Hot off the press
Robert A.
Hill
* and
Andrew
Sutherland
Department of Chemistry, Glasgow University, Glasgow, UK G12 8QQ. E-mail: bobh@chem.gla.ac.uk; andrews@chem.gla.ac.uk
First published on 14th January 2004
Abstract
A personal selection of 34 recent papers is presented covering various aspects of current developments in bioorganic chemistry and novel natural products such as manadomanzamines A and B that have activity against Mycobacterium tuberculosis, HIV-1 and AIDS opportunistic fungal infections.
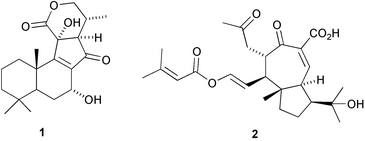
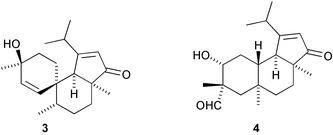
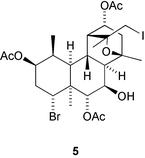
The first 13(12→11)-abeoabietane derivative, micranthin C 1, has been isolated from Isodon lophanoids var. micranthus and the structure confirmed by X-ray analysis (H.-D. Sun and co-workers, Helv. Chim. Acta, 2003, 86, 3470). The authors propose a biosynthetic pathway leading to micranthin C 1. Viburnum awabuki continues to provide interesting natural products. Vibsanin O 2, from this species, has a novel diterpenoid framework (C.-Y. Duh et al., Tetrahedron Lett., 2003, 44, 9321). Further novel diterpenoid skeletons are represented by cyanthiwigin AC 3 and AD 4 from the Jamaican sponge Myrmekioderma styx (M. T. Hamann and co-workers, Org. Lett., 2003, 5, 4575). Tasihalide A 5 is an iodinated diterpenoid from a marine cyanobacterium and red alga assemblage (R. E. Moore and co-workers, Org. Lett., 2003, 5, 4167).
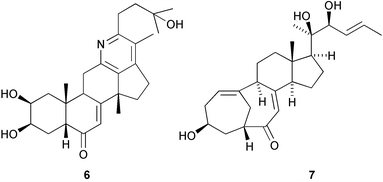
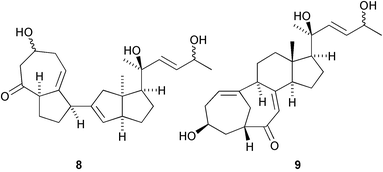
A pyridine ring-containing rearranged cholestane derivative, diploclidine 6, has been found in the leaves of Diploclisia glaucescens (Y. Fujimoto and co-workers, Tetrahedron Lett., 2003, 44, 8769). The structure of isocyclocitrinol 7, from a sponge-derived Penicillium citrinum, was determined by X-ray analysis (P. Crews and co-workers, Org. Lett., 2003, 5, 4393). In addition the structure of cyclocitrinol, previously isolated from a terrestrial Penicillium citrinum, has been revised from 8 to 9. A group of 15,21-cyclowithanolides, such as jaborosalactol 18 10, has been isolated from aerial parts of Jaborosa bergii (G. Burton and co-workers, J. Nat. Prod., 2003, 66, 1471).
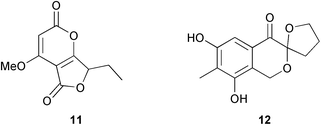
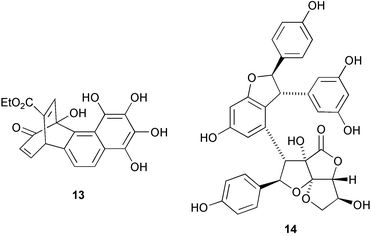
A series of tetraketide metabolites from the freshwater fungus Annulatascus triseptatus includes annularin F 11 which has a rearranged skeleton (J. B. Gloer and co-workers, J. Nat. Prod., 2003, 66, 1302). The spiroacetal terreinol 12 is a polyketide metabolite from Aspergillus terreus (A. J. Marsaioli and co-workers, Tetrahedron Lett., 2004, 45, 53). Goupiolone B 13, apparently derived via a Diels–Alder addition of tropolone and naphthalene derivatives, has been isolated from Goupia glabra (A. Estévez-Braun and co-workers, Eur. J. Org. Chem., 2003, 4243). The dimeric stilbenoid shorelactone 14, isolated from the stem bark of Shorea hemsleyana, has an interesting fused C6 unit (T. Ito et al., Helv. Chim. Acta, 2003, 86, 3394). The structure of shorelactone 14 was confirmed by X-ray analysis.
