Investigations of the marine flora and fauna of the Islands of Palau
D.
John Faulkner
a,
David J.
Newman†
*b and
Gordon M.
Cragg
b
aScripps Oceanographic Institution, La Jolla, CA 92093, USA
bNatural Products Branch, Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, NCI-Frederick, Frederick, MD 21702, USA
First published on 23rd December 2003
Abstract
Covering: 1970s to July 2003
The Islands of Palau have proven to be an excellent source of bioactive marine natural products primarily as a result of the systematic studies from the late 1970s by the research groups of Scheuer at the University of Hawaii, Faulkner at the Scripps Oceanographic Institution/University of California at San Diego, and Paul at the University of Guam. Their efforts were materially aided by the excellent facilities provided by the Government of Palau and for the last 10 years, those of the NCI's shallow water collection contractor, the Coral Reef Research Foundation. This review covers the structures and biological activities where noted, of the multitudinous marine-derived natural products isolated from the marine flora and fauna of this nation and demonstrates the enormous variety of novel structures elaborated by these organisms.
 D. John Faulkner | D. John Faulkner, born in England in 1942, received his BSc and PhD degrees from Imperial College, London, where he studied synthetic organic chemistry under the guidance of Sir Derek Barton. He received postdoctoral training from R. B. Woodward at Harvard University and W. S. Johnson at Stanford University before joining the faculty of the Scripps Institution of Oceanography, University of California at San Diego, in 1968. Recognizing the need to do something more marine, he began a new career in marine natural products chemistry. When John died in November 2002 he was Professor of Marine Chemistry. |
 David J. Newman | David Newman was born in Grays, Essex, UK, and received his D. Phil. in microbial chemistry from the University of Sussex in 1968. Following two years of postdoctoral studies on the structure of electron transport proteins at the University of Georgia, USA, he worked for a number of US-based pharmaceutical companies in natural products discovery programs, and joined the Natural Products Branch of the NCI in 1991. His scientific interests are in the discovery and history of novel natural products as drug leads in the anti-infective and cancer areas. In conjunction with Gordon Cragg, he has established collaborations between the National Cancer Institute and organizations in many countries promoting drug discovery from their natural resources. He is responsible for the marine and microbial collection programs of the NCI and in concert with Gordon Cragg (Chief, NPB), for the NCI's Open and Active Repository programs. He is both an UK Chartered Chemist and an UK Chartered Biologist and has published over 70 papers related to these interests. |
 Gordon M. Cragg | Gordon Cragg was born in Cape Town, South Africa, and obtained his D. Phil. from Oxford University in organic chemistry in 1963. After two years of postdoctoral research in natural products chemistry at the University of California, Los Angeles, he returned to South Africa where he held several academic positions before returning to the United States in 1979 to join the Cancer Research Institute at Arizona State University. In 1985, he moved to the National Cancer Institute in Bethesda, Maryland, and was appointed Chief of the Natural Products Branch in 1989. His major interests lie in the discovery of novel natural product agents for the treatment of cancer and AIDS. In 1991 he was awarded the National Institutes of Health Merit Award for his contributions to the development of the anticancer drug, taxol, and in 1998 he was elected President of the American Society of Pharmacognosy. He has established collaborations between the National Cancer Institute and organizations in many countries promoting drug discovery from their natural resources. He has published over 100 papers related to these interests. |
1 Introduction
With the untimely death of John Faulkner in November of 2002, following complications from cardiac surgery, the marine natural products world in particular and chemistry in general, lost one of its greatest exponents of the arcane art of structural determination of the extremely complex and unusual compounds elaborated by marine-dwelling organisms.John published approximately 300 scientific papers in the field of marine natural products chemistry and related topics and was instrumental, together with his colleagues at The Scripps Institute of Oceanography, where he spent all of his time after post-doctoral studies at Harvard and Stanford, in demonstrating the exquisite chemistry that marine organisms of all types elaborate as mechanisms of attack and defence.
Although not able to dive due to medical restrictions, he was an avid snorkeller and collector and was able to gather around him a large number of very talented graduate students, post-doctoral fellows, collaborators (in particular, Bob Jacobs at the University of California, Santa Barbara) and colleagues who collected marine organisms in a variety of places in the Pacific, but notably in the Islands of Palau.
This review is based on a report that John initially started with a Palauan undergraduate student, Jason Kurtei, who spent a few months with John as part of a Scripps Undergraduate Research Fellowship Program in La Jolla in 1997, and was then further developed by John but never published.
With the express consent of Meryl Faulkner, John's widow, we have taken his original manuscript, extended it to July of 2003, shortened some of the entries, extended others, and then updated and included organisms that John did not cover in the original. We have tried to keep the same general format that was used in the original report, a short series of history and highlights and then what is effectively an annotated bibliography organized by phylum/organism, where structures are shown and biological activities are reported and commented on where necessary.
We (D. J. N. & G. M. C.) should emphasize from herein that any errors are ours, not his.
2 The Islands of Palau
The Islands of Palau (also known as the Republic of Belau) are found between 7° and 8° N Latitude and 133° to 134° E Longitude (cf.Fig. 1). Originally known as the Western Caroline Islands, following their discovery by Spain in the 1500s, there was no formal incorporation into a “Spanish Empire” until 1885, when the Pope confirmed Spain's rights to the islands. In the 300 years prior to this, both English and German seafarers had established first trading posts and then coaling stations. Following the Spanish–American war, in 1899 the Carolines were sold to Imperial Germany. In 1914, when Japan declared war on Germany, the German possessions in Micronesia came under Japanese control and then they continued under Japanese control as a League of Nations mandate, finally becoming fortified islands until the end of WWII. Following WWII and a very convoluted series of associations with Micronesia and the USA, Palau became an independent nation on October 1st, 1994 as the Republic of Belau. For further information, the reader should consult the excellent history of Palau by Mandy Etpison, the daughter-in-law of the third president of Belau.1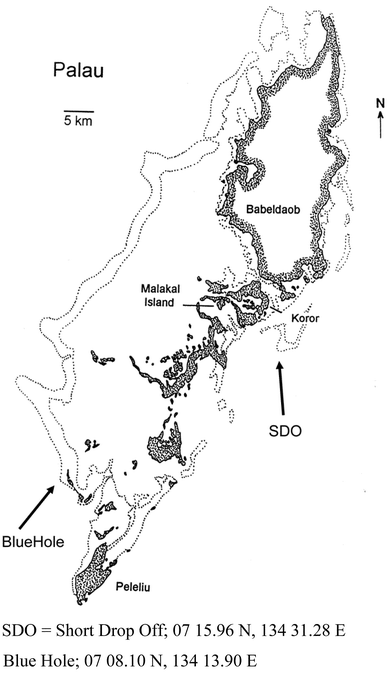 | ||
| Fig. 1 The Islands of Palau (pen and ink drawing courtesy of Dr and Mrs Patrick Colin, CRRF). | ||
Over the early part of the 20th Century, a variety of German and Japanese scientists had begun to investigate the flora and fauna of the islands, but it was really with the advent of SCUBA apparatus and the establishment of a relatively stable form of government after the 1960s that marine biologists and marine natural product chemists began to systematically study the fauna of these islands, and from 1979, John was one of these investigators.
3 Why investigate Palauan waters?
The scientific rationale is quite simple. Palau has the greatest diversity of marine habitats and organisms that can be packed into such a small geographical area. Although this sentence was originally written in 1997, the publication in 2002 by Hooper et al., confirmed this comment as in their report, the authors demonstrated that Palau, like the Great Barrier Reef in Australia, is a “biodiversity hotspot” with over 73% of the reported sponges demonstrating endemism when compared with other areas of the Pacific.2Add to this the availability of excellent diving resources, freezer facilities, and most of all, knowledgeable guides and you have an almost perfect location for the collection of marine specimens. Palau is served by reliable and frequent air transportation so that samples can be transported back to major research facilities in good condition. The laboratories of the Marine Mariculture Demonstration Center (MMDC) in the past and now those at the Coral Reef Research Foundation (CRRF, the current Collection Contractor for the NCI's shallow water collection programme) have allowed researchers to do more than just collect specimens – they can get their research started while still in a position to refine their collections for later study.
3.1 Palau's microenvironments lead to increased biodiversity
Most tourists visit Palau to dive along the precipitous reef walls that provide spectacular vistas of brightly colored fishes and the occasional thrill of encountering sharks, rays or turtles. While they are vaguely aware of brightly colored invertebrates packed along the vertical surfaces of these underwater cliffs, they seldom stop to wonder at the numbers of different plants and invertebrates to be found and give no thought at all to the distribution of the sessile inhabitants of the reef. To most divers, invertebrates are the background against which fish are photographed.Scientists see this panorama in a very different light. They notice that the community of sessile invertebrates changes somewhat with depth but often more depending on exposure to direct sunlight. The inhabitants of a pass or channel between reefs are different from those on the outer or inner faces of a reef due to changes in water flow and turbidity. Caves and tunnels provide a different set of inhabitants, which is often comprised of animals that would normally exist at much greater depths. Mud flats, mangrove swamps, shallow reefs, coral rubble and even manmade docks all provide different contributions to the overall marine biodiversity of an area. When you add to this catalog of microenvironments and their occupants, the unique communities that are found in the marine lakes of Palau, you have a paradise for scientists seeking a broad diversity of marine invertebrates. Palau, which is located not far from the presumed center of coral reef biodiversity, appears to have captured more than its fair share of invertebrate species, in particular, sponges.2
4 A brief history of marine natural product research in Palau
Marine natural product research started in the 1950s largely as a result of the availability of SCUBA but it did not really become a separate scientific discipline until about 1970, when it became an integral part of the search for new pharmaceutical agents. The search for “Drugs from the Sea” has been and still is the driving force behind the funding of research on the chemistry of marine organisms. To a lesser extent, funding agencies have also supported basic research on the chemical ecology of marine ecosystems, which concentrates on demonstrating how marine plants and invertebrates have benefited from producing toxic or deterrent chemicals.As far as we have been able to determine, the first groups to systematically collect marine organisms in Palau for chemical studies were those of Paul Scheuer from Hawaii, the Suntory Institute from Japan, and the Faulkner group from Scripps, who were housed at MMDC in the late 1970s and early 1980s. In 1979, Bill Fenical (from Scripps) led a research expedition to Palau aboard the R/V Alpha Helix but the collection of organisms was not the major activity of the expedition. The early studies led to the discovery of a number of anti-inflammatory agents, the most promising of which was manoalide. The US National Cancer Institute has had a long association with Palau, indirectly as a result of Bob Pettit's (Arizona State University) collections in the 1980s and now directly, as they support the professional collecting activities of the Coral Reef Research Foundation, who are based in Koror. Japanese scientists and ships have visited Palau quite frequently over the past 20 years but their activities have resulted in relatively few publications. For the past fifteen years, the majority of US supported natural product research from the waters of Palau has been reported by Paul Scheuer from Hawaii, Valerie Paul from Guam, and John Faulkner from Scripps.
5 Overview of therapeutic areas
5.1 Anti-inflammatory compounds
Collections from Palau played a key role in the US government's Sea Grant funded research to discover new anti-inflammatory agents from marine organisms. In particular, the discovery that manoalide 123 from the sponge Luffariella variabilis inhibited the enzyme phospholipase A2 (PLA2), which plays a key role in inflammatory conditions such as arthritis, allowed researchers to study the role of PLA2 in inflammation as well as providing the stimulus for further research to discover new agents. Despite initial expectations and hopes, manoalide was eventually dropped as a drug candidate but only after human trials had proved it to be ineffective, though there are still derivatives in preclinical and clinical studies.Manoalide is a major constituent of the sponge Luffariella variabilis that is one of the most common sponges on the reefs around Palau. The structure of manoalide was published in 1980 by Paul Scheuer's group, but without any biological activity testing. In the meantime Faulkner's group had isolated manoalide and supplied it to Robert Jacobs at the University of California, Santa Barbara, where it was first observed to be a pain-killer and was subsequently found to interrupt the biochemical sequence responsible for pain caused by inflammatory conditions such as insect stings, arthritis, burns, etc. In animal models, it was more effective than the best anti-inflammatory agents then available.
Since it was a novel agent, why did it not progress further? There were several reasons, some scientific and others more practical. The best scientific reason was that manoalide did not interact with the enzyme PLA2 in the usual manner. Rather than directly inhibiting the enzyme by binding at the active site, thus blocking its enzymatic activity, manoalide reacted with the surface of the enzyme and prevented the enzyme from moving across membranes. Thus it was neither a direct competitor nor an allosteric inhibitor. It also had other side effects as PLA2 was not the only enzyme that it bound to the surface of. Thus it was not an ideal drug candidate. In addition, since Scheuer had published the structure before any biological activity was identified, the natural product could not be patented, though a “use patent” could be awarded.
Allergan Pharmaceuticals embarked on a program to synthesize compounds that resembled manoalide, in the hope that a synthetic compound would be better than manoalide and to a certain extent they succeeded. As part of their development strategy, Allergan decided to perform a clinical trial to determine whether manoalide could be used topically for the treatment of psoriasis but that trial was not successful. Although the unsuccessful clinical trial sealed the fate of manoalide as a pharmaceutical, it has continued to be used as a biochemical reagent to block the action of phospholipase A2 and is essentially a standard reagent for this purpose.
A second anti-inflammatory agent resulted from a collaboration between academic scientists and a small biotechnology start-up company, OsteoArthritis Sciences Inc., and was again sponsored by Sea Grant. It was found that debromohymenialdisine (DBH) 164, which is a major metabolite of the sponge Stylotella aurantium, which is very common in the shallow waters of Palau, could be successfully used to treat osteoarthritis in laboratory animals. Progress in the development of DBH appeared to be going very well when the company was suddenly closed down.
