, R. H. Thomson and T. J. King, J. Chem. Soc., Perkin Trans. 1, 1976, 1696 RSC.
- D. M. Estrada, J. D. Martín and C. Pérez, J. Nat. Prod., 1987, 50, 735 CrossRef CAS.
- M. S. Ali, M. Saleem, R. Yamdagni and M. A. Ali, Nat. Prod. Lett., 2002, 16, 407 Search PubMed.
- Y. Yoshii, S. Takaichi, T. Maoka, S. Hanada and I. Inouye, J. Phycol., 2002, 38, 297 CrossRef CAS.
- A. K. Siddhanta, A. M. Goswami, B. K. Ramavat and B. Achari, J. Indian Chem. Soc., 2002, 79, 843 Search PubMed.
- Y. Takahashi, K. Itoh, M. Ishii, M. Suzuki and Y. Itabashi, Mar. Biol.(Berlin), 2002, 140, 763 Search PubMed.
- Z. Kamenarska, F. N. Yalçin, T. Ersöz, I. Çalis, K. Stefanov and S. Popov, Z. Naturforsch., C: Biosci., 2002, 57, 584 Search PubMed.
- E. Dorta, M. Cueto, A. R. Díaz-Marrero and J. Darias, Tetrahedron Lett., 2002, 43, 9043 CrossRef CAS.
- M. Suzuki, H. Yamada and K. Kurata, J. Nat. Prod., 2002, 65, 121 CrossRef CAS.
- H.-F. Tang, Y.-H. Yi, X.-S. Yao, Q.-Z. Xu, S.-Y. Zhang and H.-W. Lin, J. Asian Nat. Prod. Res., 2002, 4, 95 Search PubMed.
- S.-H. Xu, L.-S. Ding, M.-K. Wang, S.-L. Peng and X. Liao, Youji Huaxue, 2002, 22, 138 Search PubMed.
- F. Czapek, Lotos, 1912, 59, 250 Search PubMed.
- S.-E. N. Ayyad and M. Deyab, Alexandria J. Pharm. Sci., 2002, 16, 27 Search PubMed.
- J. Kimura and N. Maki, J. Nat. Prod., 2002, 65, 57 CrossRef CAS.
- E. Dorta, M. Cueto, I. Brito and J. Darias, J. Nat. Prod., 2002, 65, 1727 CrossRef CAS.
- S. Hayat, Atta-ur-Rahman, M. I. Choudhary, K. M. Khan and A. Abbaskhan, Chem. Pharm. Bull., 2002, 50, 1297 CrossRef CAS.
- Y.-C. Chen, P. I. Tsai, W. Fenical and M. E. Hay, Phytochemistry, 1992, 32, 71 CrossRef.
- S. Ohira, A Kuboki, T. Hasegawa, T. Kikuchi, T. Kutsukake and M. Nomura, Tetrahedron Lett., 2002, 43, 4641 CrossRef CAS.
- M.-N. Dave, T. Kusumi, M. Ishitsuka, T. Iwashita and H. Kakisawa, Heterocycles, 1984, 22, 2301.
- K. Aoki, M. Takahashi, M. Hashimoto, T. Okuno, K. Kurata and M. Suzuki, Biosci. Biotechnol. Biochem., 2002, 66, 1915 CrossRef CAS.
- M. Ochi, H. Kotsuki, K. Muraoka and T. Tokoroyama, Bull. Chem. Soc. Jpn., 1979, 52, 629 CAS.
- T. Laube, J. Schröder, R. Stehle and K. Seifert, Tetrahedron, 2002, 58, 4299 CrossRef CAS.
- A. R. Díaz-Marrero, M. Cueto, E. Dorta, J. Rovirosa, A. San-Martín and J. Darias, Org. Lett., 2002, 4, 2949 CrossRef CAS.
- A. R. Díaz-Marrero, J. Rovirosa, J. Darias, A. San-Martín and M. Cueto, J. Nat. Prod., 2002, 65, 585 CrossRef CAS.
- A. R. Díaz-Marrero, M. Cueto, E. Dorta, J. Rovirosa, A. San-Martín and J. Darias, Tetrahedron, 2002, 58, 8539 CrossRef CAS.
- J. Darias, J. Rovirosa, A. San-Martín, A. R. Diaz, E. Dorta and M. Cueto, J. Nat. Prod., 2001, 64, 1383 CrossRef CAS.
- V. H. Argandoña, J. Rovirosa, A. San-Martín, A. Riquelme, A. R. Díaz-Marrero, M. Cueto, J. Darias, O. Santana, A. Guadaño and A. González-Coloma, J. Agric. Food Chem., 2002, 50, 7029 CrossRef CAS.
- D. B. Stierle and J. J. Sims, Tetrahedron, 1979, 35, 1261 CrossRef CAS.
- A. San-Martín and J. Rovirosa, Biochem. Syst. Ecol., 1986, 14, 459 CrossRef CAS.
- R. J. Capon, L. M. Engelhardt, E. L. Ghisalberti, P. R. Jefferies, V. A. Patrick and A. H. White, Aust. J. Chem., 1984, 37, 537 CAS.
- J. S. Mynderse and D. J. Faulkner, J. Am. Chem. Soc., 1974, 96, 6771 CrossRef CAS.
- M. D. Higgs, D. J. Vanderah and D. J. Faulkner, Tetrahedron, 1977, 33, 2775 CrossRef CAS.
- M. Cueto, J. Darias, J. Rovirosa and A. San Martin, J. Nat. Prod., 1998, 61, 1466 CrossRef CAS.
- M. Suzuki, Y. Takahashi, Y. Mitome, T. Itoh, T. Abe and M. Masuda, Phytochemistry, 2002, 60, 861 CrossRef CAS.
- Y. Takahashi, M. Daitoh, M. Suzuki, T. Abe and M. Masuda, J. Nat. Prod., 2002, 65, 395 CrossRef CAS.
- D. Iliopoulou, C. Vagias, D. Galanakis, D. Argyropoulos and V. Roussis, Org. Lett., 2002, 4, 3263 CrossRef.
- I. Brito, M. Cueto, E. Dorta and J. Darias, Tetrahedron Lett., 2002, 43, 2551 CrossRef CAS.
- B. M. Howard and W. Fenical, Tetrahedron Lett., 1975, 1687 CrossRef CAS.
- J. Jongaramruong, A. J. Blackman, B. W. Skelton and A. H. White, Aust. J. Chem., 2002, 55, 275 CrossRef CAS.
- G. Guella, D. Skropeta, I. Mancini and F. Pietra, Z. Naturforsch., B: Chem. Sci., 2002, 57, 1147 CAS.
- D. Iliopoulou, C. Vagias, C. Harvala and V. Roussis, Phytochemistry, 2002, 59, 111 CrossRef CAS.
- D. Iliopoulou, V. Roussis, C. Pannecouque, E. De Clercq and C. Vagias, Tetrahedron, 2002, 58, 6749 CrossRef CAS.
- I. Brito, M. Cueto, A. R. Díaz-Marrero, J. Darias and A. San Martín, J. Nat. Prod., 2002, 65, 946 CrossRef CAS.
- M. L. Souto, C. P. Manríquez, M. Norte and J. J. Fernández, Tetrahedron, 2002, 58, 8119 CrossRef CAS.
- H.-D. Yoo, S. O. Ketchum, D. France, K. Bair and W. H. Gerwick, J. Nat. Prod., 2002, 65, 51 CrossRef CAS.
- V. Bultel-Poncé, S. Etahiri and M. Guyot, Bioorg. Med. Chem. Lett., 2002, 12, 1715 CrossRef CAS.
- J. P. Bergé, E. Debiton, J. Dumay, P. Durand and C. Barthomeuf, J. Agric. Food Chem., 2002, 50, 6227 CrossRef CAS.
- G. T. Carter, K. L. Rinehart Jr., H. L. Li, S. L. Kuentzel and J. L. Connor, Tetrahedron Lett., 1978, 46, 4479 CrossRef.
- Y. Liu and G. W. Gribble, J. Nat. Prod., 2002, 65, 748 CrossRef CAS.
- C. P. Falshaw, T. J. King, S. Imre, S. Islimyeli and R. H. Thomson, Tetrahedron Lett., 1980, 21, 4951 CrossRef CAS.
- R. K. Boeckman, J. Zhang and M. R. Reeder, Org. Lett., 2002, 4, 3891 CrossRef.
- D. Xiao, S. Deng and L. Zeng, Zhongshan Daxue Xuebao, Ziran Kexueban, 2002, 41, 111 Search PubMed.
- I. Kuroda, M. Musman, I. I. Ohtani, T. Ichiba, J. Tanaka, D. Garcia Gravalos and T. Higa, J. Nat. Prod., 2002, 65, 1505 CrossRef CAS.
- V. Costantino, E. Fattorusso, A. Mangoni, M. Di Rosa and A. Ianaro, J. Am. Chem. Soc., 1997, 119, 12465 CrossRef CAS.
- K. Mori, T. Tashiro, K. Akasaka, H. Ohrui and E. Fattorusso, Tetrahedron Lett., 2002, 43, 3719 CrossRef CAS.
- T. Tashiro, K. Akasaka, H. Ohrui, E. Fattorusso and K. Mori, Eur. J. Org. Chem., 2002, 3659 CrossRef CAS.
- M. Seki and K. Mori, Eur. J. Org. Chem., 2001, 3797 CrossRef CAS.