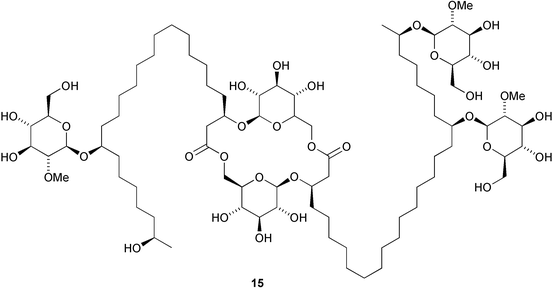
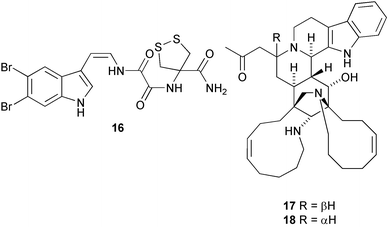
The full stereochemistry of the antiviral cycloviracin B115 has been established by total synthesis (A. Fürstner et al., J. Am. Chem. Soc., 2003, 125, 13132). The first natural product containing the amino acid 4-amino-1,2-dithiolane-4-carboxylic acid, kottamide E 16, has been isolated from the New Zealand ascidian Pycnoclavella kottae (D. R. Appleton and B. R. Copp, Tetrahedron Lett., 2003, 44, 8963). Manadomanzamines A 17 and B 18 have been isolated from an Indonesian sponge Acanthostrongylophora sp. (M. T. Hamann and co-workers, J. Am. Chem. Soc., 2003, 125, 13382). Manadomanzamines A 17 and B 18 have a novel ring structure and show activity against Mycobacterium tuberculosis, the human immunodeficiency virus and AIDS opportunistic fungal infections.
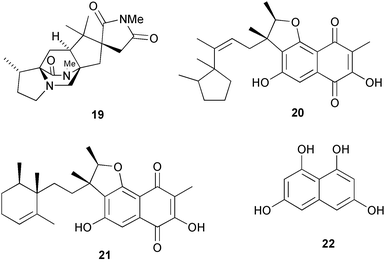
Labelling studies on asperparaline A 19, a metabolite of Aspergillus japonicus, indicate that the carbons of the spirosuccinimide ring system are derived by oxidative cleavage of a tryptophan unit (R. M. Williams and co-workers, J. Am. Chem. Soc., 2003, 125, 14692). C. M. McGinley and W. A. van der Donk have highlighted recent research concerning hydrogen atom abstraction from polyunsaturated fatty acids (Chem. Commun., 2003, 2843). Biosynthetic studies on the meroterpenoid neomarinone from a marine actinomycete have prompted a revision of the structure from 20 to 21 (B. S. Moore and co-workers, Org. Lett., 2003, 5, 4449). The biosynthetic results are consistent with neomarinone 21 being derived from a symmetrical pentaketide such as the tetrahydroxynaphthalene 22viaC-methylation and coupling to a sesquiterpenoid moiety.
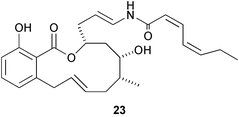
Salicylihalamide A 23 and related macrocyclic salicylic acid lactones show interesting anti-tumour properties. The chemistry and biology of this group have been reviewed by L. Yet (Chem. Rev., 2003, 103, 4283). An extensive review of the development and properties of antimalarial drugs has been published (J. Wiesner et al., Angew. Chem., Int. Ed., 2003, 42, 5274). J. Meinwald and T. Eisner have written an article emphasising the importance of natural product chemistry and the need for continuing support and development in this area (Helv. Chim. Acta, 2003, 86, 3633).