One problem in the further development of this agent is the justifiable position by the University of California that any developer must accept an obligation to share benefits arising from the marketing of DBH with the country of origin, which in this case is Palau. This is also the case with any agent identified by investigators using any of the materials collected by CRRF as part of their NCI collection contract. DBH has been reported from other sources and under the NCI programme, some benefit would have to be paid even if made by total synthesis. The ultimate fate of this agent/patent is unknown.
Although manoalide and DBH represented the best opportunities to date for development of a pharmaceutical product from Palauan marine specimens, they are by no means the only chemicals to be studied in depth by pharmacologists and molecular biologists. Palauan specimens have provided more than fifty compounds that have been studied to some level or another as potential anti-inflammatory agents by academic and industrial pharmacologists. The contributions of these studies to basic science have been extremely valuable and cannot be overlooked.
5.2 Studies of anti-cancer agents
Many of the metabolites reported from Palauan specimens are cytotoxic in as much as they kill cancer cells in tissue culture (in vitro), but very few of these compounds have proved successful in animal models (in vivo). The underlying problem is that many cytotoxins are toxic both to normal cells and cancer cells and would therefore be as damaging to the patient as to the patient's tumour. Therefore, scientists seek compounds that show selective toxicity toward the cancer cells. It is important when reading the annotated bibliography to differentiate between compounds that show real promise for the treatment of cancer (in vivo activity) and those that are reported as cytotoxic (in vitro activity).There are compounds from sponges collected in Palau that have undergone preliminary in vivo testing in animal models but, at present, no compounds from Palauan marine organisms have been/are in clinical trials as anti-cancer agents, though the number of novel structures being reported, particularly from cyanophytes (see below in the annotated bibliography), bodes well for the future.
5.3 Contributions to cellular biology
During the past few years, many cellular biologists have realized the value of marine natural products as biological probes that can be used to inhibit specific enzymes and thereby discover the inner workings of cells. They have gone from using pure compounds that were (sometimes) identified in pharmaceutical screens to the testing of extracts of marine organisms in assays developed to study specific organelles or processes that are essential to cellular organization. Two successes have been recorded in this area, ilimaquinone 98 and adociasulfate-2 170.Ilimaquinone, which was first isolated from the Palauan sponge Hippospongia metachromia by Paul Scheuer's group, causes the Golgi apparatus to break down into small particles in the same manner that it does during cell division. Collaborative research between the Faulkner laboratory at Scripps and the group of Vivek Malhotra at UCSD Biology Department has led to a better understanding of how the Golgi maintains its essential 3-dimensional structure within the cell.
Collaboration between the Faulkner laboratory and the group of Larry Goldstein at the UCSD Pharmacology Department led to the discovery of the first inhibitor of motor proteins, which are used to transport peptides along a network of microtubules from the Golgi to their final destination. The inhibitor, adociasulfate-2 (AS-2), was obtained from a Haliclona (aka Adocia) species of sponge that was collected in Palau.
6 Studies on the role of chemistry in symbiosis
This is a new area of study that has clearly benefited from the ability to do laboratory research in Palau. Carole Bewley was able to separate symbiotic microorganisms from sponge cells while in Palau.3 On her return to Scripps, she performed chemical analyses on the cell fractions and was able to demonstrate that the biologically active constituents of the sponge Theonella swinhoei were in fact produced by symbiotic microorganisms. Further research by Eric Schmidt has resulted in identification of one of the symbiotic microbes using DNA analysis after demonstrating that the microbe can grow in medium supplemented with a piece of the host sponge but not in pure culture medium (pers. comm.). Further work by Christine Salomon on the localization of dercitamide4 and cyclic peptides5 are also examples of this approach. These and others are discussed under the specific organism headings below. This essentially new approach to the problem of finding out how and why symbiotic associations occur could not have been contemplated without the proximity of laboratory facilities to the collecting site.7 Organisms (organized by Phylum, Class, Order and alphabetized by Genus/species)
In these subsequent sections, the common names of most of the dive sites, their Palauan names and the GPS coordinates are shown in Fig. 1 (the Islands themselves), Fig. 2 (common dive sites, frequently on the Eastern side of the larger islands) and in Table 1. All geographic information and maps were provided by Dr and Mrs Patrick Colin of the Coral Reef Research Foundation.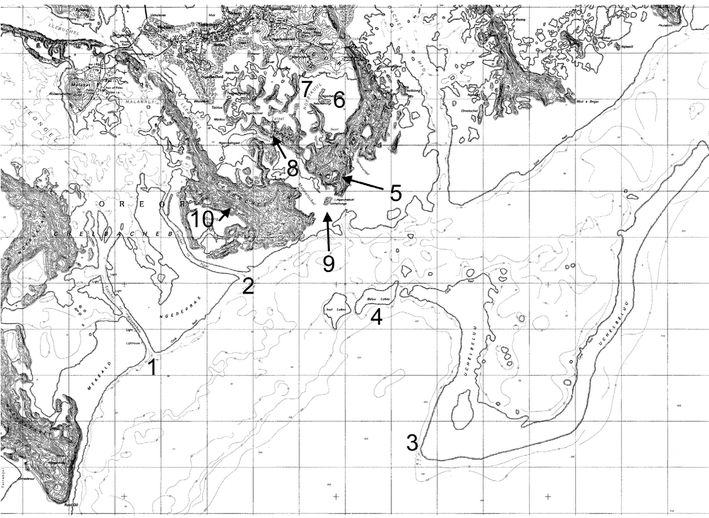 | ||
| Fig. 2 Selected dive sites in Palau. | ||
| Site | Proper Palauan name | Review names | Latitude | Longitude |
|---|---|---|---|---|
| a Very large island, cannot give a specific GPS coordinate. | ||||
| 1 | Lighthouse Channel (Toachel ra Kesebekuu) | Lighthouse Channel | 07 17.03N | 134 27.82E |
| 2 | Ngel Channel (Toachel ra Ngel) | Ngell Channel | 07 18.48N | 134 28.12E |
| 3 | Short Drop Off (Uchelbeluu Reef) | Short Drop Off or Agulpelu Reef | 07 15.96N | 134 31.28E |
| 4 | Bab el Lukes Reef | Babelukes Reef | 07 17.58N | 134 30.59E |
| 5 | Goby Lake | Big Goby Marine Lake | 07 18.76N | 134 30.10E |
| 6 | Iwayama Bay | Iwayama Bay, Arumizu Bay, Nikko Bay | 07 19.01N | 134 29.83E |
| 7 | Hotel Nikko Dock | Hotel Nikko Dock | 07 20.25N | 134 29.60E |
| 8 | Kaibakku Lake | Kaibakku Marine Lake | 07 19.47N | 134 29.42E |
| 9 | Turtle Island (Ucheliungs) | Turtle Island | 07 18.56N | 134 30.10E |
| 10 | Risong Lake | Risong Marine Lake | 07 18.74N | 134 28.04E |
| 11 | Siaes Tunnel | Siaes Tunnel | 07 18.76N | 134 13.50E |
| 12 | Ulong Channel (Ngerumekaul) | Ulong Channel (Aulong Channel) | 07 16.99N | 134 14.71E |
| 13 | Ngemelis Drop Off | Ngemelis Drop Off | 07 06.95N | 134 14.44E |
| 14 | Wonder Channel | Wonder Channel | 07 10.83N | 134 21.67E |
| 15 | Ngeruktabel Island | Urukthapel Island | nonea | none |
| 16 | Ngeruktabel Lake | Ngeruktebel Marine Lake | 07 16.80N | 134 25.92E |
| 17 | Beduliaes | Peduliaes Headland | 07 20.20N | 134 25.70E |
7.1 Chordata, Ascidacea, Enterogona
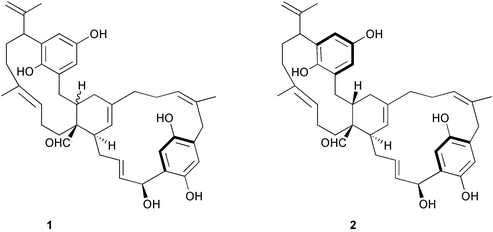
Chemistry/bioactivity: longithorols A 1 and B 2 were isolated as their pentaacetates because of stability problems and are the first examples of hydroquinones from this chemical class. They are easily converted to the corresponding quinones by exposure to air and thus no bioactivities have been reported for longithorols A and B.6
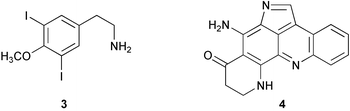
Chemistry/bioactivity: Didemnum rubeum contained large amounts of 3,5-diiodo-4-methoxyphenyl-ethylamine 3 and plakinidine D 4.7 Plakinidine D 4 was also obtained from an Indonesian Didemnum sp.7 and the isolation of both 3 and 4 was simultaneously reported from the same species by Ford and Davidson8 from samples collected in the Marshall Islands. The only reported activity was an in vitro study showing cytotoxicity of 4 against HCT-116 at 5 µg mL−1.
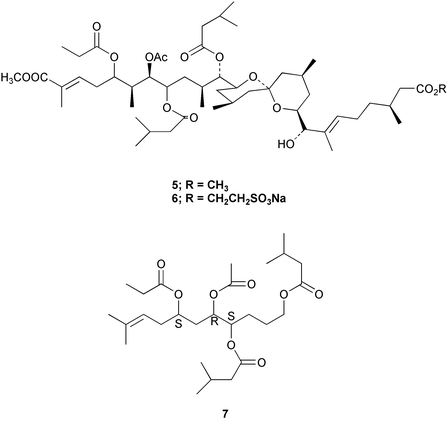
Chemistry/bioactivity: the first sample to be studied had been stored in methanol for about 10 years and when extracted gave two terpenoids, didemnaketals A and B 5.9 However, when a freshly collected specimen was investigated, only didemnaketal C 6 was isolated, which led to the conclusion that didemnaketals A and B were artifacts of the storage and extraction processes.10 The full stereochemistry of didemnaketal C 6 has not yet been elucidated. Didemnaketals A and B inhibited the enzyme, HIV-1 protease9 and though several protease inhibitors are now available, the didemnaketals were not considered drug candidates because the ester groups are too easily hydrolyzed. However, although these compounds may well be isolation artifacts, their basic structural motif has been used as the starting point for simplified analogues that appear to inhibit HIV protease by an unusual mechanism, inhibition of dimerization of the inactive HIV monomers to give the active dimeric HIV protease albeit with the most active, 7 having a high in vitroKi of 2.1 µM.11
Chemistry/bioactivity: the organic extract of this ascidian was cytotoxic in the NCI 60 cell line panel and from this material, three new β-carboline alkaloids, 2-methyleudistomin D 8, 2-methyleudistomin J 9 and 14-methyleudistomin C 10 were isolated and identified by following their bioactivities against four cell lines. In addition to these new derivatives, six known eudistomins were also found, eudistomins C, D, E, J, K and L. Of the three new compounds, 14-methyleudistomin C exhibited the most potent in vitro activity, with IC50 values of < 1 µg mL−1 against the test lines.12
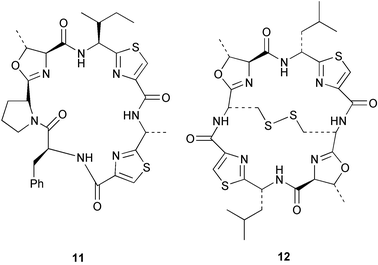
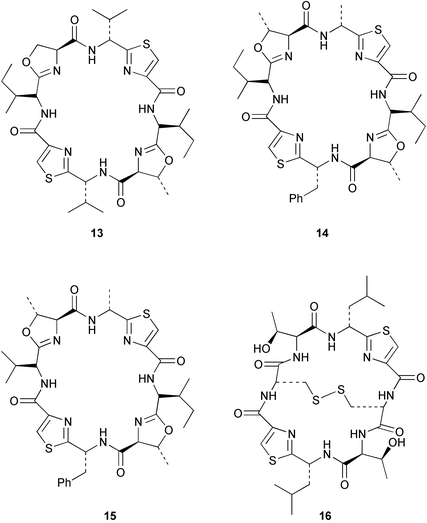
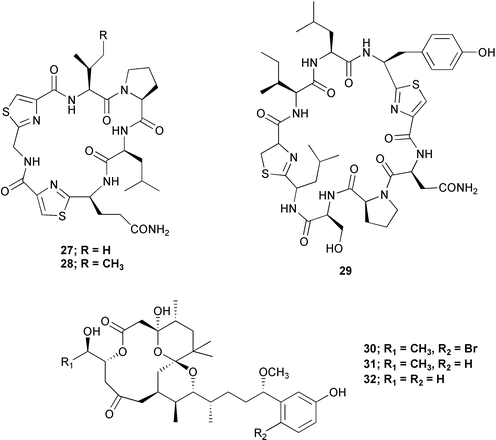
Chemistry/bioactivity: Palauan specimens of L. patella are the source of ulicyclamide 11 and ulithiacyclamide 12,14 patellamides A–C 13–15,15 preulithicyclamide 16,16 and several other closely related cyclic peptides.17,18 Most of the cyclic peptides are cytotoxic to various degrees with ulithiacyclamide 12 being the most potent.15 This compound also inhibits the Macrophage Scavenger Receptor, resulting in inhibition of the progress of atherosclerotic lesions, with an IC50 of 98 nM.16
Cellular location studies: there had been considerable discussion in the literature as to whether these peptides were produced (at least in part) by the cyanophyte but variable results were reported. In 2002, Salomon and Faulkner, using the on-site facilities of the Coral Reef Research Foundation on Koror, were able to carefully dissect examples of L. patella and subsequently show that the peptides were distributed throughout the tunic of the ascidian and were not found in any detectable quantity in intact Prochloron cells.5
These findings do not demonstrate the source of the peptides but do open up a series of questions as to how peptides could migrate from the cyanophyte into the lower tunic.