- K. C. Nicolaou, J. Li and G. Zenke, Helv. Chim. Acta, 2000, 83, 1977 CrossRef CAS.
- M. Meyer and M. Guyot, Lipids, 2002, 37, 1109 Search PubMed.
- Y. Liu, C. O. Lee, J. Hong and J. H. Jung, Bull. Korean Chem. Soc., 2002, 23, 1467 CAS.
- V. Costantino, E. Fattorusso, C. Imperatore and A. Mangoni, J. Nat. Prod., 2002, 65, 883 CrossRef CAS.
- V. Costantino, E. Fattorusso and A. Mangoni, J. Org. Chem., 1993, 58, 186 CrossRef CAS.
- R. G. Linington, M. Robertson, A. Gauthier, B. B. Finlay, R. van Soest and R. J. Andersen, Org. Lett., 2002, 4, 4089 CrossRef CAS.
- S. Aoki, K. Matsui, H. Wei, N. Murakami and M. Kobayashi, Tetrahedron, 2002, 58, 5417 CrossRef CAS.
- K. Watanabe, Y. Tsuda, Y. Yamane, H. Takahashi, K. Iguchi, H. Naoki, T. Fujita and R. W. M. van Soest, Tetrahedron Lett.,, 2000, 41, 9271 CrossRef CAS.
- J. S. Yadav and R. K. Mishra, Tetrahedron Lett., 2002, 43, 1739 CrossRef CAS.
- Y. Nakao, T. Uehara, S. Matsunaga, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 2002, 65, 922 CrossRef CAS.
- H. Li, S. Matsunaga and N. Fusetani, J. Nat. Prod., 1994, 57, 1464 CrossRef CAS.
- S. Nishimura, S. Matsunaga, M. Shibazaki, K. Suzuki, N. Harada, H. Naoki and N. Fusetani, J. Nat. Prod., 2002, 65, 1353 CrossRef CAS.
- T. N. Makarieva, E. A. Santalova, I. A. Gorshkova, A. S. Dmitrenok, A. G. Guzii, V. I. Gorbach, V. I. Svestashev and V. A. Stonik, Lipids, 2002, 37, 75 Search PubMed.
- J. S. Mynderse and R. E. Moore, Phytochemistry, 1979, 18, 1181 CrossRef CAS.
- M. R. Rao and D. J. Faulkner, J. Nat. Prod., 2002, 65, 1201 CrossRef CAS.
- J. S. Sandler, P. L. Colin, J. N. A. Hooper and D. J. Faulkner, J. Nat. Prod., 2002, 65, 1258 CrossRef CAS.
- A. Qureshi, J. Salvá, M. K. Harper and D. J. Faulkner, J. Nat. Prod., 1998, 61, 1539 CrossRef CAS.
- M. Jung, J. Ham and J. Song, Org. Lett., 2002, 4, 2763 CrossRef CAS.
- Y. Chen, P. J. McCarthy, D. K. Harmody, R. Schimoler-O'Rourke, K. Chilson, C. Selitrennikoff, S. A. Pomponi and A. E. Wright, J. Nat. Prod., 2002, 65, 1509 Search PubMed.
- S. P. Gunasekera, M. Gunasekera, G. P. Gunawardana, P. McCarthy and N. Burres, J. Nat. Prod., 1990, 53, 669 CAS.
- G. Yao and K. Steliou, Org. Lett., 2002, 4, 485 CrossRef CAS.
- C. Campagnuolo, E. Fattorusso, O. Taglialatela-Scafati, A. Ianaro and B. Pisano, Eur. J. Org. Chem., 2002, 61 CrossRef CAS.
- A. D. Patil, A. J. Freyer, M. F. Bean, B. K. Carte, J. W. Westley, R. K. Johnson and P. Lahouratate, Tetrahedron, 1996, 52, 377 CrossRef CAS.
- P. Y. Hayes and W. Kitching, J. Am. Chem. Soc., 2002, 124, 9718 CrossRef CAS.
- J.-F. Hu, H.-F. Gao, M. Kelly and M. T. Hamann, Tetrahedron, 2002, 58, 1233 CrossRef CAS.
- E. Fattorusso, O. Taglialatela-Scafati, M. Di Rosa and A. Ianaro, Tetrahedron, 2000, 56, 7959 CrossRef CAS.
- S. Sirirath, J. Tanaka, I. I. Ohtani, T. Ichiba, R. Rachmat, K. Ueda, T. Usui, H. Osada and T. Higa, J. Nat. Prod., 2002, 65, 1820 CrossRef CAS.
- A. Kijjoa, R. Watanadilok, P. Sonchaeng, P. Sawangwong, M. Pedro, M. S. J. Nascimento, A. M. S. Silva, G. Eaton and W. Herz, Z. Naturforsch., C: Biosci., 2002, 57, 732 Search PubMed.
- T. Teruya, S. Nakagawa, T. Koyama, K. Suenaga and D. Uemura, Chem. Lett., 2002, 38 CrossRef CAS.
- T. S. Bugni, G. P. Concepción, G. C. Mangalindan, M. K. Harper, R. D. James and C. M. Ireland, Phytochemistry, 2002, 60, 361 CrossRef CAS.
- S. P. Gunasekera, G. K. Paul, R. E. Longley, R. A. Isbrucker and S. A. Pomponi, J. Nat. Prod., 2002, 65, 1643 CrossRef CAS.
- R. A. Edrada, R. Ebel, A. Supriyono, V. Wray, P. Schupp, K. Steube, R. van Soest and P. Proksch, J. Nat. Prod., 2002, 65, 1168 CrossRef CAS.
- S. Kehraus, G. M. König and A. D. Wright, J. Nat. Prod., 2002, 65, 1056 CrossRef CAS.
- T. Wakimoto, S. Matsunaga, A. Takai and N. Fusetani, Chem. Biol., 2002, 9, 309 CrossRef CAS.
- A. Groweiss, J. J. Newcomer, B. R. O'Keefe, A. Blackman and M. R. Boyd, J. Nat. Prod., 1999, 62, 1691 CrossRef CAS.
- R. K. Boeckman Jr., T. J. Clark and B. C. Shook, Org. Lett., 2002, 4, 2109 CrossRef.
- R. K. Boeckman Jr., T. J. Clark and B. C. Shook, Helv. Chim. Acta, 2002, 85, 4532 CrossRef.
- G. K. Paul, S. P. Gunasekera, R. E. Longley and S. A. Pomponi, J. Nat. Prod., 2002, 65, 59 CrossRef CAS.
- A. R. Carroll, G. K. Pierens, G. Fechner, P. de Almeida Leone, A. Ngo, M. Simpson, E. Hyde, J. N. A. Hooper, S.-L. Boström, D. Musil and R. J. Quinn, J. Am. Chem. Soc., 2002, 124, 13340 CrossRef CAS.
- S. Hanessian, R. Margarita, A. Hall, S. Johnstone, M. Tremblay and L. Parlanti, J. Am. Chem. Soc., 2002, 124, 13342 CrossRef CAS.
- L. Ciasullo, A. Casapullo, A. Cutignano, G. Bifulco, C. Debitus, J. Hooper, L. Gomez-Paloma and R. Riccio, J. Nat. Prod., 2002, 65, 407 CrossRef CAS.
- H. Itokawa, K. Watanabe, S. Kawaoto and T. Inoue, Jpn. Kokai Tokkyo Koho, Pat. no. JP 63203671, 1988 Search PubMed.
- S. Kehraus, G. M. König, A. D. Wright and G. Woerheide, J. Org. Chem., 2002, 67, 4989 CrossRef CAS.
- Y. Nakao, N. Oku, S. Matsunaga and N. Fusetani, J. Nat. Prod., 1998, 61, 667 CrossRef.
- H. H. Wasserman and R. Zhang, Tetrahedron Lett., 2002, 43, 3743 CrossRef CAS.
- H. H. Wasserman and R. Zhang, Tetrahedron, 2002, 58, 6277 CrossRef CAS.
- Y. Murakami, M. Takei, K. Shindo, C. Kitazume, J. Tanaka, T. Higa and H. Fukamachi, J. Nat. Prod., 2002, 65, 259 CrossRef CAS.
- S. Deng and J. Taunton, J. Am. Chem. Soc., 2002, 124, 916 CrossRef CAS.
- F. Yokokawa, H. Sameshima, Y. In, K. Minoura, T. Ishida and T. Shioiri, Tetrahedron, 2002, 58, 8127 CrossRef CAS.
- L. T. Tan, R. T. Williamson, W. H. Gerwick, K. S. Watts, K. McGough and R. Jacobs, J. Org. Chem., 2000, 65, 419 CrossRef CAS.
- J. N. Tabudravu, M. Jaspars, L. A. Morris, J. J. Kettenes-van den Bosch and N. Smith, J. Org. Chem., 2002, 67, 8593 CrossRef CAS.
- G. R. Pettit, R. Tan, M. D. Williams, L. Tackett, J. M. Schmidt, R. L. Cerny and J. N. A. Hooper, Bioorg. Med. Chem. Lett., 1993, 3, 2869 CrossRef CAS.
- J. N. Tabudravu, L. A. Morris, J. J. Kettenes-van den Bosch and M. Jaspars, Tetrahedron, 2002, 58, 7863 CrossRef.
- A. Randazzo, G. Bifulco, C. Giannini, M. Bucci, C. Debitus, G. Cirino and L. Gomez-Paloma, J. Am. Chem. Soc., 2001, 123, 10870 CrossRef CAS.