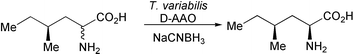
In an extension of their work on biocatalytic approaches to deracemization of α-amino acids, N. J. Turner and co-workers have shown that a range of different amino acid oxidases and chemical reducing agents can be used for the stereoinversion of β- and γ-substituted amino acids (Chem. Commun., 2003, 2636). For example, treatment of (2RS,4S)-4-methyl-2-aminohexanoic acid with D-amino acid oxidase from Trigonopsis variabilis and sodium cyanoborohydride results in the rapid formation of the (2S,4S)-stereoisomer in 66% yield and in > 99% e.e. and d.e. (Scheme 1). The Edinburgh group has also demonstrated the first lipase catalysed kinetic resolution of racemic esters on solid phase using an acylation/deacylation capture and release approach (C. E. Humphrey et al., J. Am. Chem. Soc., 2003, 125, 13952). Acylation of resin bound cyclohexane-1,3-dione with (R/S)-vinyl 3-phenylbutanoate in the presence of Chromobacterium viscosum lipase (CVL) followed by deacylation with sodium hydroxide leads to the isolation of (R)-phenylbutyric acid in modest yield and excellent e.e.'s (> 99%). The yield of the reaction was increased to 86% by subjecting the resin to a second and then third acylation reaction.
 | ||
| Scheme 1 | ||
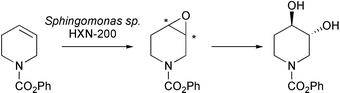
Methodology that allows trans dihydroxylation of nonactivated alkenes has been developed using an alkane degrading bacterium, Sphingomonas sp. HXN-200 (Z. Li and co-workers, J. Org. Chem., 2003, 68, 8599). This strain of the organism catalyses epoxidation and subsequent hydrolysis of the epoxide using a monooxygenase and an epoxide hydrolase respectively. Incubation of the bacterial strain with a series of N-substituted 1,2,5,6-tetrahydropyridines and 3-pyrrolines gave the trans dihydroxylated products in good yield and with > 95% e.e. (e.g.Scheme 2). The demand for chiral phosphorus containing catalysts for asymmetric synthesis has prompted the development of new approaches for their preparation. P. Kielbasinski and co-workers (Tetrahedron: Asymmetry, 2003, 14, 3379) have reported the first enzymatic desymmetrization of prochiral phosphine oxides. Reaction of diester derivatives of these phosphine oxides with pig liver esterase (PLE) leads to isolation of the (R)-monoesters in excellent yield and in good enantioselectivity.
 | ||
| Scheme 2 | ||
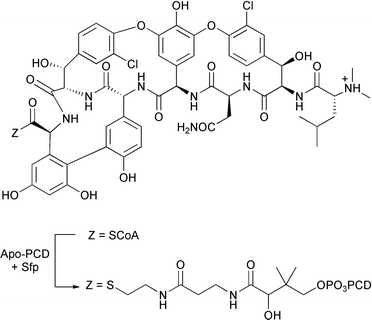
A putative intermediate of 7,8-diaminopelargonate synthase, an enzyme involved in the biosynthesis of biotin (vitamin H), has recently been characterised (R. L. Baxter and co-workers, Chem. Commun., 2003, 3498). This transaminase enzyme which uses S-adenosylmethionine (SAM) as an amine donor catalyses the transformation of (7S)-8-amino-7-oxononanoate to (7S,8R)-7,8-diaminononanoate. The deaminated by-product of SAM, the putative oxo-acid, was successfully trapped by removal of the protein by spin filtering followed by in situ reduction with sodium borohydride to give the more stable hydroxy acid. To further elucidate vancomycin biosynthesis, J. A. Robinson and co-workers have developed a new procedure of producing vancomycin-aglycone-peptide carrier domain (PCD) conjugates (Chem. Commun., 2003, 2718). Reaction of coenzyme A thioester of vancomycin aglycone with an apo-PCD from E. coli is catalysed by phosphopantetheinyl transferase Sfp from B. subtilis leading to rapid formation of the conjugate (Scheme 3). After purification, the vancomycin-aglycone-PCD conjugate was characterized by electrospray mass spectroscopy.