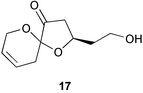
Chemistry/bioactivity: an unusual spiroketal, lissoketal 17, was isolated from this organism but no bioactivity data was reported.19
7.2 Cnidaria, Alcyonaria, Alcyonacea
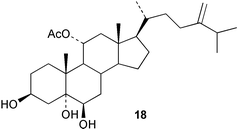
Chemistry/bioactivity: two new sterols, 11 α-acetoxy-24-methylenecholesta-3β,5α,6β-triol 18 and 11α-acetoxy-24R-methylcholesta-3β,5α,6β-triol were isolated from the soft coral. No bioactivity data was reported by the authors.20
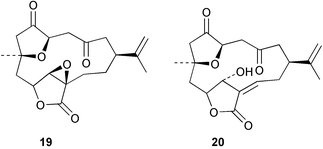
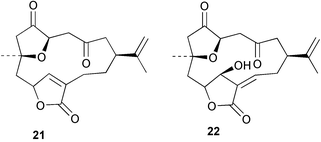
Chemistry/bioactivity: S. inelegans contained two new norcembrane diterpenes 19 and 20 with the structure of 19 being determined by X-ray crystallography. No bioactivity data was reported by the authors.21
Chemistry/bioactivity: S. numerosa contained one new norcembrane diterpene 21 and the known norcembenolide 22.22 As with 19 above, the structure of 21 was determined by X-ray crystallography.21
Chemistry/bioactivity: S. querciformis contained the known norcembenolide 22 and one new norcembrane diterpene 23, but no bioactivity information was presented.21
Chemistry/bioactivity: the Sinularia sp. contained the new norcembrane diterpene 21 but no bioactivity information was presented.21
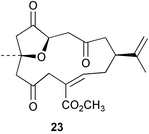
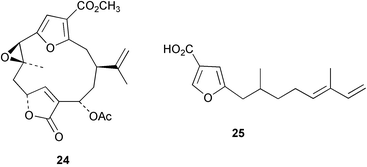
Chemistry/bioactivity: this Sinularia sp. contained 13α-hydroxypukalide 24, first isolated from S. polydactyla by Bowden et al.23 and the known24 furanosesquiterpene acid 25.25 13α-hydroxypukalide was reported to inhibit settlement of the blue mussel Mytilus edulis at 0.1 µg mL−1.
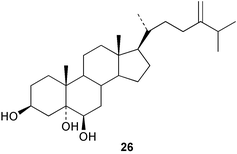
Chemistry/bioactivity: this Sinularia sp. contained five related steroidal triols (e.g.26) 24-methylenecholesta-3β,5α,6β-triol, which inhibited settlement of the blue mussel Mytilus edulis at a dose of 700 µg mL−1.26
7.3 Cyanophycota, Cyanophyceae, Nostocales
Chemistry/bioactivity: following methanolic extraction and subsequent ethyl acetate partitioning, the ethyl acetate soluble material was fractionated following activity in an HIV-integrase inhibition assay. This led to the isolation and identification of the known cyclic peptide, dolastatin 3 27 as an inhibitor of the integrase with an IC50 value of 5 mM. What was of significant interest was the discovery that this agent and other similar molecules, appear to bind to plastic wells and pipette tips. Thus concentrations delivered are probably not those reported. Two other peptides that appeared to have little to no activity in this assay were also isolated and purified, homodolastatin 3 28 and kororamide 29. In addition to these peptides, the known cytotoxic metabolites, aplysiatoxin 30, debromoaplysiatoxin 31 and oscillotoxin 32 were also isolated and purified.27
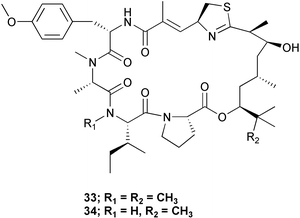
Chemistry/bioactivity: the lipophilic extract of this material was cytotoxic to KB and LoVo cell lines and following conventional isolation techniques, two very cytotoxic peptides were purified. One was apratoxin A 33 previously reported from a Lyngbya sp. collected in Apra Harbor, Guam and the other, apratoxin C 34 was novel. In both cases, their activities (IC50 values) against KB and LoVo were at the sub-nanomolar level.28
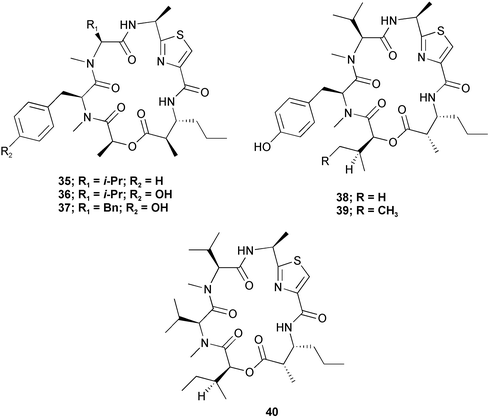
Chemistry/bioactivity: six closely related cyclic peptides, ulongamides A–F 35–40 were isolated from these samples of Lyngbya. Unlike the apratoxins with which they share the same host, and which have cytotoxic activity at the sub-nanomolar level, five of the six compounds (A–E) are cytotoxic (IC50 values) to KB/LoVo at 1–5 µM, whereas the sixth, (F), the only one without an aromatic side-chain, is not active below 10 µM.29
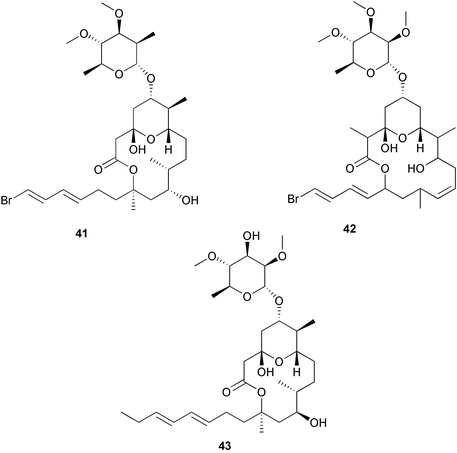
Chemistry/bioactivity: in addition to the apratoxins, ulongamides and lyngbyapeptins, this collection also yielded an unusual brominated glycosidic macrolide that was named lyngbyaloside B 41 due to its close similarity to the previously reported lyngbyaloside 42. It is also a structural analogue of the recently reported lyngbouilloside 43 that was isolated by Gerwick's group from the cyanophyte, L. bouillonii and on re-examination of the voucher specimen, this producing organism matches the description for L. bouillonii. Lyngbyaloside was weakly cytotoxic against KB (IC50 of 4.3 µM) and roughly four-fold less active versus LoVo.30
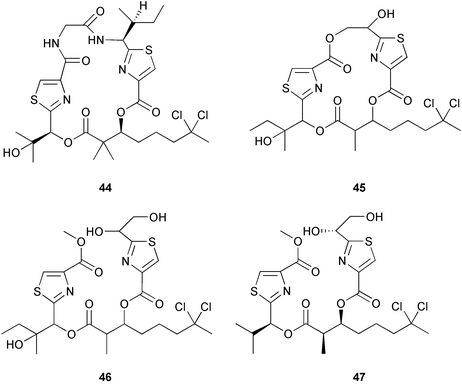
Chemistry/bioactivity: all isolations were followed using cytotoxicity against either KB or LoVo cells as the initial indicator. Whilst investigating the apratoxin producers from Guam, lyngbyabellin A 44 was found.31 This compound, together with a weaker (IC50 values of 2–5 µM) cytotoxic analogue, named lyngbyabellin C 45 were isolated from the Short Drop Off collection. What is of interest is that during the isolation of the latter compound, a methanolysis of one of the ring ester groups occurred, giving rise to a linear compound that was named as homohydroxydollabellin 46 because of its resemblance to the D. auricularia metabolite, dollabellin 47 reported by Yamada's group in 1995.32 Since in the Palauan case, the linear compound had effectively the same cytotoxicity as the cyclic precursor, and methanol was used extensively in the isolation of dollabellin, it is probable that this compound is an artifact of the isolation protocol and thus demonstrates that the actual source should be searched for once the material has been isolated from its host.
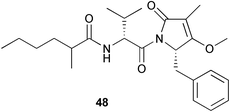
From the “mixed collection” another quite cytotoxic material (IC50's in the 0.4 to 1 µM range) was obtained. It was not encountered in any of the Guamanian collections and in only the one Palauan sample. This was the mixed amide-imide compound that was given the trivial name of palau'imide 48.31
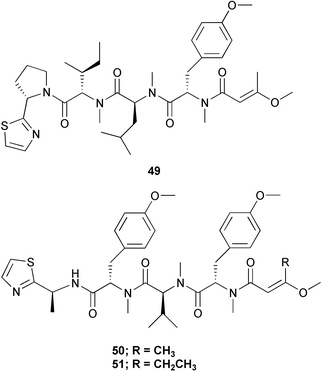
Finally, from the work-up of all of these compounds, two non-cytotoxic tetrapeptides related to the known lyngbyapeptin A 49, lyngbyapeptins B 50 and C 51 were isolated and purified from side-cuts. These latter two were not reported from any of the apratoxin-containing Guamanian samples, nor was the former compound found in the Palauan samples.31
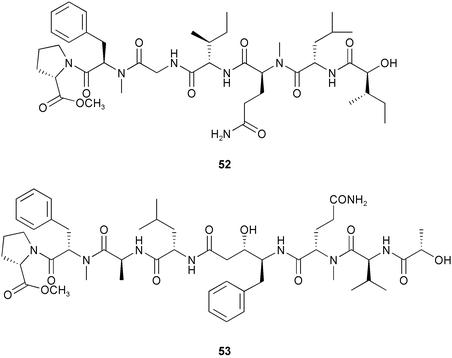
Chemistry/bioactivity: the lipophilic extract of this material demonstrated potent solid tumour selectivity and following bioactivity-driven isolation, the linear peptide tasiamide 52 (now tasiamide A) was isolated and purified demonstrating cytotoxicity against both KB and LoVo cells in the µg mL−1 range.33 This linear peptide's closest match in cyanobacterial metabolites is malevamide A which shares approximately half of the sequence. Very recently, further workup of the aqueous extract of this same collection by following bioactivity against the KB cell line yielded the linear peptide tasiamide B 53 containing the unusual amino acid, 4-amino-3-hydroxy-5-phenylpentanoic acid, that had previously been reported as a component of protease inhibitors from Candida and Streptomyces spp.34
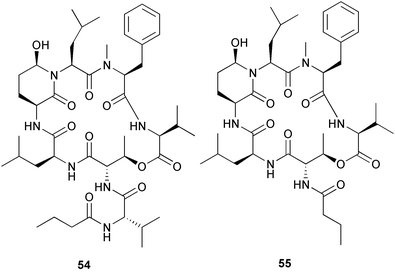
Chemistry/bioactivity: in contrast to tasiamide, the two cytotoxic peptides that were purified from this preparation were in the hydrophilic fraction. These two closely related cyclic peptides were named tasipeptin A 54 and tasipeptin B 55 and demonstrated activity against KB cells with IC50 concentrations at the 1 µM level.35
7.4 Miscellaneous bacteria/fungi
Chemistry/bioactivity: the culture filtrate contained the known compound griseoluteic acid and three new phenazine derivatives, pelagiomicins A 56–C. Of these, pelagiomicin A was reported to exhibit antimicrobial and antitumour activity.36
Chemistry/bioactivity: following isolation and subsequent fermentation in Difco marine broth, this bacterium, Pseudoalteromonas sp. F-420, produced a new lipid metabolite, korormicin 57 that was active against marine (halophilic) Gram-negative bacteria, but was not active against marine (halophilic) Gram-positive bacteria and terrestrial microorganisms irrespective of Gram type.37 This molecule might serve as a method of defining such Gram-negative marine microbes.
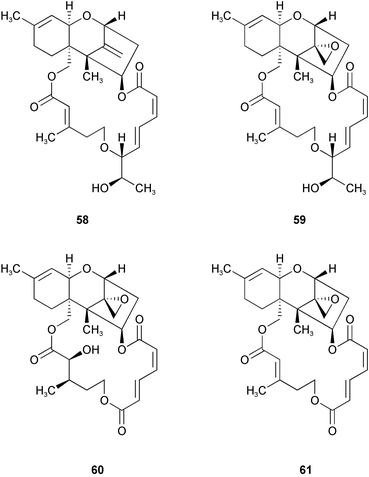
Chemistry/bioactivity: the fungus was cultivated in half-strength potato-dextrose medium in seawater and the materials isolated from an acetone/ethyl acetate treatment/extraction following their bioactivity against HL-60 and L1210 tumour lines. One new trichothecene, 12,13-deoxyroridin E 58 and three previously described trichothecenes, roridin E 59, verrucarin A 60 and verrucarin J 61 were also isolated. The novel derivative of roridin E was about 80 fold less active than the parent epoxy-containing roridin E.38
7.5 Mollusca
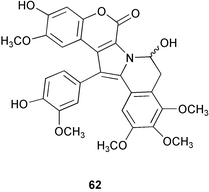
Chemistry/bioactivity: lamellarins A 62–D were isolated from specimens of Lamellaria sp. and the structure of 62 was determined by X-ray crystallography.40 Subsequently, the same compounds were isolated from the ascidian upon which the mollusc was feeding. These compounds inhibited the cell division of fertilized sea urchin eggs.