- C. Della Monica, A. Randazzo, G. Bifulco, P. Cimino, M. Aquino, I. Izzo, F. De Riccardis and L. Gomez-Paloma, Tetrahedron Lett., 2002, 43, 5707 CrossRef CAS.
- Y. Sera, K. Adachi, K. Fujii and Y. Shizuri, Mar. Biotechnol., 2002, 3, 441 CrossRef.
- R. J. Capon, J. Ford, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, J. Nat. Prod., 2002, 65, 358 CrossRef CAS.
- A. Zampella, A. Randazzo, N. Borbone, S. Luciani, L. Trevisi, C. Debitus and M. V. D'Auria, Tetrahedron Lett., 2002, 43, 6163 CrossRef CAS.
- A. Zampella, M. V. D'Auria, L. G. Paloma, A. Casapullo, L. Minale, C. Debitus and Y. Henin, J. Am. Chem. Soc., 1996, 118, 6202 CrossRef CAS.
- Y. Okada, S. Matsunaga, R. W. M. van Soest and N. Fusetani, Org. Lett., 2002, 4, 3039 CrossRef CAS.
- A. Zampella, M. V. D'Auria, L. Minale, C. Debitus and C. Roussakis, J. Am. Chem. Soc., 1996, 118, 11085 CrossRef CAS.
- B. M. Trost, O. Dirat and J. L. Gunzner, Angew. Chem., Int. Ed., 2002, 41, 841 CrossRef CAS.
- D. A. Evans, E. Hu, J. D. Burch and G. Jaeschke, J. Am. Chem. Soc., 2002, 124, 5654 CrossRef CAS.
- B. M. Trost, J. L. Gunzner, O. Dirat and Y. H. Rhee, J. Am. Chem. Soc., 2002, 124, 10396 CrossRef CAS.
- P. A. Horton, F. E. Koehn, R. E. Longley and O. J. McConnell, J. Am. Chem. Soc., 1994, 116, 6015 CrossRef CAS.
- E. Lee, H. Y. Song, J. W. Kang, D.-S. Kim, C.-K. Jung and J. M. Joo, J. Am. Chem. Soc., 2002, 124, 384 CrossRef CAS.
- E. Lee, H. Y. Song, J. M. Joo, J. W. Kang, D.-S. Kim, C.-K. Jung, C. Y. Hong, S. W. Yeong and K. Jeon, Bioorg. Med. Chem., 2002, 12, 3519 Search PubMed.
- A. Cutignano, I. Bruno, G. Bifulco, A. Casapullo, C. Debitus, L. Gomez-Paloma and R. Riccio, Eur. J. Org. Chem., 2001, 775 CrossRef CAS.
- A. B. Smith III and I. G. Safonov, Org. Lett., 2002, 4, 635 CrossRef.
- A. B. Smith III, I. G. Safonov and R. M. Corbett, J. Am. Chem. Soc., 2002, 124, 11102 CrossRef.
- J. Tanaka and T. Higa, Tetrahedron Lett., 1996, 37, 5535 CrossRef CAS.
- M. R. Rao and D. J. Faulkner, J. Nat. Prod., 2002, 65, 386 CrossRef CAS.
- K. L. Erickson, K. R. Gustafson, L. K. Pannell, J. A. Beutler and M. R. Boyd, J. Nat. Prod., 2002, 65, 1303 CrossRef CAS.
- P. Phuwapraisirisan, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2002, 65, 942 CrossRef CAS.
- M. A. Rashid, C. L. Cantrell, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2001, 64, 1341 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson, R. C. Crouch, A. Groweiss, L. K. Pannell, Q. N. Van and M. R. Boyd, Org. Lett., 2002, 4, 3293 CrossRef CAS.
- N. K. Utkina, V. A. Denisenko, M. V. Virovaya, O. V. Scholokova and N. G. Prokof'eva, J. Nat. Prod., 2002, 65, 1213 CrossRef CAS.
- M. Kuramoto, T. Fujita and N. Ono, Chem. Lett., 2002, 464 CrossRef CAS.
- D. E. Williams, P. Lassota and R. J. Andersen, J. Org. Chem., 1998, 63, 4838 CrossRef CAS.
- D. E. Williams, K. S. Craig, B. Patrick, L. M. McHardy, R. van Soest, M. Roberge and R. J. Andersen, J. Org. Chem., 2002, 67, 245 CrossRef CAS.
- D. B. Stierle and D. J. Faulkner, J. Nat. Prod., 1991, 54, 1134 CAS.
- R. C. Larock and Y. Wang, Tetrahedron Lett., 2002, 43, 21 CrossRef CAS.
- S. Tsukamoto, M. Takahashi, S. Matsunaga, N. Fusetani and R. W. M. van Soest, J. Nat. Prod., 2000, 63, 682 Search PubMed.
- W. R. F. Goundry, V. Lee and J. E. Baldwin, Tetrahedron Lett., 2002, 43, 2745 CrossRef CAS.
- M. Tsuda, K. Hirano, T. Kubota and J. Kobayashi, Tetrahedron Lett., 1999, 40, 4819 CrossRef CAS.
- J. E. Baldwin, S. P. Romeril, V. Lee and T. D. W. Claridge, Org. Lett.,, 2001, 3, 1145 CrossRef CAS.
- B. B. Snider and B. Shi, Tetrahedron Lett., 2001, 42, 1639 CrossRef CAS.
- S. P. Romeril, V. Lee, T. D. W. Claridge and J. E. Baldwin, Tetrahedron Lett., 2002, 43, 327 CrossRef CAS.
- L. Chill, T. Yosief and Y. Kashman, J. Nat. Prod., 2002, 65, 1738 CrossRef CAS.
- K. Y. Orabi, K. A. El Sayed, M. T. Hamann, D. C. Dunbar, M. S. Al-Said, T. Higa and M. Kelly, J. Nat. Prod., 2002, 65, 1782 CrossRef CAS.
- M. Nakagawa, M. Endo, N. Tanaka and L. Gen-Pei, Tetrahedron Lett., 1984, 25, 3227 CrossRef CAS.
- S.-S. Moon, J. B. MacMillan, M. M. Olmstead, T. A. Ta, I. N. Pessah and T. F. Molinski, J. Nat. Prod., 2002, 65, 249 CrossRef CAS.
- M. Yousaf, K. A. El Sayed, K. V. Rao, C. W. Lim. J.-F. Hu, M. Kelly, S. G. Franzblau, F. Zhang, O. Peraud, R. T. Hill and M. T. Hamann, Tetrahedron, 2002, 58, 7397 CrossRef CAS.
- J. M. Humphrey, Y. Liao, A. Ali, T. Rein, Y.-L. Wong, H.-J. Chen, A. K. Courtney and S. F. Martin, J. Am. Chem. Soc., 2002, 124, 8584 CrossRef CAS.
- R. J. Capon, C. Skene, D. Vuong, E. Lacey, J. H. Gill, K. Heiland and T. Friedel, J. Nat. Prod., 2002, 65, 368 CrossRef CAS.
- A. D. Patil, A. J. Freyer, B. Carte, P. B. Taylor, R. K. Johnson and D. J. Faulkner, J. Nat. Prod., 2002, 65, 628 CrossRef CAS.
- J.-F. Hu, J. A. Schetz, M. Kelly, J.-N. Peng, K. K. H. Ang, H. Flotow, C. Y. Leong, S. B. Ng, A. D. Buss, S. P. Wilkins and M. T. Hamann, J. Nat. Prod., 2002, 65, 476 CrossRef CAS.
- S. Sölter, R. Dieckmann, M. Blumenberg and W. Francke, Tetrahedron Lett., 2002, 43, 3385 CrossRef CAS.
- G. Lidgren, L. Bohlin and J. Bergmann, Tetrahedron Lett., 1986, 27, 3283 CrossRef CAS.
- A. Lieberknecht and H. Griesser, Tetrahedron Lett., 1987, 28, 4275 CrossRef CAS.
- M. J. McKay, A. R. Carroll, R. J. Quinn and J. N. A. Hooper, J. Nat. Prod., 2002, 65, 595 CrossRef CAS.
- M. Salmoun, C. Devijver, D. Daloze, J.-C. Braekman and R. W. M. van Soest, J. Nat. Prod., 2002, 65, 1173 CrossRef CAS.
- S. P. Gunasekera, P. J. McCarthy and M. Kelly-Borges, J. Nat. Prod., 1994, 57, 14537.
- B. Jiang, C.-G. Yang and J. Wang, J. Org. Chem., 2002, 67, 1396 CrossRef.
- A. Casapullo, G. Bifulco, I. Bruno and R. Riccio, J. Nat. Prod., 2000, 63, 447 CrossRef CAS.
- F. Y. Miyake, K. Yakushijin and D. A. Horne, Org. Lett., 2002, 4, 941 CrossRef CAS.
- T. Kawasaki, K. Ohno, H. Enoki, Y. Umemoto and M. Sakamoto, Tetrahedron Lett., 2002, 43, 4245 CrossRef CAS.
- A. E. Wright, S. A. Pomponi, S. S. Cross and P. McCarthy, J. Org. Chem., 1992, 57, 4772 CrossRef CAS.
- R. J. Capon, F. Rooney, L. M. Murray, E. Collins, A. T. R. Sim, J. A. P. Rostas, M. S. Butler and A. R. Carroll, J. Nat. Prod., 1998, 61, 660 CrossRef CAS.