 | ||
| Scheme 3 | ||
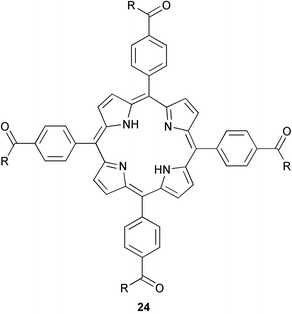
Control of polyketide chain length by the CLF subunit of the dimeric ketosynthase has recently been established (J. Am. Chem. Soc., 2003, 125, 12708). Using structure-based mutagenesis, C. Khosla and co-workers have identified certain residues in the CLF subunit which when altered lead to different polyketide chain lengths. They surmise that reducing the size of these key residues leads to an increase in size of the polyketide channel allowing two more chain-extending cycles to take place. In an effort to understand how small molecules bind to potassium channels, S. N. Gradl et al. have synthesized new four fold symmetrical tetraphenylporphyrin derivatives 24 and studied their interaction with the human Kv1.3 channel (J. Am. Chem. Soc., 2003, 125, 12668). Displacement assays showed these compounds to have Ki values in the submicromolar range and subsequent electrophysical experiments suggest these compounds bind in the outer vestibule of the channel. The authors plan to synthesize a new class of compounds based on this structure for the ultimate goal of developing novel therapeutic agents to treat autoimmune disorders.
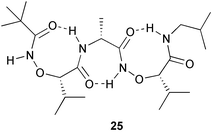
To allow the study of electron transfer in proteins, a redox active amino acid, 3,4-dihydroxyphenylalanine (DHP) has been efficiently and selectively incorporated into proteins in E. coli (P. G. Schultz and co-workers, J. Am. Chem. Soc., 2003, 125, 14662). Under anaerobic conditions, cyclic voltammetry was used to effectively demonstrate the activity of myoglobin containing DHP and its effect on the redox potential of the Fe(III) heme group. A new strategy to induce γ-turns in short peptides has been developed (D. Yang et al., J. Am. Chem. Soc., 2003, 125, 13018). Incorporation of α-aminoxy acids immediately after the α-amino acid of interest results in the formation of an eight membered ring intramolecular hydrogen bond (N–O turn) which in turn leads to formation of the γ-turn 25.
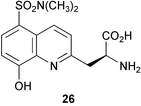
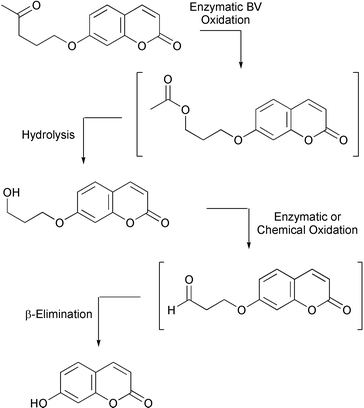
The first fluorogenic assay for detecting Baeyer–Villigerase activity in microbial cells has recently been reported (M. C. Gutierrez et al., Org. Biomol. Chem., 2003, 1, 3500). The assay uses 4-oxopentyl umbelliferyl ether as the test ketone for Baeyer–Villigerase activity (Scheme 4). On oxidation, the resulting ester undergoes spontaneous or enzymatic hydrolysis. The subsequent alcohol can be either enzymically or chemically oxidised and the aldehyde formed β-eliminates to give the fluorescent product, umbelliferone. The assay was tested on a series of different microorganisms and the results of these were confirmed by HPLC analysis. A fluorescence assay has also been developed to detect protein kinase activity (B. Imperiali and co-workers, J. Am. Chem. Soc., 2003, 125, 14248). A series of kinase probes were prepared containing the amino acid, Sox 26 which, on binding of Mg2+ generates the fluorescence signal. The Sox 26 is separated from the amino acid residue to be phosphorylated by a β-turn sequence that provides a binding pocket for the Mg2+. These probes were shown to be good substrates for their respective enzymes and this technique is applicable to threonine and tyrosine kinases as well as the usual serine kinases.
 | ||
| Scheme 4 | ||
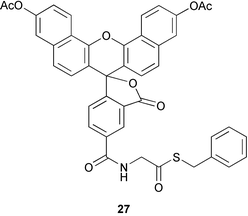
A series of small molecule probes have been synthesized for site-specific labelling of N-terminal cysteine-containing proteins (D. S. Y. Yeo et al., Chem. Commun., 2003, 2870). The probes (e.g.27) all contain a thioester functional group that undergoes reaction with an N-terminal cysteine either in vitro with purified proteins or in vivo with live bacterial cells. In vitro testing of all the probes showed 50% labelling after only 30 minutes which means this strategy should be suitable for real-time bioimaging experiments in live cells.
| This journal is © The Royal Society of Chemistry 2004 |