7.6 Mollusca, Gastropoda, Nudibranchia
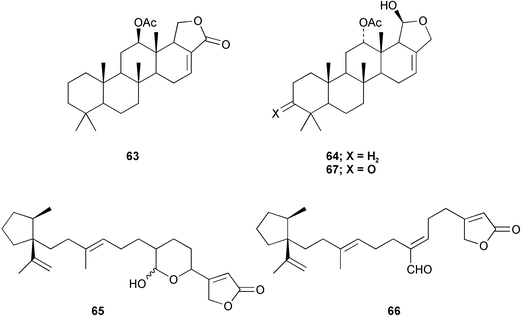
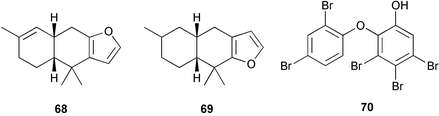
Chemistry/chemical ecology: the specimens from the marine lake contained 12-epi-scalarin 63, deoxoscalarin 64, luffariellins C 65 and D 66, and 3-ketodeoxoscalarin 67, which are all considered to be metabolites of dictyoceratid sponges. In contrast, the specimens from Iwayama Bay contained furodysin 68, furodysinin 69, and their singlet oxygen oxidation products, and the pentabrominated biphenyl ether 70, all of which have been found in species of Dysidea from the same location.41,42 Dorid nudibranchs are known to sequester allelochemicals from their sponge diets and use them for their own defense.
From this study one may conclude that the ability of C. funerea, and dorid nudibranchs in general, to distinguish allelochemicals from other metabolites is not dependent on the nudibranch being adapted to a specific food source.
7.7 Porifera
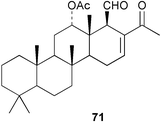
Chemistry/bioactivity: the specimen(s) contained six scalarin-type sesterterpenes (e.g.71),43 three of which had been described previously from an Australian Lendenfeldia sp.44 and it was reported that 24-methylscalaradial 71 inhibited platelet aggregation with an IC50 of 0.5 µg mL−1.
7.8 Porifera, Demospongiae, Agelascida
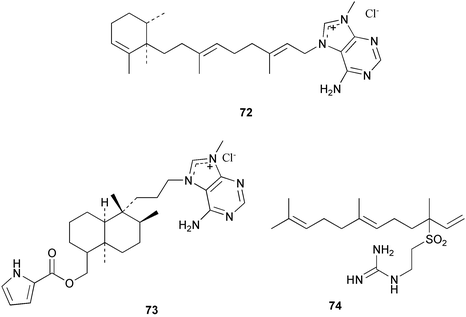
Chemistry/bioactivity: the Agelas sp. contained agelines A 72 and B 73, and agelasidine A 74 in good yields but they were difficult to separate.46 Agelines A and B are quaternary 9-methyladenine salts of diterpenes that are unstable in basic conditions whereas agelasidine A is a taurocyamine derivative of a sesquiterpene. Compounds 72 and 73 were also reported from A. nakamurai. Compounds 72 and 73 were antimicrobial and ichthyotoxic, and although the crude extract of the sponge was cytotoxic, neither of these compounds was active. Related compounds from A. mauritiana and A. nakamurai were reported to be cytotoxic and to inhibit the enzymatic activity of Na/K ATPase. There are no known uses and the instability of agelines A and B and related salts makes them unlikely candidates for commercial development.
7.9 Porifera, Demospongiae, Astrophorida
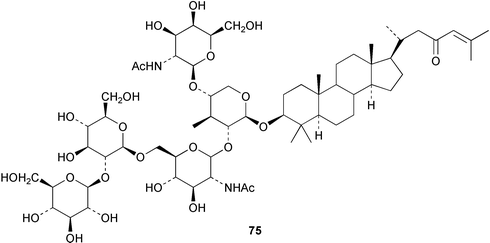
Chemistry/bioactivity: a total of nine closely related saponins called sarasinosides A1, 75, A2, A3, B1, B2, B3, C1, C2, and C3 were isolated.47,48 These saponins consist of 4 or 5 sugar units, two of which are amino-sugars, linked to a 14-nor-lanostane triterpenoid and are somewhat similar in structure to the saponins found in sea cucumbers. Like all saponins, the sarasinosides are ichthyotoxic (the saponins are thought to cause hemolysis of gill tissues) and are cytotoxic due to their action on cell membranes, where they cause irreversible non-specific lysis.
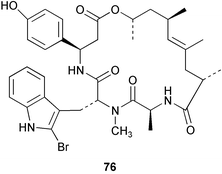
Chemistry/bioactivity: this Jaspis sp. contains jaspamide 76 as the major metabolite49 which is also known as jasplakinolide when isolated from a Fijian-derived sample of Jaspis johnstoni.50 Modified jaspamides have been reported from the sponge Jaspis splendens collected in Vanuatu.51 Jaspamide was initially reported to be a potent insecticide and antifungal agent49,50 but subsequent work by NCI scientists demonstrated that it induces actin polymerization in vitro leading to disruption of the actin skeleton.52,53 However, in spite of many attempts at NCI, no reproducible in vivo antitumour activity could be demonstrated due to the therapeutic index being very close to unity. In this respect, it is similar to the effects seen with other actin inhibitors such as the latrunculins and cytochalasins.
The question as to the actual source of these molecules is open to debate as very similar molecules, the geodiamolides, were first reported54 from a Geodia sp. in 1987 and from other taxonomically distant sponges in following years,55 Similar molecules, the chondramides, with an 18 membered macrolide ring, were reported from a terrestrial myxobacteria, by the Reichenbach group in 1995,56 with actin stabilizing activity of these compounds being reported in 1998.57
Thus, is the sponge the actual source of the jaspamides or merely a host (commensal or co-metabolic) to microbes that produce these peptides?
7.10 Porifera, Demospongiae, Axinellida
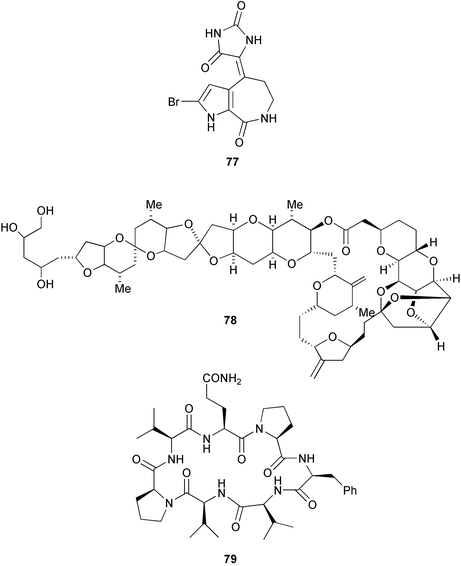
Chemistry/bioactivity: the first metabolites to be reported from this sponge were hymenialdesine, debromohymenialdesine (see Stylotella aurantium) and axinohydantoin 77, which possibly resulted from hydrolysis of hymenialdesine.58 Bioassay-guided fractionation using a leukemia cell line resulted in the isolation of very small quantities of halichondrin B 78 and homohalichondrin B, which had previously been obtained from a Japanese Halichondria sp., and three new cyclic peptides, axinastatins 1 79, 2 and 3.59,60 Although discovered using an anti-leukemia assay, the axinastatins were cytotoxic against several tumour cell lines including representative ovarian, CNS, renal, lung, colon and melanoma lines. Following the syntheses a few years later,61,62 the original report of the bioactivity of the axinastatins was questioned when it was found that the synthetic compounds had only a fraction of cytotoxicity claimed for the natural products, but the reason for the discrepancy is not known. Finally, a synthetic derivative of halichondrin B is currently in Phase I clinical trials for cancer.
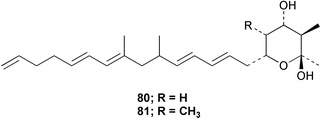
Chemistry/bioactivity: the sponge contained two homologous cyclic hemiketals 80 and 81 but without any reported bioactivity.63
7.11 Porifera, Demospongiae, Dendroceratida
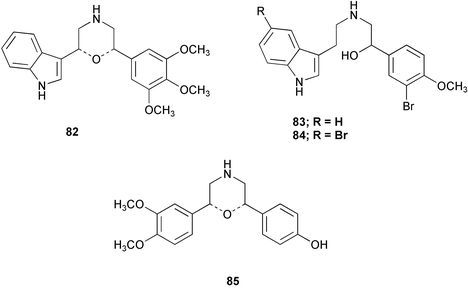
Chemistry/bioactivity: after reidentification of the sponge, four alkaloids, chelonin A 82, chelonin B 83, bromochelonin B 84 and chelonin C 85 were reported as metabolites of Chelonaplysilla sp.64 Compounds 82–84 exhibited antimicrobial activity against Bacillus subtilis and in addition, chelonin A 82 demonstrated in vivo antiinflammmatory activity.
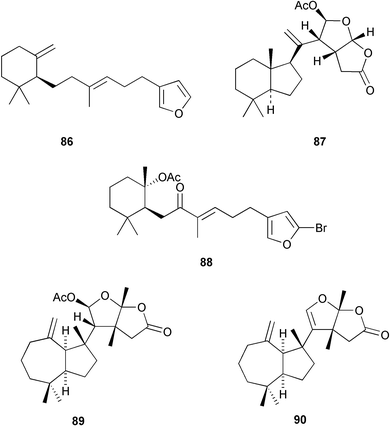
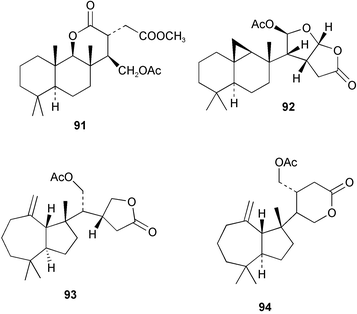
Chemistry/bioactivity: the 1981 collection contained dehydroambliol A 86, previously described from Dysidea amblia,65 norrisolide 87, a known metabolite of the spongivorous dorid nudibranch Chromodoris norrisi,66 and four new compounds, 1-bromo-8-ketoambliol A acetate 88 and dendrillolides A 89 B, and C 90.67 The 1988 collection yielded four additional compounds, dendrillolides D 91 and E 92, 12-desacetoxypolyraphin A 93 and 12-desacetoxyshahamin C 94, together with a reassessment of the structure of dendrillolides A and B, where dendrillolide A should have the original structure assigned to dendrillolide B with the structure of dendrillolide B being currently unknown.68,69 No bioactivity was recorded originally but recently Blackburn found that dendrillolide A 89 causes vesiculation of Golgi membranes (C. Blackburn, pers. comm.).
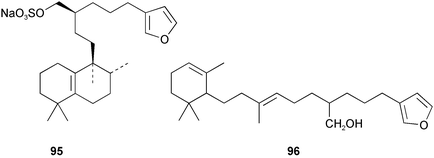
Chemistry/bioactivity: this sample contained the known compound halisulfate 3 95, which exhibited modest antimicrobial activity and had previously been reported from a Californian halichondrid sponge,70 together with the new sesterterpene igemellin 96.71
7.12 Porifera, Demospongiae, Dictyoceratida
Chemistry/bioactivity: the major metabolite in Coscinoderma sp. was suvanine 97, the structure of which was corrected as a result of studies of the Palauan specimen.72 Suvanine 97 facilitates neuromuscular transmission in indirectly stimulated rat hemidiaphram preparations (Jacobs, unpublished data), is an acetylcholinesterase inhibitor and Kimura et al.73 reported that they had isolated it by following serine protease inhibitory activity from a sample of Coscinoderma mathewsi collected in Pohnpei. They found that the activity against trypsin and thrombin was independent of the counter-ion, thus the known activity of the guanidine moiety was not the responsible factor.
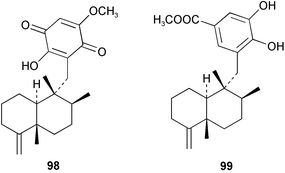
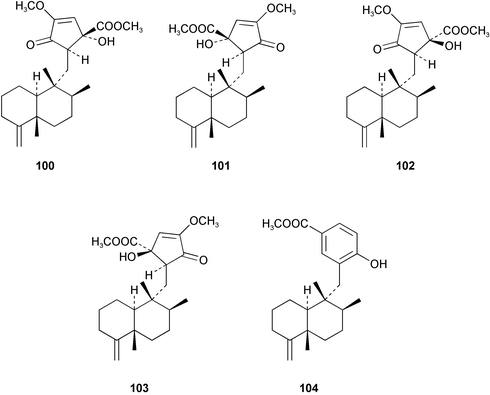
Chemistry/bioactivity: the sponge contained ilimaquinone 98 (cf. Hippospongia metachromia), dictoceratidin A 99, which had previously been isolated from a Hippospongia sp.74 and five new compounds, dactylospongenones A–D 100–103 and dictoceratidin C 104.75 No bioactivity was recorded for the new compounds although the crude extract of the sponge was antimicrobial, probably due to the presence of ilimaquinone.
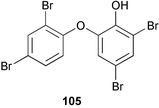
Chemistry/bioactivity: D. chlorea contained only 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol 105 which demonstrated strong antimicrobial activity.76
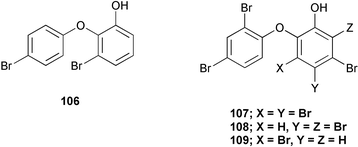
Chemistry/bioactivity: in 1972, one of the first studies of marine sponges from a secondary metabolite aspect was performed on Dysidea herbacea from Palau. In this study, Sharma and Vig78 reported the isolation of two antimicrobial agents, 2-(4′-bromophenoxy)-3-bromophenol 106 and 2-(2′,4′-dibromophenoxy)-3,4,5-tribromophenol 107. In a later study in 1981, Carte and Faulkner76 also reported the isolation of 107 together with 2-(2′,4′-dibromophenoxy)-4,5,6-tribromophenol 108 and 2-(2′,4′-dibromophenoxy)-3,5-dibromophenol 109 from the Hotel Nikko samples.