- N. K. Garg, R. Sarpong and B. M. Stoltz, J. Am. Chem. Soc., 2002, 124, 13179 CrossRef CAS.
- K. M. Meragelman, L. M. West, P. T. Northcote, L. K. Pannell, T. C. McKee and M. R. Boyd, J. Org. Chem., 2002, 67, 6671 CrossRef CAS.
- L. C. Chang, S. Otero-Quintero, J. N. A. Hooper and C. A. Bewley, J. Nat. Prod., 2002, 65, 776 CrossRef CAS.
- N. B. Perry, J. W. Blunt and M. H. G. Munro, Tetrahedron, 1988, 44, 1727 CrossRef CAS.
- H. Tohma, Y. Harayama, M. Hashizume, M. Iwata, M. Egi and Y. Kita, Angew. Chem., Int. Ed., 2002, 41, 348 CrossRef CAS.
- R. D. Charan, T. C. McKee, K. R. Gustafson, L. K. Pannell and M. R. Boyd, Tetrahedron Lett., 2002, 43, 5201 CrossRef CAS.
- Z. Thale, T. Johnson, K. Tenney, P. J. Wenzel, E. Lobkovsky, J. Clardy, J. Media, H. Pietraszkiewicz, F. A. Valeriote and P. Crews, J. Org. Chem., 2002, 67, 9384 CrossRef CAS.
- M. Tsuda, H. Uemoto and J. Kobayashi, Tetrahedron Lett., 1999, 40, 5709 CrossRef CAS.
- M. K. Gurjar and S. Bera, Org. Lett., 2002, 4, 3569 CrossRef CAS.
- B. Jiang, J.-F. Liu and S.-Y. Zhao, Org. Lett., 2002, 4, 3951 CrossRef CAS.
- G. R. Pettit, J. McNulty, D. L. Herald, D. L. Doubek, J. C. Chapuis, J. M. Schmidt, L. P. Tackett and M. R. Boyd, J. Nat. Prod., 1997, 60, 180 CrossRef CAS.
- S. A. Fedoreyev, N. K. Utkina, S. G. Ilyin, M. V. Reshetnyak and O. B. Maximov, Tetrahedron Lett., 1986, 27, 3177 CrossRef.
- K. J. Wiese, K. Yakushijin and D. A. Horne, Tetrahedron Lett., 2002, 43, 5135 CrossRef CAS.
- M. Assmann and M. Köck, Z. Naturforsch., C: Biosci., 2002, 57, 153 Search PubMed.
- G. Groszek, D. Kantoci and G. R. Pettit, Liebigs Ann. Chem., 1995, 715 Search PubMed.
- A. D. Patil, A. J. Freyer, L. Killmer, G. Hofmann and R. K. Johnson, Nat. Prod. Lett., 1997, 9, 201 Search PubMed.
- K. Inaba, H. Sato, M. Tsuda and J. Kobayashi, J. Nat. Prod., 1998, 61, 693 CrossRef.
- A. C. B. Sosa, K. Yakushijin and D. A. Horne, J. Org. Chem., 2002, 67, 4498 CrossRef CAS.
- M. D'Ambrosio, A. Guerriero, C. Debitus, O. Ribes, J. Pusset, S. Leroy and F. Pietra, J. Chem. Soc., Chem. Commun., 1993, 1305 RSC.
- K. S. Feldman and J. C. Saunders, J. Am. Chem. Soc., 2002, 124, 9060 CrossRef CAS.
- K. S. Feldman, J. C. Saunders and M. L. Wrobleski, J. Org. Chem., 2002, 67, 7096 CrossRef CAS.
- X. Fu, J. R. Barnes, T. Do and F. J. Schmitz, J. Nat. Prod., 1997, 60, 497 CrossRef CAS.
- R. K. Akee, T. R. Carroll, W. Y. Yoshida, P. J. Scheuer, T. J. Stout and J. Clardy, J. Org. Chem., 1990, 55, 1944 CrossRef CAS.
- S. Nakamura, I. Kawasaki, M. Kunimura, M. Matsui, Y. Noma, M. Yamashita and S. Ohta, J. Chem. Soc., Perkin Trans. 1, 2002, 1061 RSC.
- H. Gross, S. Kehraus, G. M. König, G. Woerheide and A. D. Wright, J. Nat. Prod., 2002, 65, 1190 CrossRef CAS.
- M. Assmann and M. Köck, Z. Naturforsch., C: Biosci., 2002, 57, 157 Search PubMed.
- J. C. Braekman, D. Daloze, R. Travares, E. Hajdu and R. W. M. Van Soest, J. Nat. Prod., 2000, 63, 193 CrossRef CAS.
- K. Nagasawa, A. Georgieva, H. Koshino, T. Nakata, T. Kita and Y. Hashimoto, Org. Lett., 2002, 4, 177 CrossRef CAS.
- M. Tsuda, T. Endo, K. Watanabe, J. Fromont and J. Kobayashi, J. Nat. Prod., 2002, 65, 1670 CrossRef CAS.
- J. N. Tabudravu, V. G. H. Eijsink, G. W. Gooday, M. Jaspars, D. Komander, M. Legg, B. Synstad and D. M. F. van Aalten, Bioorg. Med. Chem., 2002, 10, 1123 Search PubMed.
- E. Quiñoá and P. Crews, Tetrahedron Lett., 1987, 28, 3229 CrossRef CAS.
- J. N. Tabudravu and M. Jaspars, J. Nat. Prod., 2002, 65, 1798 CrossRef CAS.
- B. M. Saeki, A. C. Granato, R. G. S. Berlinck, A. Magalhães, A. B. Schefer, A. G. Ferreira, U. S. Pinheiro and E. Hajdu, J. Nat. Prod., 2002, 65, 796 CrossRef CAS.
- J. C. Coll, P. S. Kearns, J. A. Rideout and V. Sankar, J. Nat. Prod., 2002, 65, 753 CrossRef CAS.
- I. A. Zuleta, M. L. Vitelli, R. Baggio, M. T. Garland, A. M. Seldes and J. A. Palermo, Tetrahedron, 2002, 58, 4481 CrossRef CAS.
- S. Hirsch, A. Rudi, Y. Kashman and Y. Loya, J. Nat. Prod., 1991, 54, 92 CrossRef.
- K. A. Alvi, M. C. Diaz, P. Crews, D. L. Slate, R. H. Lee and R. Moretti, J. Org. Chem., 1992, 57, 6604 CrossRef CAS.
- K. Igushi, A. Sahashi, J. Kohno and Y. Yamada, Chem. Pharm. Bull., 1990, 38, 1121.
- T. Ling, E. Poupon, E. J. Rueden, S. H. Kim and E. A. Theodorakis, J. Am. Chem. Soc., 2002, 124, 12261 CrossRef CAS.
- P. Djura, D. B. Stierle, B. Sullivan, D. J. Faulkner, E. Arnold and J. Clardy, J. Org. Chem., 1980, 45, 1435 CrossRef CAS.
- M. Nakamura, A. Suzuki, M. Nakatani, T. Fuchikami, M. Inoue and T. Katoh, Tetrahedron Lett., 2002, 43, 6929 CrossRef CAS.
- H. Mitome, T. Nagasawa, H. Miyaoka, Y. Yamada and R. W. M van Soest, Tetrahedron, 2002, 58, 1693 CrossRef CAS.
- H. R. Bokesch, A. C. Stull, L. K. Pannell, T. C. McKee and M. R. Boyd, Tetrahedron Lett., 2002, 43, 3079 CrossRef CAS.
- M. Iwashima, I. Terada, K. Iguchi and T. Yamori, Chem. Pharm. Bull., 2002, 50, 1286 CrossRef CAS.
- M. M. Uy, S. Ohta, M. Yanai, E. Ohta, T. Hirata and S. Ikegami, Bioorg. Med. Chem. Lett., 2002, 12, 3037 CrossRef CAS.
- J. Peng, S. G. Franzblau, F. Zhang and M. T. Hamann, Tetrahedron Lett., 2002, 43, 9699 CrossRef CAS.
- N. V. Petrichtcheva, C. Duque, A. Dueñas, S. Zea, N. Hara and Y. Fujimoto, J. Nat. Prod., 2002, 65, 851 CrossRef CAS.
- C. J. Barrow, J. W. Blunt and M. H. G. Munro, Aust. J. Chem., 1988, 41, 1755 CAS.
- K. Oesterreich, I. Klein and D. Spitzner, Synlett., 2002, 10, 1712.
- T. Okino, E. Yoshimura, H. Hirota and N. Fusetani, Tetrahedron Lett., 1995, 36, 8637 CrossRef CAS.
- H. Miyaoka, H. Shida, N. Yamada, H. Mitome and Y. Yamada, Tetrahedron Lett., 2002, 43, 2227 CrossRef CAS.
- J.-R. Rho, H.-S. Lee, C. J. Sim and J. Shin, Tetrahedron, 2002, 58, 9585 CrossRef.
- J. Peng, K. Walsh, V. Weedman, J. D. Bergthold, J. Lynch, K. L. Lieu, I. A. Braude, M. Kelly and M. T. Hamann, Tetrahedron, 2002, 58, 7809 CrossRef.
- L. Ciasullo, A. Cutignano, A. Casapullo, R. Puliti, C. A. Mattia, C. Debitus, R. Riccio and L. Gomez-Paloma, J. Nat. Prod., 2002, 65, 1210 CrossRef CAS.