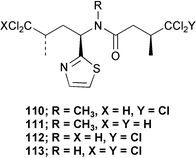
In contrast, the Kaibakku Marine Lake specimen contained 10-dechloro-N-methyl-dysideathiazole 110, 9,10-didechloro-N-methyldysideathiazole 111, 10-dechloro-dysideathiazole 112, and dysideathiazole 113 and the Ngemelis specimen yielded dysideapyrrolidone and 111.79
Symbiosis studies: using a specimen of D. herbacea from the Hotel Nikko Dock site, Unson et al.80 demonstrated that 2-(2′,4′-dibromophenoxy)-4,6-dibromophenol 105 was localized in, and presumably produced by, the cyanobacterium Oscillatoria spongeliae. Crystals of 105 were also found just beneath the surface of the sponge: it is assumed that the cyanobacteria release such large quantities of 105 (which is essentially insoluble in water), that it crystallizes on exposure to seawater.
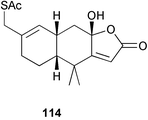
Chemistry/bioactivity: 15-acetylthiofurodysinin lactone 114 was isolated as a result of bioassay-driven isolation as a partial agonist using a human lung LTB4 receptor-binding assay linked to calcium mobilization.81 Although the scientists at then Smith Kline & French, now Glaxo SmithKline, performed QSAR studies these were never published.
Another example of this genus was also collected in 1981 at a depth of 20 m at Short Drop Off and was subsequently stored in methanol for 15 years prior to work-up. A voucher specimen was deposited in the SIO Benthic Invertebrate Collection (#P 1172).
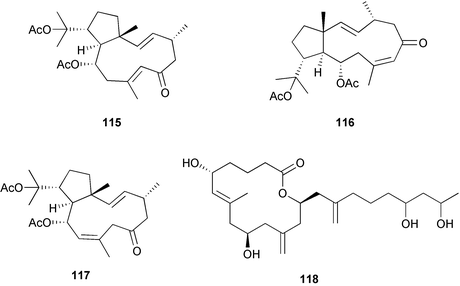
Chemistry/bioactivity: three new dolabellane diterpenes 115–117 and an unstable 14-membered macrolide, arenolide 118 were isolated from this sponge. All demonstrated modest cytotoxicity, but the isolation of these classes of compounds from this genus was previously unknown. Extensive reinspection of the sponge sample demonstrated that it was an example of a Dysidea sp. and did not appear to contain any gorgonian or brown algal contaminants/symbionts. A possible answer is that this particular sponge had adsorbed materials from its surroundings, a scenario that has been proposed to account for the presence of red algal metabolites in sponges.82
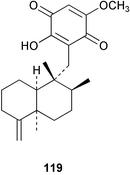
Chemistry/bioactivity: the sponge contained a 6 : 4 mixture of ilimaquinone 98 (see Dactylospongia sp.) and 5-epi-ilimaquinone83119, with the latter compound inhibiting fish-feeding at 5 pg mg−1 of pellet.
Chemistry/bioactivity: the merosesquiterpene ilimaquinone 98 was first isolated from a specimen of H. metachromia from Hawaii84 whereas the bioactivity studies referred to below used ilimaquinone isolated from Palauan specimens of H. metachromia and Dactylospongia elegans. Ilimaquinone is generally regarded as a nuisance compound because it is active in too many bioassays. It has been patented for use against cancer and HIV but it is unlikely to be used in treating humans because of its non-specific activity. However, as a result of demonstrating that ilimaquinone causes reversible breakdown of the Golgi membranes and disruption of the microtubule network in normal rat kidney cells,85 it is now being used as a biochemical probe to study the dynamics of Golgi membranes and their role in sorting, modifying and distributing proteins.
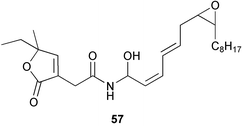
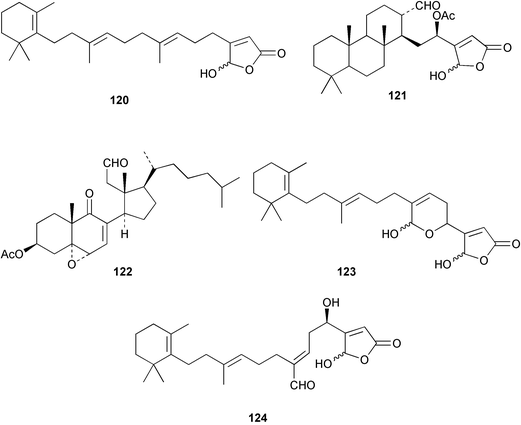
Chemistry/bioactivity: the first specimen (from 1985) contained luffariellolide 120 as a major constituent comprising 14.5% by dry weight86 and luffolide 121 as a minor metabolite.87 In contrast, the second specimen (from 1995) contained three 9,11-secosterols, luffasterols A 122–C, as well as manoalide 123 and secomanoalide 124.88 Both 120 and 121 inhibited PLA2 and were anti-inflammatory agents whilst luffasterol A 122 demonstrated activity in the NCI's 60 cell line panel.
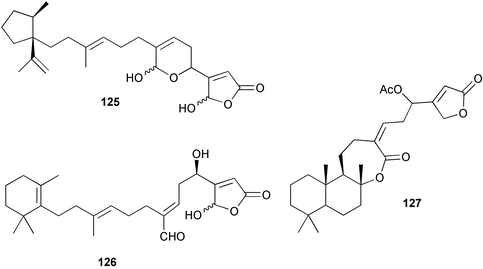
Chemistry/bioactivity: Scheuer and coworkers89,90 reported the isolation of manoalide 123, secomanoalide 124 and (E)- and (Z)-neomanoalides, but did not report their important anti-inflammatory activity. After examining a number of specimens of L. variabilis, Kernan et al.91 found that although most individuals contained manoalide and secomanoalide, some contained luffariellins A 125 and B 126 and others contained mixtures of all four compounds. Further research on L. variabilis from Palau92 resulted in the discovery of luffalactone 127 and (4E,6E)-dehydromanoalide.
Although manoalide was first reported as an antimicrobial agent, its most important activity was as an anti-inflammatory agent. The groups of Jacobs93–96 and Dennis97,98 independently established that manoalide inhibits the enzyme phospholipase A2, which is involved in the initial step of the inflammatory response. Since manoalide was present at a level of about 1% dry weight from a common sponge, it was regarded as a good candidate for drug development and its use as an anti-inflammatory agent was patented by the University of California. It was tested by Allergan Corp. as a topical treatment for psoriasis but the formulation used did not allow sufficient drug to pass through the skin and subsequently Allergan continued work on synthetic compounds based on the manoalide structure. All subsequent trials were not performed with the natural product.
The apparent failure of manoalide as a potential drug did not discourage additional research on details of its mechanism as a phospholipase A2 (PLA2) inhibitor.96,99–102 PLA2 is the enzyme responsible for the hydrolysis of membrane-bound phospholipids to release arachidonic acid that is subsequently converted into the prostaglandins and leukotrienes that ultimately cause the pain experienced in bee stings, psoriasis, arthritis and other inflammatory conditions. It was shown that manoalide undergoes an irreversible chemical reaction with lysine units on the interfacial binding site on PLA2 and thus prevents the correct binding between PLA2 and membrane-bound phospholipids.103
Manoalide is now widely used as a biochemical reagent to inhibit PLA2, although at higher concentrations it inhibits other lysine-rich enzymes. Interestingly, manoalide also reacts with the lysine residues in sponge fibers to form an orange complex that stains the fibers. The biological activity of luffariellin A 125 is identical to that of manoalide 123, while secomanoalide 124 and luffariellin B 126 are less active. A structure–activity study of manoalide and its derivatives has been reported104 together with a review of the work through 1992.105 As mentioned earlier, there are derivatives based on the manoalide pharmacophore that are still in different research phases in industry.
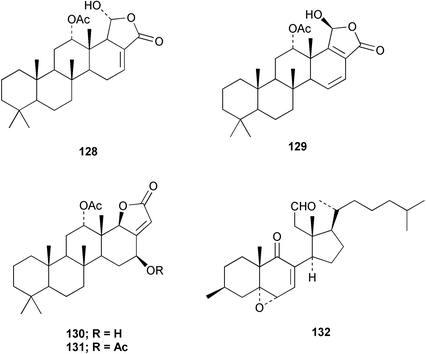
Chemistry/bioactivity: S. matamata contained scalarin 128, 12α-acetoxy-19β-hydroxyscalara-15,17-dien-20,19-olide 129, 12α-acetoxy-16β-hydroxyscalarolbutenolide 130, 12α,16β-diacetoxyscalarolbutenolide 131, and a new 9,11-secosterol, 3β-hydroxy-5α,6α-epoxy-9-oxo-9,11-seco-5α-cholest-7-en-11-al 132.106 Unpublished research by Dr Robert Jacobs and coworkers (UC Santa Barbara) suggested that 129 exhibited significant anti-inflammatory activity.
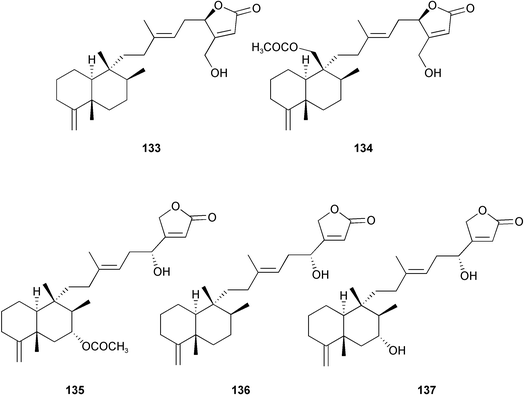
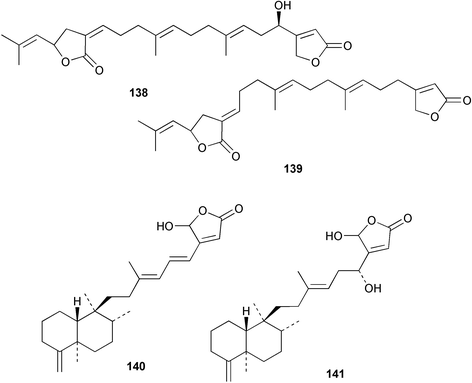
Chemistry/bioactivity: the crude organic extract of this sponge demonstrated activity against breast and melanoma lines and following bioactivity-driven isolations, five new sesterterpenes, thorectandrols A–E 133–137 were reported.107,108 In addition, the known compounds luffarin R 138, luffarin V 139 and palauolide 140 and palauolol 141 were also isolated. Both 140 and 141 were shown to have IC50 values in the range of 1–100 µg mL−1 depending upon cell line. The original reports of the latter two agents did not report this type of activity (vide infra).
7.13 Porifera, Demospongiae, Halichondrida
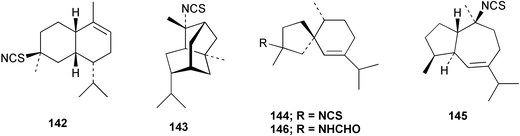
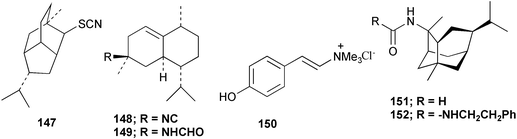
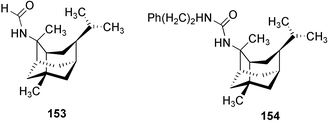
Chemistry/bioactivity: this sponge (previously called T. aplysinoides) was the source of the first naturally occurring sesquiterpene thiocyanate 142 plus three sesquiterpene isothiocyanates 143–145 and a formamide 146.109 Subsequent studies of A. aplysinoides uncovered another thiocyanate 147,110 an isonitrile 148, a formamide 149, a tyrosine derivative 150, two unrelated diterpenes, neoverrucosan-5β-ol and homoverrucosan-5β-ol111 and the formamide 151 and urea 152,112 with the structures of 141 and 151 being determined by X-ray analysis. Although sesquiterpene isonitriles, formamides and isothiocyanates are believed to be feeding deterrents, no such studies with this particular group of compounds have been reported. Though the crude extract of another sample of this sponge exhibited DNA damaging activity, the active constituent(s) was/were not reported but two novel nitrogenous sesterterpenes were isolated and purified, 2-(formylamino)trachyopsane 153 and N-phenethyl-N-2-trachyopsanylurea 154.112
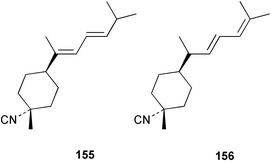
Chemistry/bioactivity: Halichondria cf. lendenfeldi contained two sesquiterpene isonitriles, 3-isocyanotheonellin 155 and 3-isocyanobisabolane-8,10-diene 156 and the corresponding formamides, 3-formamidotheonellin and 3-formamidobisabolane-8,10-diene.113 No bioactivity data was determined as the sponge was being studied as a possible food source for nudibranchs of the genus Phyllida.
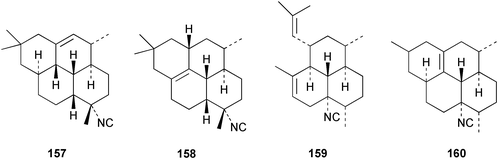
Chemistry/bioactivity: this sponge contained four diterpene isonitriles, 7-isocyano-1-cycloamphilectene 157, 7-isocyano-11-cycloamphilectene 158, 8-isocyano-10,14-amphilectadiene 159, and 8-isocyano-1(12)-cycloamphilectene 160, that were identified by X-ray analyses.114 As with other isonitriles, compounds 159 and 160 were mildly antimicrobial against Gram positive bacteria.
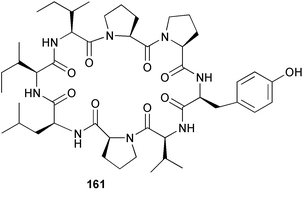
Chemistry/bioactivity: the cyclic peptide hymenistatin 1 161 was obtained in very low yield after an extensive bioassay guided fractionation.115 Owing to the low yield, hymenistatin 1 was subsequently synthesized.116 The compound was isolated by following P388 activity and provided a 30% life extension against this murine leukemia.
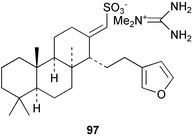
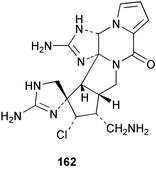
Chemistry/bioactivity: the bisguanidine alkaloid palau'amine 162 was isolated from the 1991 collection of S. agminata118 with five additional related metabolites being reported by workers from the same group in 1998 when the taxonomy was revized.117 Palau'amine is relatively non-toxic in mice but is cytotoxic to P-388 and A549 cells. It is also antimicrobial and antifungal and showed promise in an immunomodulatory assay. It should be noted that the dust from the dried sponge caused a powerful allergic reaction in man, manifested as shortness of breath for 4 hours.
There have been several syntheses of this alkaloid in the past but recently, synthetic routes to palau'amine precursors via construction of the spirocyclic core,119via formation of triazacyclopent(cd)-pentalenes120 or via modifications of a phakellin synthesis121 have been reported. These have the potential to construct variations on the structures by synthetic methodologies that could lead to determination of SAR characteristics.
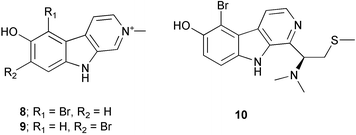
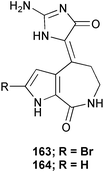
Chemistry/bioactivity: S. aurantium is a good source of hymenialdisine 163 and debromohymenialdisine 164, compounds that were first described from Axinella verrucosa and Acanthella auriantica by Cimino et al.,122 and also from Hymeniacidon aldis,123 and Phakellia flabellata.124 In addition, the 10E-geometrical isomers were reported as minor constituents of the sponge in 1996.125 Debromohymenialdisine (DBH) has been identified as a potent anti-inflammatory agent, which led to its being patented for the treatment of rheumatoid arthritis by SmithKline Beecham (SKB, now Glaxo SmithKline) and for osteoarthritis (by OsteoArthritis Sciences Inc., now defunct) and as a protein kinase C inhibitor by SKB. At least two separate syntheses have been published.126,127
7.14 Porifera, Demospongiae, Haplosclerida
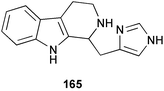
Chemistry/bioactivity: using a bioassay for cathepsin K (a cysteine protease implicated as playing a role in osteoporosis), a new tryptamine derived alkaloid, haploscleridamine 165 was isolated and demonstrated activity in the assay with an IC50 value of 26 µM.128
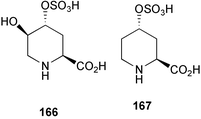
Chemistry/bioactivity: using the aqueous extract of the sponge and following activity by inhibition of 3H-CGP39653 binding to N-methyl-D-aspartic acid (NMDA) receptors from rat brain, a novel amino acid named cribonic acid 166 that demonstrated good activity (IC50 value of 83 nM) was isolated and purified. This compound was also active in vivo in mice following direct intracerebroventricular injection and gave an estimated ED50 of 29 pM per mouse.129 What is also of interest is that the des-hydroxy compound 167 was also isolated from two non-Palauan Micronesian sponges, A. carteri and S. aurantium but was not found in the Palauan sample. This compound was almost as active as the hydroxyl analogue but what is of particular note is that this compound 167 was first reported from a phytochemical study of the legume Pletophorum africanum,130 thus the actual source of the agent may be non-invertebrate.
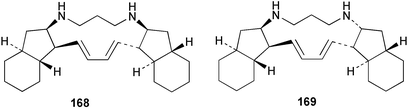
Chemistry/bioactivity: the sponge contains good quantities of two isomeric alkaloids, haliclonadiamine 168 and papua'amine 169, which were first described from a specimen of Haliclona sp. from Papua New Guinea131 occurring in a ratio of approximately 2 : 1 in the Palauan specimen with the structure of haliclonadiamine being confirmed by X-ray crystallographic analysis of the corresponding diacetate.132 There was some confusion about the physical and spectral properties of the compounds because they can form both mono- and di-hydrochloride salts, but there have been several syntheses of these alkaloids reported. Both haliclonadiamine 168 and papua'amine 169 inhibited Candida albicans, Bacillus subtilis and Staphylococcus aureus at 1–5 µg.disc−1. Papua'amine also demonstrated reasonably effective antifungal activity against the dermatophyte, Trichophyton mentagrophytes at 10 µg.disc−1. In addition, recent work has demonstrated that papua'amine 169 caused vesiculation of the Golgi membranes (see Hippospongia metachromia) and work is still continuing on this aspect.
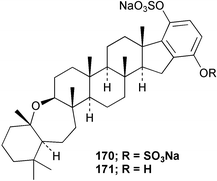
Chemistry/bioactivity: Haliclona (aka Adocia) sp. contains six hexaprenyl hydroquinone sulfates exemplified by adociasulfates 2 170 and 6 171. Adociasulfate 2 and the corresponding mono-sulfate, adociasulfate 6, are the first known natural product inhibitors of kinesin motor proteins, which are responsible for the transport of chemicals along microtubules within the cell,133,134 with the only other published inhibitors being a synthetic small molecule from a chemical genetics program (monastrol), nucleotide analogues or organic dyestuffs.135
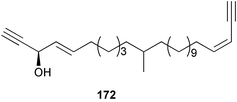
Chemistry/bioactivity: the Haliclona sp. contained three new acetylenes, (3R,4E,23Z)-3-hydroxy-11-methylhexacosa-4,23-diene-1,25-diyne 172, (3Z,23Z)-methylhexacosa-3,23-diene-1,25-diyne, and (3Z)-14-methyldocosa-3-en-1-yne, but no bioactivity assays were performed.136
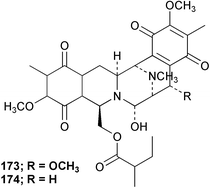
Chemistry/bioactivity: the Reniera sp. from Palau contained two new alkaloids, renieramycins E 173 and F 174 which were potent antimicrobial agents but unstable.137 The base structure of these materials is very close to that of the saframycins from a terrestrial streptomycete and to safracin B from a marine pseudomonad.
7.15 Porifera, Demospongiae, Homosclerophorida
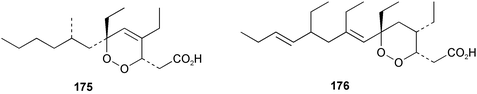
Chemistry/bioactivity: both specimens of P. aff. agulospiculatus contained the cyclic peroxide 175 while the 1993 specimen also contained the cyclic peroxide 176, which indicated that this earlier specimen was probably contaminated with a closely related Plakortis species, which was subsequently found among the Palau collections made in 1997. In addition to these cyclic peroxides, a series of related furans was also isolated. Both peroxides inhibited the proliferation of Leishmania mexicana promastigotes with 175 having an LD50 of 0.29 µg mL−1 and peroxide 176 being approximately three-fold less effective with an LD50 of 1.00 µg mL−1.138 What may be of interest is that a number of marine-derived cyclic peroxides have been reported to demonstrate anti-malarial activity as well.
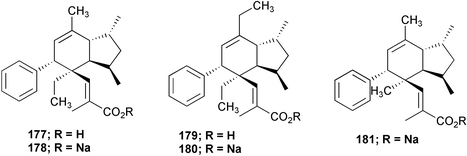
Chemistry/bioactivity: the extracts yielded the known compound, plakotenin 177, its sodium salt 178, homo-plakotenin as both the free acid 179 and sodium salt 180 and the sodium salt of nor-plakotenin 181. Of these five compounds, plakotenin, its sodium salt and homo-plakotenin were found to inhibit the proliferation of rheumatoid synovial fibroblasts by 36 to 77% at a concentration of 1 µg mL−1 in an assay performed at SmithKline Beecham.139
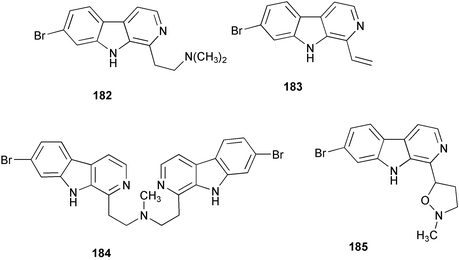
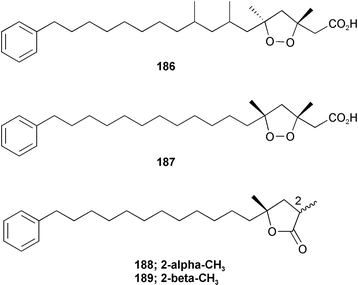
Chemistry/bioactivity: using activity against the human colon tumour cell line HCT-116 as the assay, eight new compounds were isolated and purified, of which the first seven demonstrated activities in the 0.4–15 µM range for IC50 values.140 The compounds were plakortamines A–D 182–185, epiplakinic acids G 186 and H 187 and two related γ-lactones, (2S*,4R*)-2,4-dimethyl-4-hydroxy-16-phenylhexadecanoic acid 1,4-lactone 188 and (2R*,4R*)-2,4-dimethyl-4-hydroxy-16-phenylhexadecanoic acid 1,4-lactone 189.
7.16 Porifera, Demospongiae, Lithistida
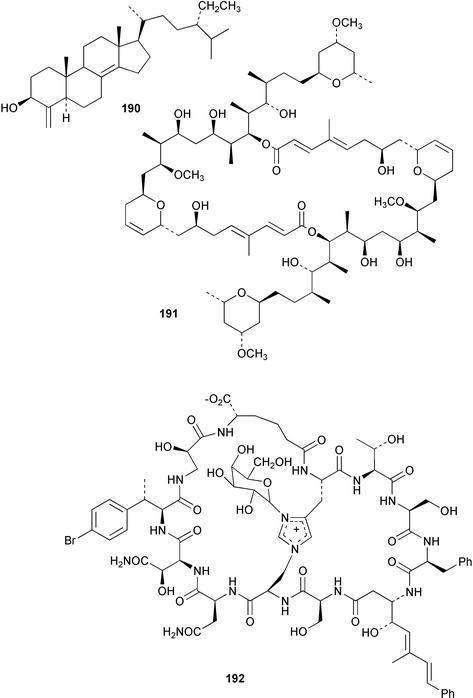
Chemistry/bioactivity: the Palauan T swinhoei contains theonellasterol 190,141 the cytotoxic macrolide swinholide A 191, and the cyclic peptide theopalauamide 192.142 Swinholide A was reported to demonstrate strong antitumour activity143 whilst theopalauamide demonstrated antifungal activity.
Symbiosis studies: using novel techniques including fixation of the sponge cellular contents and subsequent HPLC and NMR studies on the isolated cells, Bewley et al.3 demonstrated that both 191 and 192 were shown to be associated with symbiotic unicellular bacteria and filamentous fungi, respectively. Identification of the microbes has not been formally reported as yet.
7.17 Porifera, Demospongiae, Petrosida
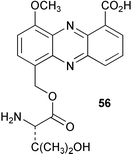
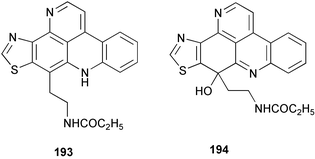
Chemistry/bioactivity: the major metabolite of O. sagittaria is dercitamide 193, which was first isolated from a deep-water sample of a Stelletta sp.144 and also from a tunicate of the genus Cystodytes.145 In addition to dercitamide, whilst working on the cellular location of that compound, the minor metabolite sagitol 194 was isolated and identified.146 Dercitamide was reported to be a cytotoxic and immunosuppressive agent144 and very similar compounds of the same pyridoacridine class were found to be topoisomerase II inhibitors. In contrast, sagitol was inactive in cytotoxicity assays.
Cellular location studies: since dercitamide (also known as kuanoniamine C) was found in both sponges and ascidians, it had been proposed that the compound was produced by a symbiotic microorganism. Studies using confocal microscopy to detect the natural fluorescence of 193, following cell separation techniques and chemical analysis, showed that it was localized exclusively in bacteria-free sponge inclusional cells, and was probably not produced by extracellular bacteria and then transferred to the sponge cells.4
Finally, there is a recent paper by Skyler and Heathcock describing the “Family Tree” of the pyridinoacridine metabolites that should be consulted for its predictions as to as yet undiscovered/unsynthesized compounds of this general structural class.147
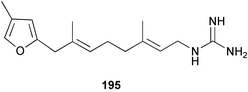
Chemistry/bioactivity: this Siphonodictyon sp. contained relatively large amounts of siphonodictidine 195, an unusual guanidinosesquiterpene that inhibits photosynthesis and respiration in Acropora formosa causing death of the coral polyps.148 The siphonodictidine appears to be released in a mucous secretion that inhibits the growth of coral polyps around the oscular chimney.
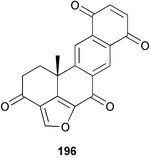
Chemistry/bioactivity: the structure of halenaquinone 196 was determined by single crystal X-ray analysis,149 and the compound exhibited modest in vitro activity versusS. aureus and B. subtilis.