- Y. Liu, J. Hong, C.-O. Lee, K. S. Im, N. D. Kim, J. S. Choi and J. H. Jung, J. Nat. Prod., 2002, 65, 1307 CrossRef CAS.
- Y. Liu, B. H. Bae, N. Alam, J. Hong, C. J. Sim, C. Lee, K. S. Im and J. H. Jung, J. Nat. Prod., 2001, 64, 1301 CrossRef CAS.
- K. S. Craig, D. E. Williams, I. Hollander, E. Frommer, R. Mallon, K. Collins, D. Wojciechowicz, A. Tahir, R. van Soest and R. J. Andersen, Tetrahedron Lett., 2002, 43, 4801 CrossRef CAS.
- M. Tsuda, T. Endo, Y. Mikami, J. Fromont and J. Kobayashi, J. Nat. Prod., 2002, 65, 1507 CrossRef CAS.
- R. D. Charan, T. C. McKee and M. R. Boyd, J. Nat. Prod., 2002, 65, 492 CrossRef CAS.
- S. de Rosa, A. Crispino, A. de Giulio and C. Iodice, J. Nat. Prod., 1995, 58, 1776 CrossRef CAS.
- A. K. Cheung and M. L. Snapper, J. Am. Chem. Soc., 2002, 124, 11584 CrossRef CAS.
- D. T. A. Youssef, R. K. Yamaki, M. Kelly and P. J. Scheuer, J. Nat. Prod., 2002, 65, 2 CrossRef CAS.
- M. C. Roy, J. Tanaka, N. de Voogd and T. Higa, J. Nat. Prod., 2002, 65, 1838 CrossRef CAS.
- C. C. Stessman, R. Ebel, A. J. Corvino and P. Crews, J. Nat. Prod., 2002, 65, 1183 CrossRef CAS.
- J.-F. Hu, M. Kelly and M. T. Hamann, Steroids, 2002, 67, 743 CrossRef CAS.
- G. Santafé, V. Paz, J. Rodríguez and C. Jiménez, J. Nat. Prod., 2002, 65, 1161 CrossRef CAS.
- L. Yang and R. J. Andersen, J. Nat. Prod., 2002, 65, 1924 CrossRef CAS.
- R. A. Keyzers, P. T. Northcote and V. Webb, J. Nat. Prod., 2002, 65, 598 CrossRef CAS.
- T. Miyamoto, K. Kodama, Y. Aramaki, R. Higuchi and R. W. M. Van Soest, Tetrahedron Lett., 2001, 42, 6349 CrossRef CAS.
- B. Liu and W. Zhou, Tetrahedron Lett., 2002, 43, 4187 CrossRef CAS.
- S. Aoki, Y. Naka, T. Itoh, T. Furukawa, R. Rachmat, S. Akiyama and M. Kobayashi, Chem. Pharm. Bull., 2002, 50, 827 CrossRef CAS.
- N. Borbone, S. De Marino, M. Iorizzi, F. Zollo, C. Debitus, G. Esposito and T. Iuvone, J. Nat. Prod., 2002, 65, 1206 CrossRef CAS.
- A. I. Kalinovsky, A. S. Antonov, S. S. Afiyatullov, P. S. Dimitrenok, E. V. Evtuschenko and V. A. Stonik, Tetrahedron Lett., 2002, 43, 523 CrossRef CAS.
- J. L. McCormick, T. C. McKee, J. H. Cardellina II, M. Leid and M. R. Boyd, J. Nat. Prod., 1996, 59, 1047 CrossRef CAS.
- D. Tasdemir, G. C. Mangalindan, G. P. Concepción, S. M. Verbitski, S. Rabindran, M. Miranda, M. Greenstein, J. N. Hooper, M. K. Harper and C. M Ireland, J. Nat. Prod., 2002, 65, 210 CrossRef CAS.
- D. E. Williams, A. Tahir and R. J. Andersen, J. Nat. Prod., 1999, 62, 653 CrossRef CAS.
- D. Enders and T. Schüßeler, Synthesis, 2002, 2280 CrossRef CAS.
- M. L. Ciavatta, G. Scognamiglio, E. Trivellone, T. Bisogno and G. Cimino, Tetrahedron, 2002, 58, 4943 CrossRef CAS.
- K. Takada, Y. Nakao, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2002, 65, 411 CrossRef CAS.
- M. Vanisree and G. V. Subbaraju, Asian J. Chem., 2002, 14, 957 Search PubMed.
- R. Parvataneni and P. V. S. Rao, J. Indian Chem. Soc., 2002, 79, 732 Search PubMed.
- X.-X. He, R.-L. Yang, J.-Y. Su and L.-M. Zeng, Zhongshan Daxue Xuebao, Ziran Kexueban, 2002, 41, 114 Search PubMed.
- P. S. Parameswaran, C. G. Naik, M. Govenkar and V. R. Hegde, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2002, 41, 1093 Search PubMed.
- C.-Y. Duh, S.-C. Chien, P.-Y. Song, S.-K. Wang, A. A. H. El-Gamal and C.-F. Dai, J. Nat. Prod., 2002, 65, 1853 CrossRef CAS.
- J. A. Palermo, M. F. Rodríguez Brasco, C. Spagnuolo and A. M. Seldes, J. Org. Chem., 2000, 65, 4482 CrossRef CAS.
- B. Witulski, A. Zimmermann and N. D. Gowans, Chem. Commun., 2002, 2984 RSC.
- G.-H. Wang, A. F. Ahmed, Y.-H. Kuo and J.-H. Sheu, J. Nat. Prod., 2002, 65, 1033 CrossRef CAS.
- J.-Y. Su, Y.-L. Zhong and L.-M. Zeng, J. Nat. Prod., 1993, 56, 288 CrossRef CAS.
- F. Coelho and G. Diaz, Tetrahedron, 2002, 58, 1647 CrossRef CAS.
- G.-H. Wang, A. F. Ahmed, J.-H. Sheu, C.-Y. Duh, Y.-C. Shen and L.-T. Wang, J. Nat. Prod., 2002, 65, 887 CrossRef.
- A. Rudi, S. Levi, Y. Benayahu and Y. Kashman, J. Nat. Prod., 2002, 65, 1672 CrossRef CAS.
- N. S. Reddy, T. V. Goud and Y. Venkateswarlu, J. Nat. Prod., 2002, 65, 1059 CrossRef.
- A. D. Rodríguez and I. I. Rodríguez, Tetrahedron Lett., 2002, 43, 5601 CrossRef CAS.
- A. D. Rodríguez, E. González and S. D. Huang, J. Org. Chem., 1998, 63, 7083 CrossRef CAS.
- H. Miyaoka, D. Honda, H. Mitome and Y. Yamada, Tetrahedron Lett., 2002, 43, 7773 CrossRef CAS.
- C.-Y. Duh, A. A. H. El-Gamal, C.-J. Chu, S.-K. Wang and C.-F. Dai, J. Nat. Prod., 2002, 65, 1535 CrossRef CAS.
- M. Iwashima, I. Terada, K. Okamoto and K. Iguchi, J. Org. Chem., 2002, 67, 2977 CrossRef CAS.
- C.-Y. Duh, A. A. H. El-Gamal, S.-K. Wang and C.-F. Dai, J. Nat. Prod., 2002, 65, 1429 CrossRef CAS.
- K. Mori, K. Iguchi, N. Yamada, Y. Yamada and Y. Inouye, Chem. Pharm. Bull., 1988, 36, 2840 CAS.
- K. Iguchi, H. Sawai, H. Nishimura, M. Fujita and T. Yamori, Bull. Chem. Soc. Jpn., 2002, 75, 131 CrossRef CAS.
- H. Miyaoka, Y. Isaji, Y. Kajiwara, Y. Kunimune and Y. Yamada, Tetrahedron Lett., 1998, 39, 6503 CrossRef CAS.
- M. Iwashima, Y. Matsumoto, Y. Takenaka, K. Iguchi and T. Yamori, J. Nat. Prod., 2002, 65, 1441 CrossRef CAS.
- Y. Uchio, S. Eguchi, M. Nakayama and T. Hase, Chem. Lett., 1982, 277 CAS.
- Y.-P. Shi, A. D. Rodríguez, C. L. Barnes, J. A. Sánchez, R. G. Raptis and P. Baran, J. Nat. Prod., 2002, 65, 1232 CrossRef CAS.
- A. D. Rodríguez and H. Dhasmana, J. Nat. Prod., 1993, 56, 564 CrossRef CAS.
- A. D. Rodríguez, I. C. Piña, J. J. Soto, D. R. Rojas and C. L. Barnes, Can. J. Chem., 1995, 73, 643 CAS.
- A. D. Rodríguez and A. L. Acosta, J. Nat. Prod., 1998, 61, 40 CrossRef CAS.
- A. Longeon, M.-L. Bourguet-Kondracki and M. Guyot, Tetrahedron Lett., 2002, 43, 5937 CrossRef CAS.
- N. B. Pham, M. S. Butler and R. J. Quinn, J. Nat. Prod., 2002, 65, 1147 CrossRef CAS.
- J.-H. Sheu, A. F. Ahmed, R.-T. Shiue, C.-F. Dai and Y.-H. Kuo, J. Nat. Prod., 2002, 65, 1904 CrossRef CAS.
- C.-W. Lin, J.-Y. Su and L.-M. Zeng, Chem. Res. Chin. Univ., 2002, 18, 189 Search PubMed.