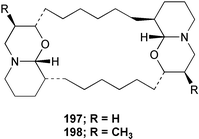
Chemistry/bioactivity: the major metabolite isolated from this sponge was araguspongine C 197, which had previously been described from an Okinawan specimen of Xestospongia sp. by Kobayashi et al.150 who reported that it exhibited vasodilative properties in an isolated rat artery model, together with a new minor metabolite, 3β,3β′-dimethylxestospongine C 198.151
7.18 Porifera, Demospongiae, Poecilosclerida
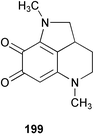
Chemistry/Bioactivity: two pyrroloquinones, damirones A 199 and B, were isolated from this sponge but no bioactivity was reported.
7.19 Porifera, Demospongiae, Verongida
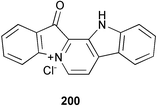
Chemistry/bioactivity: a compound called palauolide 140 was obtained from the 1980 (mixed) collection of sponges.153 In contrast, from the 1985 collection palauolol 141 was found to be the major metabolite of the of Fascaplysinopsis sp. and was shown to dehydrate to obtain palauolide.154 This sample also contained the known metabolite fascaplysin 200, which had been reported in 1988 from a Fijian collection of Fascaplysinopsis reticulata.155 Both 140 and 141 inhibit phospholipase A2 and antimicrobial activity was reported for 140 and 200.
8 Conclusion
The work that is presented above is just an example of the chemical, biochemical, microbiological and marine biological riches that are present in one atoll in the Central South Pacific Ocean. As a result of the geographic location and the ease of access, together with the support of the Government of the Republic of Belau, scientists have been permitted to investigate the waters of this country.Due to the presence of the NCI's shallow water collection contractor, the Coral Reef Research Foundation in Palau for the last 10 years, Palau has what is probably the best inventory of taxonomically identified marine fauna of any country when expressed on a square kilometre basis. Even with that, there are still immense areas that have not been investigated, particularly in the microbial environment.
One has only to look at the vastly different chemical entities that have been found from what is effectively less than 3 kilograms of cyanophytes (cf Lyngbya and Symploca above), to realize the immensity of the scientific investigations yet to come and the potential for discovery of novel pharmaceutical agents and biological probes is definitely proven by the work listed above.
The Government of Belau is justifiably reticent in awarding research collection permits to non-citizens for such studies and it was one of John's proudest accomplishments that he held one of the first permits awarded. The foresight of the Government of Belau in giving John such a collection permit is amply demonstrated by the extensive investigations performed by his group over the period 1979–2002 as shown in this review.
This work will be continued both by his students from over the years and by others at Scripps and other universities/institutions, who, like John, have the best interests of Palau at heart, as any samples from their collections that may ultimately be commercialized will have as part of any development programme, a requirement that Palau must benefit.
References
- M. Etpison, Palau, Portrait of Paradise, NECO Marine Corp., Palau, 1997 Search PubMed.
- J. N. A. Hooper, J. A. Kennedy and R. J. Quinn, Biodiv. Conserv., 2002, 11, 851 Search PubMed.
- C. A. Bewley, N. D. Holland and D. J. Faulkner, Experientia, 1996, 52, 716 Search PubMed.
- C. E. Salomon, T. Deerinck, M. H. Ellisman and D. J. Faulkner, Mar. Biol., 2001, 139, 313 Search PubMed.
- C. E. Salomon and D. J. Faulkner, J. Nat. Prod., 2002, 65, 689 CrossRef CAS.
- X. Fu, M. L. G. Ferreira and F. J. Schmitz, J. Nat. Prod., 1999, 62, 1306 CrossRef CAS.
- C. J. Smith, D. A. Venables, C. M. Ireland, C. Hopmann, C. E. Salomon, D. J. Faulkner, J. Jompa and A. Tahir, J. Nat. Prod., 1997, 60, 1048 CrossRef CAS.
- P. W. Ford and B. S. Davidson, J. Nat. Prod., 1997, 60, 1051 CrossRef CAS.
- B. C. M. Potts, D. J. Faulkner, J. A. Chan, G. C. Simolike, P. Offen, M. Hemling and T. A. Francis, J. Am. Chem. Soc., 1991, 113, 6321 CrossRef CAS.
- J. Pika and D. J. Faulkner, Nat. Prod. Lett., 1995, 7, 291 Search PubMed.
- X. Fan, G. R. Flentke and D. H. Rich, J. Am. Chem. Soc., 1998, 120, 8893 CrossRef CAS.
- M. A. Rashid, K. R. Gustafson and M. R. Boyd, J. Nat. Prod., 2001, 64, 1454 CrossRef CAS.
- M. L. Dionisio-Sese, M. Ishikura, T. Maruyama and S. Miyachi, Mar. Biol., 1997, 128, 455 CrossRef CAS.
- C. Ireland and P. J. Scheuer, J. Am. Chem. Soc., 1980, 102, 5688 CrossRef CAS.
- C. M. Ireland, A. D. Durso, Jr., R. A. Newman and M. P. Hacker, J. Org. Chem., 1982, 47, 1807 CrossRef CAS.
- A. D. Patil, A. J. Freyer, L. Killmer, C. Chambers and R. K. Johnson, Nat. Prod. Lett., 1997, 9, 181 Search PubMed.
- J. M. Wasylyk, J. E. Biskupiak, C. E. Costello and C. M. Ireland, J. Org. Chem., 1983, 48, 4445 CrossRef CAS.
- D. F. Sesin, S. J. Gaskell and C. M. Ireland, Bull. Soc. Chim. Belg., 1986, 95, 853 CAS.
- C. Hopmann and D. J. Faulkner, Tetrahedron Lett., 1997, 38, 169 CrossRef CAS.
- Q. Lu and D. J. Faulkner, Nat. Prod. Lett., 1997, 10, 231 Search PubMed.
- A. Sato, W. Fenical, Q. Zheng and J. Clardy, Tetrahedron Lett., 1985, 41, 4303 CrossRef CAS.
- B. F. Bowden, J. C. Coll, S. J. Mitchell, J. Mulden and G. J. Stokie, Aust. J. Chem., 1978, 31, 2049 CAS.
- B. F. Bowden, J. C. Coll and A. D. Wright, Aust. J. Chem., 1989, 42, 757 CAS.
- B. F. Bowden, J. C. Coll, E. D. de Silva, M. S. L. de Costa, P. J. Djura, M. Mahendran and D. M. Tapiolas, Aust. J. Chem., 1983, 36, 371 CAS.
- S. Mizobuchi, K. Kon-ya, K. Adachi, M. Sakai and W. Miki, Fish. Sci., 1994, 60, 345 Search PubMed.
- S. Mizobuchi, K. Adachi and W. Miki, Fish. Sci., 1996, 62, 98 Search PubMed.
- S. S. Mitchell, D. J. Faulkner, K. Rubins and F. D. Bushman, J. Nat. Prod., 2000, 63, 279 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, Bioorg. Med. Chem., 2002, 10, 1973 Search PubMed.
- H. Luesch, P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 996 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, G. G. Harrigan, J. P. Doom, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 1945 CrossRef CAS.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, Tetrahedron, 2002, 56, 7959 CrossRef CAS.
- H. Sone, T. Kondo, M. Kiryu, H. Ishiwata, M. Ojika and K. Yamada, J. Org. Chem., 1995, 60, 4774 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2002, 65, 1336 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 1006 CrossRef CAS.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2003, 66, 620 CrossRef CAS.
- N. Imamura, M. Nishijima, T. Takadera, K. Adachi, M. Sakai and H. Sano, J. Antibiot., 1997, 50, 8 CAS.
- K. Yoshikawa, T. Takadera, K. Adachi, M. Nishijima and H. Sano, J. Antibiot., 1997, 50, 949 CAS.
- M. Namikoshi, K. Akano, S. Meguro, I. Kasuga, Y. Mine, T. Takahashi and H. Kobayashi, J. Nat. Prod., 2001, 64, 396 CrossRef CAS.
- M. Suzuki, Y. Nakagawa, S. Harayama and S. Yamamoto, Int. J. Syst. Evol. Microbiol., 2001, 51, 1639 Search PubMed.
- R. J. Andersen, D. J. Faulkner, C.-h. He, G. D. Van Duyne and J. Clardy, J. Am. Chem. Soc., 1985, 107, 5492 CrossRef CAS.
- B. Carte, M. R. Kernan, E. B. Barrabee, D. J. Faulkner, G. K. Matsumoto and J. Clardy, J. Org. Chem., 1986, 51, 3528 CrossRef CAS.
- M. R. Kernan, E. B. Barrabee and D. J. Faulkner, Comp. Biochem. Physiol., B: Biochem. Mol. Biol., 1988, 89, 275 Search PubMed.
- M. Nakagawa, Y. Hamamoto, M. lshihama, S. Hamasaki and M. Endo, Tetrahedron Lett., 1987, 28, 431 CrossRef CAS.
- R. Kazlauskas, P. T. Murphy and R. J. Wells, Aust. J. Chem., 1982, 35, 51 CAS.
- J. C. Braekman, D. Daloze, C. Soller and R. W. M. Van Soest, Biochem. Syst. Ecol., 1992, 20, 417 CrossRef CAS.
- R. J. Capon and D. J. Faulkner, J. Am. Chem. Soc., 1984, 106, 1819 CrossRef CAS.
- L. Kitagawa, M. Kobayashi, Y. Okamoto, M. Yoshikawa and Y. Hamamoto, Chem. Pharm. Bull., 1987, 35, 5036.
- M. Kobayashi, Y. Okamoto and I. Kitagawa, Chem. Pharm. Bull., 1991, 39, 2867 CAS.
- T. M. Zabriskie, J. A. Klocke, C. M. Ireland, A. H. Marcus, T. F. Molinski, D. J. Faulkner, C. Xu and J. Clardy, J. Am. Chem. Soc., 1986, 108, 3123 CrossRef.
- P. Crews, L. V. Manes and M. Boehler, Tetrahedron Lett., 1986, 27, 2797 CrossRef CAS.
- A. Zampella, C. Giannini, C. Debitus, C. Roussakis and M. V. D'Auria, J. Nat. Prod., 1999, 62, 332 CrossRef CAS.
- M. R. Bubb, A. M. Senderowicz, E. A. Sausville, K. L. Duncan and E. D. Korn, J. Biol. Chem., 1994, 269, 14869 CAS.
- A. M. Senderowicz, G. Kaur, E. Sainz, C. Laing, W. D. Inman, J. Rodriguez, P. Crews, L. Malspeis and M. R. Grever, J. Nat. Can. Inst., 1995, 87, 46 Search PubMed.
- W. R. Chan, W. F. Tinto, P. S. Manchand and L. J. Todaro, J. Org. Chem., 1987, 52, 3091 CrossRef CAS.
- J. E. Coleman, R. W. M. Van Soest and R. J. Andersen, J. Nat. Prod., 1999, 62, 1137 CrossRef CAS.
- B. Kunze, R. Jansen, F. Sasse, G. Hofle and H. Reichenbach, J. Antibiot., 1995, 48, 1262 CAS.
- F. Sasse, B. Kunze, T. M. A. Gronewold and H. Reichenbach, J. Nat. Can. Inst., 1998, 90, 1559 Search PubMed.
- G. R. Pettit, C. L. Herald, J. E. Leet, R. Gupta, D. E. Schaufelberger, R. B. Bates, P. J. Clelow, D. L. Doubek, K. P. Manfredi, K. Riltzler, J. M. Schmidt, L. P. Tackett, F. B. Ward, M. Bruck and F. Camou, Can. J. Chem., 1990, 68, 1621.
- G. R. Pettit, C. L. Herald, M. R. Boyd, J. E. Leet, C. Dufresne, D. L. Doubek, J. M. Schmidt, R. L. Cemy, J. N. A. Hooper and K. Riltzler, J. Med. Chem., 1991, 34, 3339 CrossRef CAS.
- G. R. Pettit, F. Gao, R. L. Cerny, D. L. Doubek, L. P. Tackett, J. M. Schmidt and J.-C. Chapius, J. Med. Chem., 1994, 37, 1165 CrossRef CAS.
- G. R. Pettit, J. W. Holman and G. M. Boland, J. Chem. Soc., Perkin Trans. 1, 1996, 2411 RSC.
- O. Mechnich and H. Kessler, Tetrahedron Lett., 1996, 37, 5355 CrossRef CAS.
- C. M. Cerda-Garcia-Rojas and D. J. Faulkner, Tetrahedron Lett., 1995, 51, 1087 CAS.
- S. C. Bobzin and D. J. Faulkner, J. Org. Chem., 1991, 56, 4403 CrossRef CAS.
- R. Walker and D. Faulkner, J. Org. Chem., 1981, 46, 1098 CrossRef CAS.
- J. E. Hocklowski, D. J. Faulkner, G. K. Matsumoto and J. Clardy, J. Org. Chem., 1983, 48, 1141 CrossRef.
- B. Sullivan and D. J. Faulkner, J. Org. Chem., 1984, 49, 3204 CrossRef CAS.
- T. F. Molinski, D. J. Faulkner, H. Cun-heng, G. D. Van Duyne and J. Clardy, J. Org. Chem., 1986, 51, 4564 CrossRef.
- S. C. Bobzin and D. J. Faulkner, J. Org. Chem., 1989, 54, 5727 CrossRef CAS.
- M. R. Kernan and D. J. Faulkner, J. Org. Chem., 1988, 53, 4574 CrossRef CAS.
- G. Liu, J. Pika and D. J. Faulkner, Nat. Prod. Lett., 1995, 7, 297 Search PubMed.