- J. H. Kwak, F. J. Schmitz and G. C. Williams, J. Nat. Prod., 2002, 65, 704 CrossRef CAS.
- O. Taglialatela-Scafati, U. Deo-Jangra, M. Campbell, M. Roberge and R. J. Andersen, Org. Lett., 2002, 4, 4085 CrossRef.
- D. Banjoo, B. S. Mootoo, R. S. Ramsewak, R. Sharma, A. J. Lough, S. McLean and W. F. Reynolds, J. Nat. Prod., 2002, 65, 314 CrossRef CAS.
- Y.-C. Shen, Y.-C. Lin and M. Y. Chiang, J. Nat. Prod., 2002, 65, 54 CrossRef CAS.
- D. Friedrich and L. A. Paquette, J. Nat. Prod., 2002, 65, 126 CrossRef CAS.
- L. A. Paquette, The Chemical Record, 2001, 1, 311 Search PubMed.
- V. A. Stonik, I. I. Kapustina, A. I. Kalinovsky, P. S. Dmitrenok and B. B. Grebnev, Tetrahedron Lett., 2002, 43, 315 CrossRef CAS.
- G.-H. Wang, J.-H. Sheu, C.-Y. Duh and M. Y. Chiang, J. Nat. Prod., 2002, 65, 1475 CrossRef CAS.
- P. Radhika, P. V. S. Rao, V. Anjaneyulu, R. N. Asolkar and H. Laatsch, J. Nat. Prod., 2002, 65, 737 CrossRef CAS.
- N. González, J. Rodríguez, R. G. Kerr and C. Jiménez, J. Org. Chem., 2002, 67, 5117 CrossRef CAS.
- C. Anta, N. González, G. Santafé, J. Rodríguez and C. Jiménez, J. Nat. Prod., 2002, 65, 766 CrossRef CAS.
- C.-Y. Duh, A. A. H. El-Gamal, C.-Y. Chiang, C.-J. Chu, S.-K. Wang and C.-F. Dai, J. Nat. Prod., 2002, 65, 1882 CrossRef CAS.
- C. Anta, N. González, J. Rodríguez and C. Jiménez, J. Nat. Prod., 2002, 65, 1357 CrossRef CAS.
- M. J. Ortega, E. Zubía, S. Rodríguez, J. L. Carballo and J. Salvá, Eur. J. Org. Chem., 2002, 3250 CrossRef CAS.
- X.-X. He, J.-Y. Su, L.-M. Zeng, X.-P. Yang and Y.-J. Liang, Huaxue Xuebao, 2002, 60, 334 CAS.
- Y. Tomono, H. Hirota, Y. Imahara and N. Fusetani, J. Nat. Prod., 1999, 62, 1538 CrossRef CAS.
- M. Linker and W. Kreiser, Helv. Chim. Acta, 2002, 85, 1096 CrossRef CAS.
- Z.-Y. Shao, D.-Y. Zhu and Y.-W. Guo, J. Nat. Prod., 2002, 65, 1675 CrossRef CAS.
- J. Tanaka, A. Trianto, M. Musman, H. H. Issa, I. I. Ohtani, T. Ichiba, T. Higa, W. Y. Yoshida and P. J. Scheuer, Tetrahedron, 2002, 58, 6259 CrossRef CAS.
- J. Tanaka, T. Higa, K. Tachibana and T. Iwashita, Chem. Lett., 1982, 1295 CAS.
- T. Higa, J. Tanaka and K. Tachibana, Tetrahedron Lett., 1981, 22, 2777 CrossRef CAS.
- N. Alam, J. Hong, C.-O. Lee, J. S. Choi, K. S. Im and J. H. Jung, Chem. Pharm. Bull., 2002, 50, 661 CrossRef CAS.
- A. Fontana, M. L. Ciavatta and G. Cimino, J. Org. Chem., 1998, 63, 2845 CrossRef CAS.
- I. S. Marcos, A. B. Pedrero, M. J. Sexmero, D. Diez, P. Basabe, F. A. Hernandez, H. B. Broughton and J. G. Urones, Synlett, 2002, 105 CrossRef CAS.
- A. Suksamrarn, A. Jankam, B. Tarnchompoo and S. Putchakarn, J. Nat. Prod., 2002, 65, 1194 CrossRef CAS.
- N. Lindquist, J. Nat. Prod., 2002, 65, 681 CrossRef CAS.
- S.-Y. Zhang, Y.-H. Yi, H.-F. Tang, Q.-Z. Xu, Z.-R. Zou and L. Li, Dier Junyi Daxue Xuebao, 2002, 23, 250 Search PubMed.
- P. Macek and D. Lebez, Toxicon, 1988, 26, 441 CrossRef CAS.
- M. G. Hinds, W. Zhang, G. Anderluh, P. E. Hansen and R. S. Norton, J. Mol. Biol., 2002, 315, 1219 CrossRef CAS.
- Q. Hong, I. Gutiérrez-Aguirre, A. Barlic, P. Malovrh, K. Kristan, Z. Podlesek, P. Macek, D. Turk, J. M. González-Mañas, J. H. Lakey and G. Anderluh, J. Biol. Chem., 2002, 277, 41916 CrossRef CAS.
- H. Nagai, N. Oshira, K. Takuwa-Kuroda, S. Iwanaga, M. Nozaki and T. Nakajima, Biosci. Biotechnol. Biochem., 2002, 66, 2621 CrossRef CAS.
- M. M. Monastyrnaya, T. A. Zykova, O. V. Apalikova, T. V. Shwets and E. P. Kozlovskaya, Toxicon, 2002, 40, 1197 CrossRef CAS.
- A. Fürstner, A. Leitner, M. Méndez and H. Krause, J. Am. Chem. Soc., 2002, 124, 13856 CrossRef.
- T. J. Speed and D. M. Thamattoor, Tetrahedron Lett., 2002, 43, 367 CrossRef CAS.
- N. Alam, J. Hong, C. O. Lee, K. S. Im, B. W. Son, J. S. Choi, W. C. Choi and J. H. Jung, J. Nat. Prod., 2001, 64, 956 CrossRef CAS.
- B. H. Bae, K. S. Im, W. C. Choi, J. Hong, C.-O. Lee, J. S. Choi, B. W. Son, J.-I. Song and J. H. Jung, J. Nat. Prod., 2000, 63, 1511 CrossRef CAS.
- M. Zhang, K. Long, H. Wu and K. Ma, J. Nat. Prod., 1994, 57, 155 CrossRef CAS.
- W. K. Liu, N. L. Y. Wong, H. M. Huang, J. K. C. Ho, W. H. Zhang and C. T. Che, Life Sci., 2002, 70, 843 CrossRef CAS.
- E. P. Loret, R. M. S. del Valle, P. Mansuelle, F. Sampieri and H. Rochat, J. Biol. Chem., 1994, 269, 16785 CAS.
- F. Bosmans, A. Aneiros and J. Tytgat, FEBS Lett., 2002, 532, 131 CrossRef CAS.
- P. Wulff, J. S. Carlé and C. J. Christophersen, J. Chem. Soc., Perkin Trans. 1, 1981, 2895 RSC.
- L. Peters, G. M. König, H. Terlau and A. D. Wright, J. Nat. Prod., 2002, 65, 1633 CrossRef CAS.
- N. Lysek, E. Rachor and T. Lindel, Z. Naturforsch., C: Biosci., 2002, 57, 1056 Search PubMed.
- J. L. C. Wright, J. Nat. Prod., 1984, 47, 893 CrossRef CAS.
- J. S. Carlé and C. Christophersen, J. Am. Chem. Soc., 1979, 101, 4012 CrossRef CAS.
- P. B. Holst, U. Anthoni, C. Christophersen and P. H. Nielsen, J. Nat. Prod., 1994, 57, 997 CrossRef CAS.
- M. S. Morales-Ríos, N. F. Santos-Sánchez, O. R. Suárez-Castillo and P. Joseph-Nathan, Magn. Reson. Chem., 2002, 40, 677 CrossRef CAS.
- C. K. Narkowicz, A. J. Blackman, E. Lacey, J. H. Gill and K. Heiland, J. Nat. Prod., 2002, 65, 938 CrossRef CAS.
- S.-J. Jeong, R. Higuchi, T. Miyamoto, M. Ono, M. Kuwano and S. F. Mawatari, J. Nat. Prod., 2002, 65, 1344 CrossRef CAS.
- S. Eisenbarth, M. Gehling, A. Harder and B. Steffan, Tetrahedron, 2002, 58, 8461 CrossRef CAS.
- M. S. Morales-Ríos, O. R. Suárez-Castillo and P. Joseph-Nathan, Tetrahedron, 2002, 58, 1479 CrossRef CAS.
- C. C. Hughes and D. Trauner, Angew. Chem., Int. Ed., 2002, 41, 4556 CrossRef CAS.
- B. D. Morris and M. R. Prinsep, J. Nat. Prod., 1999, 62, 688 CrossRef CAS.
- A. J. Blackman and D. J. Matthews, Heterocycles, 1985, 23, 2829 CAS.
- M. Ramirez Osuna, G. Aguirre, R. Somanathan and E. Molins, Tetrahedron: Asymmetry, 2002, 13, 2261 CrossRef CAS.
- M. L. Ciavatta, E. Trivellone, G. Villani and G. Cimino, Tetrahedron Lett., 1993, 34, 6791 CrossRef CAS.
- R. A. Sampson and M. V. Perkins, Org. Lett., 2002, 4, 1655 CrossRef CAS.
- M. V. Perkins and R. A. Sampson, Org. Lett., 2001, 3, 123 CrossRef CAS.
- M. A. Calter and W. Liao, J. Am. Chem. Soc., 2002, 124, 13127 CrossRef CAS.
- M. Norte, F. Cataldo, A. G. González, M. L. Rodríguez and C. Ruiz-Perez, Tetrahedron, 1990, 46, 1669 CrossRef CAS.
- I. Paterson, D. Y.-K. Chen and A. S. Franklin, Org. Lett., 2002, 4, 391 CrossRef CAS.
- J. E. Hochlowski, J. C. Coll, D. J. Faulkner, J. E. Biskupiak, C. M. Ireland, Q.-T. Zheng, C.-H. He and J. Clardy, J. Am. Chem. Soc., 1984, 106, 6748 CrossRef.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, S. Magno, M. Di Rosa, A. Ianaro and R. Poletti, J. Am. Chem. Soc., 2002, 124, 13114 CrossRef CAS.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, S. Magno and R. Poletti, Chem. Res. Toxicol., 2002, 15, 979 CrossRef CAS.
- S. J. Fahey and M. J. Garson, J. Chem. Ecol., 2002, 28, 1773 Search PubMed.
- D. R. Appleton, M. A. Sewell, M. V. Berridge and B. R. Copp, J. Nat. Prod., 2002, 65, 630 CrossRef CAS.
- J. Kimura, Y. Takada, T. Inayoshi, Y. Nakao, G. Goetz, W. Y. Yoshida and P. J. Scheuer, J. Org. Chem., 2002, 67, 1760 CrossRef CAS.
- J. Rajaganapathi, K. Kathiresan and T. P. Singh, Mar. Biotechnol., 2002, 4, 447 CrossRef CAS.
- J. M. McIntosh, C. Dowell, M. Watkins, J. E. Garrett, D. Yoshikami and B. M. Olivera, J. Biol. Chem., 2002, 277, 33610 CrossRef CAS.
- S. W. Ayer and R. J. Andersen, Experientia, 1983, 39, 255 Search PubMed.
- T. Barsby, R. G. Linington and R. J. Andersen, Chemoecology, 2002, 12, 199 Search PubMed.
- B. J. Burreson, P. J. Scheuer, J. Finer and J. Clardy, J. Am. Chem. Soc., 1975, 97, 4763 CrossRef CAS.
- A. Srikrishna and P. R. Kumar, Tetrahedron Lett., 2002, 43, 1109 CrossRef CAS.
- K. L. McPhail, M. T. Davies-Coleman, R. C. B. Copley and D. S. Eggleston, J. Nat. Prod., 1999, 62, 1618 CrossRef CAS.
- R. C. B. Copley, M. T. Davies-Coleman, D. R. Edmonds, D. J. Faulkner and K. L. McPhail, J. Nat. Prod., 2002, 65, 580 CrossRef CAS.
- J. A. Findlay and G. Li, Can. J. Chem., 2002, 80, 1697 CrossRef.
- M. Gavagnin, N. Ungur, E. Mollo, J. Templado and G. Cimino, Eur. J. Org. Chem., 2002, 1500 CrossRef CAS.
- A. Spinella, E. Zubía, E. Martinez, J. Ortea and G. Cimino, J. Org. Chem., 1997, 62, 5471 CrossRef CAS.
- A. Spinella, T. Caruso and C. Coluccini, Tetrahedron Lett., 2002, 43, 1681 CrossRef CAS.
- T. Caruso and A. Spinella, Tetrahedron: Asymmetry, 2002, 13, 2071 CrossRef CAS.
- N. K. Gulavita, E. D. da Silva, M. R. Hagadone, P. Karuso, P. J. Scheuer, G. D. van Duyne and J. Clardy, J. Org. Chem., 1986, 51, 5136 CrossRef CAS.
- Y. Kitano, T. Ito, T. Suzuki, Y. Nogata, K. Shinshima, E. Yoshimura, K. Chiba, M. Tada and I. Sakaguchi, J. Chem. Soc., Perkin Trans. 1, 2002, 2251 RSC.
- H. Ishiwata, T. Nemoto, M. Ojika and K. Yamada, J. Org. Chem., 1994, 59, 4710 CrossRef CAS.
- H. Ishiwata, H. Sone, H. Kigoshi and K. Yamada, J. Org. Chem., 1994, 59, 4712 CrossRef CAS.
- R. Bai, D. G. Covell, C. Liu, A. K. Ghosh and E. Hamel, J. Biol. Chem., 2002, 277, 32165 CrossRef CAS.
- M. Fujita, Y. Nakao, S. Matsunaga, T. Nishikawa and N. Fusetani, J. Nat. Prod., 2002, 65, 1936 CrossRef CAS.
- T. C. McKee, D. L. Galinis, L. K. Pannell, J. H. Cardellina II, J. Laasko, C. M. Ireland, L. Murray, R. J. Capon and M. R. Boyd, J. Org. Chem., 1998, 63, 7805 CrossRef CAS.
- R. Shen, C. T. Lin and J. A. Porco, J. Am. Chem. Soc., 2002, 124, 5650 CrossRef CAS.
- A. Aiello, E. Fattorusso, A. Mangoni and M. Menna, Eur. J. Org. Chem., 2002, 1047 CrossRef CAS.
- T. Rezanka and V. M. Dembitsky, Eur. J. Org. Chem., 2002, 2400 CrossRef CAS.
- J.-F. Biard, C. Roussakis, J.-M. Kornprobst, D. Gouiffes-Barbin, J.-F. Verbist, P. Cotelle, M. P. Foster, C. M. Ireland and C. Debitus, J. Nat. Prod., 1994, 57, 1336 CrossRef CAS.
- P. Wipf, Y. Uto and S. Yoshimura, Chem. Eur. J., 2002, 8, 1670 CrossRef CAS.
- B. C. M. Potts, D. J. Faulkner, J. A. Chan, G. C. Simolike, P. Offen, M. E. Hemling and T. A. Francis, J. Am. Chem. Soc., 1991, 113, 6321 CrossRef CAS.
- J. Pika and D. J. Faulkner, Nat. Prod. Lett., 1995, 7, 291 Search PubMed.
- C. E. Salomon, D. H. Williams, E. Lobkovsky, J. C. Clardy and D. J. Faulkner, Org. Lett., 2002, 4, 1699 CrossRef CAS.
- N. González, J. Rodríguez and C. Jiménez, J. Org. Chem., 1999, 64, 5705 CrossRef CAS.
- H. Kiyota, D. J. Dixon, C. K. Luscombe, S. Hettstedt and S. V. Ley, Org. Lett., 2002, 4, 3223 CrossRef CAS.
- J. A. Tincu and S. W. Taylor, J. Nat. Prod., 2002, 65, 377 CrossRef CAS.
- A. Arrault, A. Witczak-Legrand, P. Gonzalez, N. Bontemps-Subielos and B. Banaigs, Tetrahedron Lett., 2002, 43, 4041 CrossRef CAS.
- C. E. Salomon and D. J. Faulkner, J. Nat. Prod., 2002, 65, 689 CrossRef CAS.
- W. S. Jang, K. N. Kim, Y. S. Lee, M. H. Nam and I. H. Lee, FEBS Lett., 2002, 521, 81 CrossRef CAS.
- I.-H. Lee, C. Zhao, T. Nguyen, L. Menzel, A. J. Waring, M. A. Sherman and R. I. Lehrer, J. Peptide Res., 2001, 58, 445 CrossRef CAS.
- L. P. Menzel, I. H. Lee, B. Sjostrand and R. I. Lehrer, Dev. Comp. Immunol., 2002, 26, 505 Search PubMed.
- A. D. Wright, E. Goclik, G. M. König and R. Kaminsky, J. Med. Chem., 2002, 45, 3067 CrossRef CAS.
- T. Ozawa, S. Aoyagi and C. Kibayashi, J. Org. Chem., 2001, 66, 3338 CrossRef CAS.
- R. A. Davis, A. R. Carroll and R. J. Quinn, J. Nat. Prod., 2002, 65, 454 CrossRef CAS.
- J. F. Biard, S. Guyot, C. Roussakis, J. F. Verbist, J. Vercauteren, J. F. Weber and K. Boukef, Tetrahedron Lett., 1994, 35, 2691 CrossRef CAS.
- M. Jugé, N. Grimaud, J. F. Biard, M. P. Sauviat, M. Nabil, J. F. Verbist and J. Y. Petit, Toxicon, 2001, 39, 1231 CrossRef CAS.
- P. Sun, C. Sun and S. M. Weinreb, J. Org. Chem., 2002, 67, 4337 CrossRef CAS.
- H. Abe, S. Aoyagi and C. Kibayashi, Angew. Chem., Int. Ed., 2002, 41, 3017 CrossRef CAS.
- R. A. Davis, W. Aalbersberg, S. Meo, R. M. da Rocha and C. M. Ireland, Tetrahedron, 2002, 58, 3263 CrossRef CAS.
- S. Urban, J. W. Blunt and M. H. G. Munro, J. Nat. Prod., 2002, 65, 1371 CrossRef CAS.
- M. J. Ortega, E. Zubía, J. M. Ocaña, S. Naranjo and J. Salvá, Tetrahedron, 2000, 56, 3963 CrossRef CAS.