- L. V. Manes, P. Crews, M. R. Kernan, D. J. Faulkner, F. R. Fronczek and R. D. Gandour, J. Org. Chem., 1988, 53, 570 CrossRef CAS.
- J. Kimura, E. Ishizuka, Y. Nakao, W. Y. Yoshida, P. J. Scheuer and M. Kelly-Borges, J. Nat. Prod., 1998, 61, 248 CrossRef CAS.
- H. Nakamura, S. Deng, J. Kobayashi, Y. Ohizumi and Y. Hirata, Tetrahedron Lett., 1986, 42, 4197 CrossRef CAS.
- D. M. Kushlan, D. J. Faulkner, L. Parkanyi and J. Clardy, Tetrahedron Lett., 1989, 45, 3307 CrossRef CAS.
- B. Carte and D. J. Faulkner, Tetrahedron Lett., 1981, 37, 2335 CAS.
- P. L. Colin and C. Arneson, Tropical Pacific Invertebrates, Coral Reef Press, Beverly Hills, 1995 Search PubMed.
- G. M. Sharma and B. Vig, Tetrahedron Lett., 1972, 1715 CrossRef CAS.
- M. D. Unson, C. B. Rose, D. J. Faulkner, L. S. Brinen, J. Rios Steiner and J. Clardy, J. Org. Chem., 1993, 58, 6336 CrossRef CAS.
- M. D. Unson, N. D. Holland and D. J. Faulkner, Mar. Biol., 1994, 119, 1 CAS.
- B. Carte, S. Mong, B. Poehland, H. Sarau, J. W. Westley and D. J. Faulkner, Tetrahedron Lett., 1989, 30, 2725 CrossRef CAS.
- Q. Lu and D. J. Faulkner, J. Nat. Prod., 1998, 61, 1096 CrossRef CAS.
- B. Carte, C. B. Rose and D. J. Faulkner, J. Org. Chem., 1985, 50, 2785 CrossRef CAS.
- R. T. Luibrand, T. R. Erdman, J. J. Vollmer, P. J. Scheuer, J. Finer and J. Clardy, Tetrahedron Lett., 1979, 35, 609 CrossRef CAS.
- P. A. Takizawa, J. K. Yucel, B. Viet, D. J. Faulkner, T. Deerinck, G. Soto, M. Ellisman and V. Malhotra, Cell, 1993, 73, 1079 CAS.
- K. F. Albizati, T. Holman, D. J. Faulkner, K. B. Glaser and R. S. Jacobs, Experientia, 1987, 43, 949 Search PubMed.
- M. R. Kernan, D. J. Faulkner, L. Parkanyi, J. Clardy, M. S. de Carvalho and R. S. Jacobs, Experientia, 1989, 45, 388 Search PubMed.
- M. V. R. Reddy, M. K. Harper and D. J. Faulkner, J. Nat. Prod., 1997, 60, 41 CrossRef CAS.
- E. D. de Silva and P. J. Scheuer, Tetrahedron Lett., 1980, 21, 1611 CrossRef CAS.
- E. D. de Silva and P. J. Scheuer, Tetrahedron Lett., 1980, 21, 3147 CrossRef CAS.
- M. R. Kernan, D. J. Faulkner and R. S. Jacobs, J. Org. Chem., 1987, 52, 3081 CrossRef CAS.
- B. C. M. Potts, R. J. Capon and D. J. Faulkner, J. Org. Chem., 1992, 57, 2965 CrossRef CAS.
- J. C. de Freitas, L. A. Blankmeier and R. S. Jacobs, Experientia, 1984, 40, 864 Search PubMed.
- R. S. Jacobs, P. Culver, R. Langdon, T. O'Brien and S. White, Tetrahedron Lett., 1985, 41, 981 CrossRef CAS.
- K. B. Glaser and R. S. Jacobs, Biochem. Pharmacol., 1986, 35, 449 CrossRef CAS.
- K. B. Glaser and R. S. Jacobs, Biochem. Pharmacol., 1987, 36, 2079 CrossRef CAS.
- D. Lombardo and E. A. Dennis, J. Biol. Chem., 1985, 260, 7234 CAS.
- R. A. Deems, D. Lombardo, B. P. Morgan, E. D. Mihelich and E. A. Dennis, Biochem. Biophys. Acta, 1987, 917, 258 Search PubMed.
- C. F. Bennett, S. Mong, M. A. Clarke, L. I. Kruse and S. T. Crooke, Biochem. Pharmacol., 1987, 36, 733 CrossRef CAS.
- P. B. Jacobson, L. A. Marshall, A. Sung and R. S. Jacobs, Biochem. Pharmacol., 1990, 39, 1557 CrossRef CAS.
- L. J. Reynolds, E. D. Mihelich and E. A. Dennis, J. Biol. Chem., 1991, 266, 16512 CAS.
- A. R. Ortiz, M. T. Pisabarro and F. Gago, J. Med. Chem., 1993, 36, 1866 CrossRef CAS.
- B. C. M. Potts, D. J. Faulkner, M. S. de Carvahlo and R. S. Jacobs, J. Am. Chem. Soc., 1992, 114, 5093 CrossRef CAS.
- K. B. Glaser, M. S. de Carvalho, R. S. Jacobs, M. R. Kernan and D. J. Faulkner, Mol. Pharmacol., 1989, 36, 782 Search PubMed.
- B. C. M. Potts, D. J. Faulkner and R. S. Jacobs, J. Nat. Prod., 1992, 55, 1701 CrossRef CAS.
- Q. Lu and D. J. Faulkner, J. Nat. Prod., 1997, 60, 195 CrossRef CAS.
- R. D. Charan, T. C. McKee and M. R. Boyd, J. Nat. Prod., 2001, 64, 661 CrossRef CAS.
- R. D. Charan, T. C. McKee and M. R. Boyd, J. Nat. Prod., 2002, 65, 492 CrossRef CAS.
- H. He, D. J. Faulkner, J. S. Shumsky, K. Hong and J. Clardy, J. Org. Chem., 1989, 54, 2511 CrossRef CAS.
- H. He, J. Salvo, R. F. Catalos and D. J. Faulkner, J. Org. Chem., 1992, 57, 3191 CrossRef CAS.
- R. S. Compagnone and D. J. Faulkner, J. Nat. Prod., 1995, 58, 145 CrossRef CAS.
- A. D. Patil, A. J. Freyer, R. Reichwein, M. F. Bean, L. Faucette, R. K. Johnson, R. C. Haltiwanger and D. S. Eggleston, J. Nat. Prod., 1997, 60, 507 CrossRef CAS.
- K. E. Kassuhlke, C. M. Potts and D. J. Faulkner, J. Org. Chem., 1991, 56, 3747 CrossRef CAS.
- T. F. Molinski, D. J. Faulkner, G. D. Van Duyne and J. Clardy, J. Org. Chem., 1987, 52, 3334 CrossRef CAS.
- G. R. Pettit, P. J. Clewlow, C. Dufresne, D. J. Doubek, R. L. Cerny and K. Ratzler, Can. J. Chem., 1990, 68, 708 CAS.
- R. K. Konat, D. F. Mierke, H. Kessler, B. Kutscher, M. Bernd and R. Voegeli, Helv. Chim. Acta, 1993, 76, 1649 CAS.
- R. B. Kinnel, H.-P. Gehrken, R. Swali, G. Skoropowski and P. J. Scheuer, J. Org. Chem., 1998, 63, 3281 CrossRef CAS.
- R. B. Kinnel, H.-P. Gehrken and P. J. Scheuer, J. Am. Chem. Soc., 1993, 115, 3376 CrossRef CAS.
- A. S. Dilley and D. Romo, Org. Lett., 2001, 3, 1535 CrossRef CAS.
- G. Belanger, F.-T. Hong, L. E. Overman, B. N. Rogers, J. E. Tellew and W. C. Trenkle, J. Org. Chem., 2002, 67, 7880 CrossRef CAS.
- K. G. Poullennec and D. Romo, J. Am. Chem. Soc., 2003, 125, 6344 CrossRef CAS.
- G. Cimino, S. De Rosa, S. De Stefano, L. Mazzarella, R. Puliti and G. Sodano, Tetrahedron Lett., 1982, 23, 767 CrossRef CAS.
- I. Kitagawa, M. Kobayashi, K. Kitanaka, M. Kido and Y. Kyogoku, Chem. Pharm. Bull., 1983, 31, 2321 CAS.
- G. W. Sharma, J. S. Buyer and M. W. Pomerantz, J. Chem. Soc., Chem. Commun., 1980, 435 RSC.
- D. H. Williams and D. J. Faulkner, Nat. Prod. Lett., 1996, 9, 57 Search PubMed.
- H. Annoura and T. Tatsuoka, Tetrahedron Lett., 1995, 36, 413 CrossRef CAS.
- Y. Xu, K. Yakushijin and D. A. Home, J. Org. Chem., 1997, 62, 456 CrossRef CAS.
- A. D. Patil, A. J. Freyer, B. Carte, P. B. Taylor, R. K. Johnson and D. J. Faulkner, J. Nat. Prod., 2002, 65, 628 CrossRef CAS.
- R. Sakai, H. Matsubara, K. Shimamoto, M. Jimbo, H. Kamiya and M. Namikoshi, J. Nat. Prod., 2003, 66, 784 CAS.
- S. V. Evans, T. K. M. Shing, R. T. Aplin, L. E. Fellows and G. W. J. Fleet, Phytochemistry, 1985, 24, 2593 CrossRef CAS.
- B. J. Baker, P. J. Scheuer and J. N. Shoolery, J. Am. Chem. Soc., 1988, 110, 965 CrossRef CAS.
- E. Fahy, T. F. Molinski, M. K. Harper, B. W. Sullivan, D. J. Faulkner, L. Parkanyi and J. Clardy, Tetrahedron Lett., 1988, 29, 3427 CrossRef.
- C. L. Blackburn, C. Hopmann, R. Sakowicz, M. S. Berdelis, L. S. B. Goldstein and D. J. Faulkner, J. Org. Chem., 1999, 64, 5564 CrossRef CAS.
- R. Sakowicz, M. S. Berdelis, K. Ray, C. L. Blackburn, C. Hopmann, D. J. Faulkner and L. S. B. Goldstein, Science, 1998, 280, 292 CrossRef CAS.
- D. J. Newman, G. M. Cragg, S. Holbeck and E. A. Sausville, Curr. Cancer Drug Targs., 2002, 2, 279 Search PubMed.
- D. H. Williams and D. J. Faulkner, J. Nat. Prod., 1996, 59, 1099 CrossRef CAS.
- H. He and D. J. Faulkner, J. Org. Chem., 1989, 54, 5822 CrossRef CAS.
- R. S. Compagnone, I. C. Pisa, H. R. Rangel, F. Dagger, A. I. Suarez, M. V. R. Reddy and D. J. Faulkner, Tetrahedron Lett., 1998, 54, 3057 CrossRef CAS.
- A. Qureshi, C. S. Stevenson, C. A. Albert, R. S. Jacobs and D. J. Faulkner, J. Nat. Prod., 1999, 62, 1205 CrossRef CAS.
- J. S. Sandler, P. L. Colin, J. N. A. Hooper and D. J. Faulkner, J. Nat. Prod., 2002, 65, 1256 CrossRef CAS.
- E. Kho, D. K. Imagawa, M. Rohmer, Y. Kashman and C. Djerassi, J. Org. Chem., 1981, 46, 1836 CrossRef CAS.
- E. W. Schmidt, C. A. Bewley and D. J. Faulkner, J. Org. Chem., 1998, 63, 1254 CrossRef CAS.
- M. Kobayashi, K. Kawazoe, T. Okamoto, T. Sasaki and I. Kitagawa, Chem. Pharm. Bull., 1994, 42, 19 CAS.
- G. P. Gunawardana, S. Khomoto and N. S. Burres, Tetrahedron Lett., 1989, 30, 4359 CrossRef CAS.
- G. P. Gunawardana, F. E. Koehn, A. Y. Lee, J. Clardy, H. He and D. J. Faulkner, J. Org. Chem., 1992, 57, 1523 CrossRef CAS.
- C. E. Salomon and D. J. Faulkner, Tetrahedron Lett., 1996, 37, 9147 CrossRef CAS.
- D. Skyler and C. H. Heathcock, J. Nat. Prod., 2002, 65, 1573 CrossRef CAS.
- B. Sullivan, D. J. Faulkner and L. Webb, Science, 1983, 221, 1175 CAS.
- D. M. Roll, P. J. Scheuer, G. K. Matsumoto and J. Clardy, J. Am. Chem. Soc., 1983, 105, 6177 CrossRef CAS.
- M. Kobayashi, K. Kawazoe and I. Kitagawa, Chem. Pharm. Bull., 1989, 37, 1676 CAS.
- M. V. R. Reddy and D. J. Faulkner, Nat. Prod. Lett., 1997, 11, 53 Search PubMed.
- R. W. M. van Soest, S. Zea and M. Kielman, Bijdragen Dierkunde, 1994, 64, 163 Search PubMed.
- B. Sullivan and D. J. Faulkner, Tetrahedron Lett., 1982, 23, 907 CrossRef CAS.
- E. W. Schmidt and D. J. Faulkner, Tetrahedron Lett., 1996, 37, 3951 CrossRef CAS.
- D. M. Roll, C. M. Ireland, H. S. M. Lu and J. Clardy, J. Org. Chem., 1988, 53, 3276 CrossRef CAS.
Footnote |
| † Address for correspondence: Natural Products Branch, NCI, PO Box B, Frederick, MD 21702, USA. E-mail: dn22a@nih.gov |
| This journal is © The Royal Society of Chemistry 2004 |