- F. Bellina, C. Anselmi and R. Rossi, Tetrahedron Lett., 2002, 43, 2023 CrossRef CAS.
- T. Janosik, A.-L. Johnson and J. Bergman, Tetrahedron, 2002, 58, 2813 CrossRef CAS.
- H. Sato, M. Tsuda, K. Watanabe and J. Kobayashi, Tetrahedron, 1998, 54, 8687 CrossRef CAS.
- D. R. Appleton, M. J. Page, G. Lambert, M. V. Berridge and B. R. Copp, J. Org. Chem., 2002, 67, 5402 CrossRef CAS.
- Y. R. Torres, T. S. Bugni, R. G. S. Berlinck, C. M. Ireland, A. Magalhães, A. G. Ferreira and R. M. da Rocha, J. Org. Chem., 2002, 67, 5429 CrossRef CAS.
- D. R. Appleton, A. N. Pearce, G. Lambert, R. C. Babcock and B. R. Copp, Tetrahedron, 2002, 58, 9779 CrossRef CAS.
- Nilar, P. J. Sidebottom, B. K. Carté and M. S. Butler, J. Nat. Prod., 2002, 65, 1198 CrossRef.
- S. M. Verbitski, C. L. Mayne, R. A. Davis, G. P. Concepcion and C. M. Ireland, J. Org. Chem., 2002, 67, 7124 CrossRef CAS.
- A. Rudi, I. Goldberg, Z. Stein, F. Frolow, Y. Benayahu, M. Schleyer and Y. Kashman, J. Org. Chem., 1994, 59, 999 CrossRef CAS.
- A. Rudi, T. Evan, M. Aknin and Y. Kashman, J. Nat. Prod., 2000, 63, 832 CrossRef CAS.
- A. T. Kreipl, C. Reid and W. Steglich, Org. Lett., 2002, 4, 3287 CrossRef CAS.
- J. Ham and H. Kang, Bull. Korean Chem. Soc., 2002, 23, 163 CAS.
- P. Schupp, P. Proksch and V. Wray, J. Nat. Prod., 2002, 65, 295 CrossRef CAS.
- N. Funato, H. Takayanagi, Y. Konda, Y. Toda, Y. Harigaya, Y. Iwai and S. Omura, Tetrahedron Lett., 1994, 35, 1251 CrossRef CAS.
- P. Schupp, C. Eder, P. Proksch, V. Wray, B. Schneider, M. Herderich and V. Paul, J. Nat. Prod., 1999, 62, 959 CrossRef CAS.
- K. Suwanborirux, K. Charupant, S. Amnuoypol, S. Pummangura, A. Kubo and N. Saito, J. Nat. Prod., 2002, 65, 935 CrossRef CAS.
- A. Endo, A. Yanagisawa, M. Abe, S. Tohma, T. Kan and T. Fukuyama, J. Am. Chem. Soc., 2002, 124, 6552 CrossRef CAS.
- M. J. Uddin, S. Kokubo, K. Ueda, K. Suenaga and D. Uemura, Chem. Lett., 2002, 1028 CrossRef CAS.
- L. Garrido, E. Zubía, M. J. Ortega and J. Salvá, J. Nat. Prod., 2002, 65, 1328 CrossRef CAS.
- M. Yoshida, M. Murata, K. Inaba and M. Morisawa, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 14831 CrossRef CAS.
- X. Fu, M. B. Hossain, D. van der Helm and F. J. Schmitz, J. Am. Chem. Soc., 1994, 116, 12125 CrossRef CAS.
- X. Fu, M. B. Hossain, D. van der Helm and F. J. Schmitz, J. Am. Chem. Soc., 1995, 117, 9381 CrossRef CAS.
- M. E. Layton, C. A. Morales and M. D. Shair, J. Am. Chem. Soc., 2002, 124, 773 CrossRef CAS.
- S. Fukuzawa, S. Matsunaga and N. Fusetani, Tetrahedron, 1995, 51, 6707 CrossRef CAS.
- S. Lee, T. G. LaCour, D. Lantrip and P. L. Fuchs, Org. Lett., 2002, 4, 313 CrossRef CAS.
- S. Lee and P. L. Fuchs, Org. Lett., 2002, 4, 317 CrossRef CAS.
- A. M. Fernandez, H.-Y. He, L. A. McDonald, P. Lassota, C. Discafani, E. F. Sorensen, M. C. Edler, L. R. Barrows, J. C. Clardy and C. M. Ireland, Pure Appl. Chem., 1998, 70, 2130.
- M. C. Edler, A. M. Fernandez, P. Lassota, C. M. Ireland and L. R. Barrows, Biochem. Pharmacol., 2002, 63, 707 CrossRef CAS.
- H. Kang and W. Fenical, Tetrahedron Lett., 1996, 37, 2369 CrossRef CAS.
- S. A. Abas, M. B. Hossain, D. van der Helm, F. J. Schmitz, M. Laney, R. Cabuslay and R. C. Schatzman, J. Org. Chem., 1996, 61, 2709 CrossRef CAS.
- A. M. Popov, V. L. Novikov, O. S. Radchenko and G. B. Elyakov, Dokl. Biochem. Biophys., 2002, 385, 213 Search PubMed.
- N. Takada, M. Watanabe, K. Suenaga, K. Yamada, M. Kita and D. Uemura, Tetrahedron Lett., 2001, 42, 6557 CrossRef CAS.
- M. Kita, M. Watanabe, N. Takada, K. Suenaga, K. Yamada and D. Uemura, Tetrahedron, 2002, 58, 6405 CrossRef CAS.
- D. Uemura, A. Takada, K. Suenaga, K. Yamada, M. Watanabe and S. Nakagawa, Jpn. Kokai Tokkyo Koho, 2002, 2002241362 Search PubMed.
- S. Kawatake, K. Nakamura, M. Inagaki and R. Higuchi, Chem. Pharm. Bull., 2002, 50, 1091 CrossRef CAS.
- S. Kawatake, M. Inagaki, R. Isobe, T. Miyamoto and R. Higuchi, Chem. Pharm. Bull., 2002, 50, 1386 CrossRef CAS.
- M. E. Díaz de Vivar, A. M. Seldes and M. S. Maier, Lipids, 2002, 37, 597 Search PubMed.
- K. Yamada, K. Sasaki, Y. Harada, R. Isobe and R. Higuchi, Chem. Pharm. Bull., 2002, 50, 1467 CrossRef CAS.
- Y. Murata and N. U. Sata, J. Agric. Food Chem., 2000, 48, 5557 CrossRef CAS.
- N. U. Sata, R. Kuwahara and Y. Murata, Tetrahedron Lett., 2002, 43, 115 CrossRef CAS.
- D. Takahashi, T. Maoka, M. Tsushima, K. Fujitani, M. Kozuka, T. Matsuno and T. Shingu, Chem. Pharm. Bull., 2002, 50, 1609 CrossRef CAS.
- I. I. Kapustina, L. P. Ponomarenko, O. P. Moiseenko and V. A. Stonik, Chem. Nat. Compd., 2001, 37, 515 Search PubMed.
- E. V. Levina, P. V. Andriyashchenko, A. I. Kalinovsky, P. S. Dmitrenok and V. A. Stonik, Russ. J. Bioorg. Chem., 2002, 28, 189 Search PubMed.
- E. V. Levina, P. V. Andriyashchenko, A. I. Kalinovsky, P. S. Dmitrenok, V. A. Stonik and N. G. Prokof'eva, Russ. Chem. Bull., 2002, 51, 535 CrossRef CAS.
- W. Wang, F. Li, N. Alam, Y. Liu, J. Hong, C.-K. Lee, K. S. Im and J. H. Jung, J. Nat. Prod., 2002, 65, 1649 CrossRef CAS.
- J. Qi, M. Ojika and Y. Sakagami, Bioorg. Med. Chem., 2002, 10, 1961 Search PubMed.
- H. D. Chludil, A. M. Seldes and M. S. Maier, J. Nat. Prod., 2002, 65, 153 CrossRef CAS.
- P. Radhika, V. Anjaneyulu, P. V. S. Rao, T. N. Makarieva and A. I. Kalinovosky, Indian J. Chem. Sect. B, 2002, 41, 1276.
- H. D. Chludil, C. C. Muniain, A. M. Seldes and M. S. Maier, J. Nat. Prod., 2002, 65, 860 CrossRef CAS.
- V. R. Hegde, T.-M. Chan, H. Pu, V. P. Gullo, M. G. Patel, P. Das, N. Wagner, P. S. Parameswaran and C. G. Naik, Bioorg. Med. Chem. Lett., 2002, 12, 3203 CrossRef CAS.
- N. Asai, N. Fusetani, S. Matsunaga and J. Sasaki, Tetrahedron, 2000, 56, 9895 CrossRef CAS.
- N. Asai, N. Fusetani and S. Matsunaga, J. Nat. Prod., 2001, 64, 1210 CrossRef CAS.
- Y. Masuda, M. Yoshida and K. Mori, Biosci. Biotechnol. Biochem., 2002, 66, 1531 CrossRef CAS.
- E. Peña-Cabrera and L. S. Liebeskind, J. Org. Chem., 2002, 67, 1689 CrossRef CAS.
- D. C. Rowley, M. S. T. Hansen, D. Rhodes, C. A. Sotriffer, H. Ni, J. A. McCammon, F. D. Bushman and W. Fenical, Bioorg. Med. Chem., 2002, 10, 3619 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |