Preparation of ordered SBA-15 mesoporous silica containing chelating groups. Study of the complexation of EuIII inside the pore channels of the materials
Robert J. P.
Corriu
*a,
Ahmad
Mehdi
a,
Catherine
Reyé
a,
Chloé
Thieuleux
a,
Anatoly
Frenkel
b and
Alain
Gibaud
c
aLaboratoire de Chimie Moléculaire et Organisation du Solide (CNRS UMR 5637), Université de Montpellier II, Place E. Bataillon, F-34095, Montpellier cedex 5, France. E-mail: reye@univ-montp2.fr; Fax: +33 (0)4 67 14 38 52; Tel: +33 (0)4 67 14 30 38
bDepartment of Physics, Yeshiva University, 245 Lexington Avenue, New York, NY 10016, USA. E-mail: frenkel@bnl.gov
cLaboratoire de Physique de l'Etat Condensé (CNRS UMR 6087), Université du Maine, 72085, Le Mans cedex 09, France. E-mail: gibaud@univ-lemans.fr; Fax: +33 (0)2 43 83 35 18; Tel: +33 (0)2 43 83 32 62
First published on 31st October 2003
Abstract
Ordered mesoporous silica containing 3-chloropropyl groups was prepared by a direct synthetic approach involving hydrolysis and co-condensation of tetraethylorthosilicate (TEOS) and 3-chloropropyltrimethoxysilane in the presence of the triblock copolymer P123 as the structure-directing agent and under acidic conditions. Nucleophilic displacement of chloro groups by cyclam moieties (cyclam![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4,8,11-tetraazacyclotetradecane) was then achieved almost quantitatively. Subsequent treatment of solids containing different amounts of cyclam moieties with an ethanolic solution of europium (III) chloride gave rise to 1∶1 EuIII/cyclam complexes. The EXAFS studies have shown that EuIII adopts an octahedral geometry.
1,4,8,11-tetraazacyclotetradecane) was then achieved almost quantitatively. Subsequent treatment of solids containing different amounts of cyclam moieties with an ethanolic solution of europium (III) chloride gave rise to 1∶1 EuIII/cyclam complexes. The EXAFS studies have shown that EuIII adopts an octahedral geometry.
Introduction
The preparation of hybrid materials containing strongly chelated metal or lanthanide cations is a challenge worth pursuing as most transition metal and lanthanide salts exhibit physical properties (optical or magnetic, for example) that could be of interest for materials with specific properties. For this purpose, we have incorporated cyclam moieties within a silica matrix, cyclam (1,4,8,11-tetraazacyclotetradecane) being well-known for its remarkable binding ability towards transition metals.1,2 That was done by hydrolysis and polycondensation of the tetrasilylated cyclam 1.3 The incorporation of EuIII ions inside these solids was investigated and surprisingly, while complexation of EuIII by the precursor 1 is not possible in solution, it was shown that direct incorporation of EuIII within the hybrid material containing tetra-N-propyl cyclam moieties occurred, in a ratio of one EuIIIper two cyclam moieties. This result suggested that the cyclam moieties are in close proximity within the hybrid material, allowing the complexation of one EuIII between two tetraaza macrocycles.4 This is in agreement with the previous study of the incorporation of CuII into the same material, a study that revealed the presence of Cu–Cu interactions by ESR spectroscopy.5 These results showed that the coordination mode of europium salts within the bulk is not the same as in solution, prompting us to study the coordination mode of EuIII with cyclam moieties located inside the channel pores of ordered mesoporous hybrid materials in order to determine if it is similar to that in solution or in the bulk.In this paper, we describe the preparation of ordered SBA-15 mesoporous silica containing N-propylcyclam moieties. These materials are obtained by post-modification of ordered SBA-15 mesoporous silica functionalized with chloropropyl groups prepared by the direct synthetic approach.6–12 Successful complexation of europium(III) salt by the N-propylcyclam moieties located inside the channel pores of these materials was further achieved, giving rise to 1∶1 EuIII/cyclam complexes. The results of EXAFS experiments that were performed to obtain quantitative information on the coordination mode of EuIII within the channel pores of materials are discussed.
Results
1 Preparation of materials
First, we prepared ordered SBA-15 mesoporous silica containing chloropropyl groups. For this purpose, co-hydrolysis and polycondensation of a mixture of 3-chloropropyltrimethoxysilane and n equiv. (n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 15 and 30) of tetraethylorthosilicate (TEOS) was carried out in the presence of the triblock copolymer P123 (EO20PO70EO20) as template (Scheme 1). The procedure was the same as the one described for the preparation of ordered SBA-15 mesoporous silica containing phosphonic acid diethyl ester groups.12 The template was removed from the as-synthesized material by Soxhlet extraction with ethanol heated under reflux for 24 h to give the samples denoted as Cl-SBAn
(n
9, 15 and 30) of tetraethylorthosilicate (TEOS) was carried out in the presence of the triblock copolymer P123 (EO20PO70EO20) as template (Scheme 1). The procedure was the same as the one described for the preparation of ordered SBA-15 mesoporous silica containing phosphonic acid diethyl ester groups.12 The template was removed from the as-synthesized material by Soxhlet extraction with ethanol heated under reflux for 24 h to give the samples denoted as Cl-SBAn
(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 15 and 30): Cl to specify the presence of chloropropyl groups inside the pore channels and n to indicate the number of equivalents of TEOS used. The composition of the final materials was inferred from the results of elemental analysis of Si and Cl. They were found to be close to those for the original mixtures as indicated in Table 1.
9, 15 and 30): Cl to specify the presence of chloropropyl groups inside the pore channels and n to indicate the number of equivalents of TEOS used. The composition of the final materials was inferred from the results of elemental analysis of Si and Cl. They were found to be close to those for the original mixtures as indicated in Table 1.
 | ||
| Scheme 1 | ||
| Cl-SBA9 | Cl-SBA15 | Cl-SBA30 | |
|---|---|---|---|
| a Calculated from the desorption branch by using the BJH method. b Calculated from the elemental analysis. In parentheses, theoretical values. | |||
| S BET/m2 g−1 | 1160 | 1000 | 810 |
| V p/cm3 g−1 | 1.13 | 1.05 | 1.11 |
| D p a/Å | 47 | 54 | 61 |
| d 100/Å | 91.9 | 96.6 | 106.5 |
| a o/Å | 106.1 | 111.5 | 123.0 |
| Wall thickness/Å | 59.1 | 57.5 | 60.0 |
| [Cl group content]b/mmol g−1 | 1.24 | 0.87 | 0.51 |
| (1.49) | (0.97) | (0.51) | |
Small-angle X-ray scattering patterns of the different samples shown in Fig. 1 exhibit a single reflection. According to Pinnavaia et al., materials of the same kind presenting scattering patterns with only one reflection can be considered as having the p6m 2D hexagonal symmetry.13,14 The identification of the first Bragg peak as the reflection on the (100) scattering planes of the 2D hexagonal structure allows the determination of the hexagonal lattice parameter ao (see Table 1).
 | ||
| Fig. 1 Small-angle X-ray scattering patterns of Cl-SBA15, Cycl-SBA15 and Cycl,Eu-SBA15 | ||
The N2 adsorption-desorption isotherms of materials Cl-SBAn are very similar. They are type IV isotherms, characteristic of mesoporous materials with a narrow pore size distribution (Fig. 2). The textural data of the materials are given in Table 1. It is worth noting that the SBET, pore volumes and mean pore diameters of these materials are very large, the mean pore diameters increasing as n increases.
 | ||
| Fig. 2 Nitrogen adsorption-desorption isotherms of Cl-SBA9, Cycl-SBA9 and BJH pore size distribution plot (insert) of Cl-SBA9 | ||
Nucleophilic displacement of chloro groups by cyclam moieties was subsequently achieved by treating the Cl-SBAn solids with an acetonitrile solution containing 1.4 equiv. of cyclam heated under reflux for 48 h in the presence of a large excess of triethylamine. The solids were then filtered off and copiously washed with hot methanol, acetone and ether to afford Cycl-SBAn (Scheme 1). The excess of cyclam remaining in the filtrate was determined by conductimetry measurement, which allowed the content of cyclam incorporated in the solid to be inferred. Some relevant physical data of these solids are given in Table 2. These results (Table 2) were confirmed by the elemental analyses of Si, Cl and N. Thus, the substitution reaction was almost quantitative, except for the Cl-SBA9 material, which is the most concentrated in organic groups, for which the yield of substitution was about 80%. This is probably due to steric constraints. That indicates that the chloro groups in the materials of Cl-SBAn are mostly accessible due to the rather large pore size.
| Cycl-SBA9 | Cycl-SBA15 | Cyl-SBA30 | |
|---|---|---|---|
| a Calculated from the desorption branch by using the BJH method. b Calculated from the elemental analysis. | |||
| S BET/m2 g−1 | 627 | 688 | 464 |
| V p/cm3 g−1 | 0.52 | 0.76 | 0.82 |
| D p a/Å | 36 | 47 | 61 |
| d 100/Å | 92 | 100 | 106 |
| a o/Å | 106 | 115 | 122 |
| Wall thickness/Å | 70 | 68 | 61 |
| [Cyclam]b mmol g−1 | 0.93 | 0.70 | 0.48 |
| % Substitution (µanal.) | 80 | 92 | 98 |
| % Substitution (titration) | 80 | 92 | 95 |
The SAXS patterns of the different Cycl-SBAn samples are very similar to those of the starting materials. They exhibit the diffraction peak corresponding to the d100 spacing, indicating that the hexagonal structure is maintained (Fig. 1). The N2 adsorption-desorption isotherms for Cycl-SBAn are of type IV, characteristic of mesoporous materials with a narrow pore size distribution (Fig. 2). The surface area, total pore volume and pore size decreased notably after grafting, as expected,15 which is consistent with the presence of a significant amount of organic groups on the surface of the pore channels. Thus, it is possible to graft almost quantitatively a rather bulky group without modification of the structure. As one of the main characteristics of materials prepared by the direct synthetic approach is the regular distribution of organic groups within the channel pores,16 we can consider that the cyclam moieties are also regularly distributed inside the channel pores.
2 Complexation of EuIII by cyclam moieties within the Cycl-SBAn materials
We then investigated complexation of EuCl3 by the cyclam moieties located inside the channel pores of the Cycl-SBAn materials. Lanthanide complexes exhibit coordination numbers ranging from six to twelve (in the solid state and in solution), with eight and nine being the most common.17 Indeed, it is worth noting that in solution, complexation of europium(III) salts by cyclam derivatives is possible on condition that the cyclam derivatives are tetra-N-substituted by chelating arms (amido groups, for example), which allows a 4N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4O coordination.4
4O coordination.4
The Cycl-SBAn materials were treated with an excess of anhydrous EuCl3 [2 equiv. of EuIIIper cyclam moiety] in ethanol heated under reflux for 24 h (Scheme 1). The resulting Cycl,Eu-SBAn solids were copiously washed with ethanol in order to eliminate the non-complexed salt. The filtrate containing the excess of EuCl3 was titrated by complexometry measurements. The ratio of EuIIIper cyclam moiety was inferred from these results. It was found to be exactly 1∶1 except for Cycl,Eu-SBA9, for which the ratio EuIII∶cyclam moiety was found to be 1∶0.9. Results of elemental analyses of EuIII, Cl and N gave rise to exactly the same EuIII∶cyclam ratio as that found by titration. It is worth noting that the incorporation of EuIII does not take place in the absence of cyclam moieties inside the pore channels. This important point was demonstrated as follows: Cl-SBA9 was treated with an ethanolic solution of EuCl3 heated under reflux for 14 h. After filtration of the solid and washing with ethanol, titration of the filtrate by complexometry revealed that the entire amount of EuCl3 remained in solution. This indicates that the complexation of EuIII is essentially due to the N-propylcyclam moieties located inside the channel pores of the Cycl-SBAn materials.
3 EXAFS study
Fig. 3 shows the EuIII L3-edge EXAFS of Eu in the Cycl,Eu-SBAn materials and their corresponding Fourier transforms. Experimental data were fitted within the r range corresponding to the first nearest neighbour peak between 1.8 and 2.5 Å (Fig. 4). This yields a total number of independent data points equal to 2ΔkΔr/π![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) +
+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≈
≈![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.6,18 that is, greater than the number of variables (4). Best values of the structural parameters corresponding to these fits are listed in Table 3.
5.6,18 that is, greater than the number of variables (4). Best values of the structural parameters corresponding to these fits are listed in Table 3.
 | ||
| Fig. 3 Eu L3-edge EXAFS in (a) k space and (b) r space for Cycl,Eu-SBAn. | ||
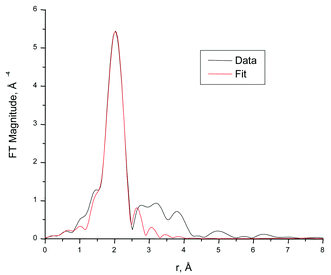 | ||
| Fig. 4 Fourier transform magnitudes of the EXAFS data and fit for Cycl,Eu-SBA9. | ||
| Cycl,Eu-SBA9 | Cycl,Eu-SBA15 | Cycl,Eu-SBA30 | |
|---|---|---|---|
| N | 6.2(4) | 6.8(1.1) | 6.5(1.0) |
| R/Å | 2.483(6) | 2.47(1) | 2.47(1) |
| σ 2/Å2 | 0.0080(10) | 0.0085(22) | 0.0080(20) |
To examine whether Cl or O enter the first shell of the Eu atom, we tested models in which a nitrogen atom in the first nearest neighbour site to Eu was substituted by Cl or O. We varied coordination numbers, distances and disorder of the Eu–Cl (or Eu–O) pairs separately from the Eu–N pairs. Because the number of parameters would exceed the number of relevant independent data points if just one data set was used in the fit, we performed the analysis for all three data sets concurrently, by imposing several physically reasonable constraints between the variables describing different samples. For example, all ΔE0 variables were constrained to be the same during the fits, as well as the σ2 of same bonds. These experiments allowed to rule out the possibility of Cl entering the first shell of Eu because the best-fit value of the coordination numbers of the Eu–Cl pairs is consistent with zero within the uncertainties.
We could not discriminate between the oxygen and nitrogen atoms as possible nearest neighbours for Eu because their backscattering amplitudes and phases are very similar. In both models, the total number of nearest neighbours to Eu was found to be equal to 6 and the interatomic distances are the same for all samples studied. Furthermore, the visual examination of the raw data (Fig. 3) allows us to conclude that there is no evidence of Eu–Eu correlation in the EXAFS data. This means that if such a correlation exists, it is very disordered and its contribution to EXAFS, therefore, is below the level of statistical noise (which can be estimated from the amplitude of the Fourier transform of the EXAFS data at high r).
Discussion
The EXAFS data revealed that the coordination of EuIII is the same within all the materials studied (Cycl,Eu-SBAn for n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 15, 30), the coordination number being six. As we know that there is one EuIII per cyclam moiety in all the materials studied, we propose a coordination of EuIII by the four N atoms originating from one cyclam moiety and two oxygen atoms in the axial direction, forming an octahedron. The oxygen donors can originate from water molecules contained in the materials and/or SiOH groups remaining at the surface of the pores (Scheme 2). We have no data allowing to discriminate between these two sources of oxygen donors.
9, 15, 30), the coordination number being six. As we know that there is one EuIII per cyclam moiety in all the materials studied, we propose a coordination of EuIII by the four N atoms originating from one cyclam moiety and two oxygen atoms in the axial direction, forming an octahedron. The oxygen donors can originate from water molecules contained in the materials and/or SiOH groups remaining at the surface of the pores (Scheme 2). We have no data allowing to discriminate between these two sources of oxygen donors.
 | ||
| Scheme 2 | ||
It is worth noting that the geometry that we propose has been previously observed in [Mn(CN)2(C10H24N4)]·ClO4 and trans-[Mn(CN)2(cyclam)]·ClO4.19 In this MnIII-cyclam complex, both Mn–C distances in the axial direction are very close to the four in-plane Mn–N distances, ranging from 2.00 to 2.03 Å. In the EuIII complex, all the six Eu–nearest neighbour distances must also be similar, as in the case of the MnIII-cyclam complex, which means that the octahedron is not significantly distorted in the axial direction. Indeed, a tetragonal distortion (elongation or shortening of the axial Eu–O bond relative to the in-plane Eu–N bond) by as little as 0.15 Å would result in a static contribution to the average Eu–nearest neigbours bond length disorder of ca. 0.005 Å2. This is a too-large contribution to the total (both static and thermal) σ2 (Table 3) to be physically reasonable.
Interestingly, the negative results of the EXAFS data modelling that assumed a Eu–Cl contribution to the EXAFS spectrum convincingly prove that no complexation of EuCl3 occurred in solution in the presence of N-triethoxysilylpropylcyclam. This is in agreement with our experimental results, which prove that there is no complexation in solution between EuCl3 and N-triehoxysilylpropylcyclam.
In conclusion, we have shown that the coordination mode of EuIII ions in the pore channels of mesoporous hybrid materials containing chelating units is the same whatever the chelating group concentration within the channel pores. This is a good indication of the regular distribution of cyclam moieties within the channel pores. A coordination number of six was found, which is different from both that observed in solution (no formation of complex between EuIII and N-triethoxysilylpropylcyclam) and that in the bulk (1 EuIII for 2 cyclam units).4 These results illustrate the versatility of the coordination chemistry in the solid, which deserves to be further explored.
Experimental
General procedures
All reactions were carried out under argon by using a vacuum line. Solvents were dried and distilled just before use. The triblock copolymer [EO20PO70EO20 with PEO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(ethylene oxide) and PPO
poly(ethylene oxide) and PPO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) poly(propylene oxide)] Pluronic P123, 3-chloropropyltrimethoxysilane, tetraethylorthosilicate (TEOS) and EuCl3 salt were purchased from Aldrich and used as supplied. 1,4,8,11-Tetraazacyclotetradecane (cyclam) was obtained from LIMSAG (CNRS UMR 5633, Université de Bourgogne, Dijon). The CP MAS 29Si solid state NMR spectra were recorded on a Bruker FTAM 300 as well as the CP MAS 13C solid state NMR spectra, in the latter case by using the TOSS technique. In both cases, the repetition times were 5 and 10 s with contact times of 5 and 2 ms. Chemical shifts (ppm) are referenced to Me4Si. Specific surface areas were determined by the Brunauer–Emmett–Teller (BET) method on a Micromeritics ASAP 2010 analyser and the average pore diameters were calculated by the BJH method. Elemental analyses were carried out by the “Service Central de Micro-Analyse du CNRS” (Vernaison, France). SAXS experiments were carried out on a high resolution Bonse–Hart camera with two germanium channel cuts for very small q values. The wavelength was 1.542 Å
(CuKα radiation).
poly(propylene oxide)] Pluronic P123, 3-chloropropyltrimethoxysilane, tetraethylorthosilicate (TEOS) and EuCl3 salt were purchased from Aldrich and used as supplied. 1,4,8,11-Tetraazacyclotetradecane (cyclam) was obtained from LIMSAG (CNRS UMR 5633, Université de Bourgogne, Dijon). The CP MAS 29Si solid state NMR spectra were recorded on a Bruker FTAM 300 as well as the CP MAS 13C solid state NMR spectra, in the latter case by using the TOSS technique. In both cases, the repetition times were 5 and 10 s with contact times of 5 and 2 ms. Chemical shifts (ppm) are referenced to Me4Si. Specific surface areas were determined by the Brunauer–Emmett–Teller (BET) method on a Micromeritics ASAP 2010 analyser and the average pore diameters were calculated by the BJH method. Elemental analyses were carried out by the “Service Central de Micro-Analyse du CNRS” (Vernaison, France). SAXS experiments were carried out on a high resolution Bonse–Hart camera with two germanium channel cuts for very small q values. The wavelength was 1.542 Å
(CuKα radiation).
Preparation of materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 9, 15 and 30).
The materials Cl-SBAn
(n
9, 15 and 30).
The materials Cl-SBAn
(n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 15 and 30) were prepared according to the same procedure. Preparation of Cl-SBA9 is given as an example.
9, 15 and 30) were prepared according to the same procedure. Preparation of Cl-SBA9 is given as an example.
The triblock copolymer (EO20PO70EO20) Pluronic P123 (4.0 g) was dissolved in 160 mL of an aqueous solution of HCl (pH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) =
=![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5). The resulting clear solution was then added to a mixture of 3-chloropropyltrimethoxysilane (0.84 g, 4.49 mmol) and tetraethylorthosilicate (8.41 g, 40.41 mmol). The mixture was vigorously stirred for 3 h at room temperature until a transparent solution appeared. The solution was transferred into a hot oil bath at 60
1.5). The resulting clear solution was then added to a mixture of 3-chloropropyltrimethoxysilane (0.84 g, 4.49 mmol) and tetraethylorthosilicate (8.41 g, 40.41 mmol). The mixture was vigorously stirred for 3 h at room temperature until a transparent solution appeared. The solution was transferred into a hot oil bath at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and NaF (76.0 mg, 1.80 mmol) was then immediately added to induce the polycondensation. A white precipitate appeared within a few minutes and the resulting suspension was further stirred for 2 days at 60
°C and NaF (76.0 mg, 1.80 mmol) was then immediately added to induce the polycondensation. A white precipitate appeared within a few minutes and the resulting suspension was further stirred for 2 days at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. The resulting white powder was filtered off and the surfactant was selectively removed by Soxhlet extraction over ethanol for 24 h.
°C. The resulting white powder was filtered off and the surfactant was selectively removed by Soxhlet extraction over ethanol for 24 h.
Cl-SBA9. After drying at 120
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C overnight under vacuum, 2.89 g (94%) of Cl-SBA9 was obtained as a white powder. 13C NMR (75 MHz, CP-MAS)
δ: 9.3 (CH2Si), 16.1 (CH3CH2OSi), 26.4 (CH2CH2Si), 46.0 (ClCH2), 59.8 (CH3CH2OSi). 29Si NMR (60 MHz, CP-MAS)
δ: −66.7 (T3), −92.0 (Q2), −101.4 (Q3), −109.4 (Q4). IR (KBr)
ν/cm−1: 3450 (H-bonded Si–OH), 2981–2909 (Csp3–H), 1636 (H2O bonded), 1200–1000 (Si–O–Si)
°C overnight under vacuum, 2.89 g (94%) of Cl-SBA9 was obtained as a white powder. 13C NMR (75 MHz, CP-MAS)
δ: 9.3 (CH2Si), 16.1 (CH3CH2OSi), 26.4 (CH2CH2Si), 46.0 (ClCH2), 59.8 (CH3CH2OSi). 29Si NMR (60 MHz, CP-MAS)
δ: −66.7 (T3), −92.0 (Q2), −101.4 (Q3), −109.4 (Q4). IR (KBr)
ν/cm−1: 3450 (H-bonded Si–OH), 2981–2909 (Csp3–H), 1636 (H2O bonded), 1200–1000 (Si–O–Si)
Cl-SBA15. Cl-SBA15 (2.97 g, 98%) was obtained as a white powder. 13C NMR (75 MHz, CP-MAS) δ: 9.1 (CH2Si), 15.9 (CH3CH2OSi), 26.2 (CH2CH2Si), 45.5 (ClCH2), 59.7 (CH3CH2OSi). 29Si NMR (60 MHz, CP-MAS) δ: −64.5 (T3), −92.0 (Q2), −101.4 (Q3), −109.6 (Q4). IR (KBr) ν/cm−1: 3426 (H-bonded Si–OH), 2981–2909 (Csp3–H), 1630 (H2O bonded), 1200–1000 (Si–O–Si).
Cl-SBA30. Cl-SBA30 (2.94 g, 96%) was obtained as a white powder. 29Si NMR (60 MHz, CP-MAS) δ: −64.3 (T3), −91.7 (Q2), −101.2 (Q3), −109.0 (Q4). IR (KBr) ν/cm−1: 3450 (H-bonded Si–OH), 1630 (H2O bonded), 1200–1000 (Si–O–Si).
Preparation of Cycl-SBA9. A mixture of cyclam (0.42 g, 2.09 mmol), Cl-SBA9 (1.00 g, 1.49 mmol) in 90 mL of acetonitrile and triethylamine (1.1 g, 10.9 mmol) was heated under reflux and stirring for 2 days. The white solid was quantatitatively recovered by filtration and washed 5 times with hot chloroform and 3 times with hot ethanol to remove the excess of cyclam. After drying at 120
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C overnight under vacuum, 1.10 g (95%) of Cycl-SBA9 was obtained as a white powder. The excess of cyclam contained in the reaction filtrate was titrated by conductimetry. 13C NMR (75 MHz, CP-MAS)
δ: 9.8 (CH2Si), 26.2 (CH2CH2N), 40.0–60.0 (NCH2). 29Si NMR (60 MHz, CP-MAS)
δ: −61.9 (T2), −66.0 (T3), −101.6 (Q3), −110.0 (Q4). IR (KBr)
ν/cm−1: 3420 (H-bonded Si–OH), 2953–2859 (Csp3–H), 1634 (H2O bonded), 1200–1000 (Si–O–Si).
°C overnight under vacuum, 1.10 g (95%) of Cycl-SBA9 was obtained as a white powder. The excess of cyclam contained in the reaction filtrate was titrated by conductimetry. 13C NMR (75 MHz, CP-MAS)
δ: 9.8 (CH2Si), 26.2 (CH2CH2N), 40.0–60.0 (NCH2). 29Si NMR (60 MHz, CP-MAS)
δ: −61.9 (T2), −66.0 (T3), −101.6 (Q3), −110.0 (Q4). IR (KBr)
ν/cm−1: 3420 (H-bonded Si–OH), 2953–2859 (Csp3–H), 1634 (H2O bonded), 1200–1000 (Si–O–Si).
Cycl-SBA15. Cycl-SBA15 was obtained in 95% yield as a white powder. 13C NMR (75 MHz, CP-MAS) δ: 9.7 (CH2Si), 26.2 (CH2CH2N), 40.0–60.0 (NCH2). 29Si NMR (60 MHz, CP-MAS) δ: −61.9 (T2), −66.0 (T3), −99.7 (Q3), −108.0 (Q4). IR (KBr) ν/cm−1: 3421 (H-bonded Si–OH), 2941–2842 (Csp3–H), 1640 (H2O bonded), 1200–1000 (Si–O–Si).
Cycl-SBA30. Cycl-SBA30 was obtained in 94% yield as a white powder. 29Si NMR (60 MHz, CP-MAS) δ: −66.0 (T3), −99.8 (Q3), −108.1 (Q4).
Preparation of Cycl,Eu-SBA9. Cycl-SBA9 (0.25 g, 0.37 mmol) was added to an ethanolic solution (10 mL) of EuCl3 (0.20 g, 0.76 mmol). The resulting suspension was heated overnight under reflux with stirring. The solid was quantitatively recovered by filtration and washed with ethanol and acetone several times. After drying at 120
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C overnight under vacuum, 0.34 g (97%) of Cycl,Eu-SBA9 was obtained as a white powder. IR (KBr)
ν/cm−1: 3428 (H-bonded Si–OH), 2962–2864 (Csp3–H), 1634 (H2O bonded), 1200–1000 (Si–O–Si).
°C overnight under vacuum, 0.34 g (97%) of Cycl,Eu-SBA9 was obtained as a white powder. IR (KBr)
ν/cm−1: 3428 (H-bonded Si–OH), 2962–2864 (Csp3–H), 1634 (H2O bonded), 1200–1000 (Si–O–Si).
EXAFS experiment
For each of the Cycl,Eu-SBAn samples, the powder was pressed into a rectangular wafer. The wafers were approximately 0.5 mm thick and satisfied the condition that Δμ·x![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≤
≤![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, where x is the effective sample thickness and Δμ is the jump in absorption coefficient at the Eu L3 absorption edge (6977 eV). The EXAFS measurements were taken in transmission mode at the X16C beamline of the National Synchrotron Light Source (Brookhaven National Laboratory, Upton, NY). X-Ray absorption spectra were collected using a double-crystal Si(111) monochromator. To reject higher order harmonics of the beam, the monochromator crystals were 30% detuned. The energy range was from the 6777 eV edge to 7618 eV (which corresponds to the Eu L2 edge). The incident beam intensity was measured in a 15 cm long ionization chamber filled with N2 while the transmitted beam intensity was measured in a 30 cm long chamber filled with a 1∶2 Ar–He mixture. After subtracting the smooth atomic background from the measured X-ray absorption coefficients, the edge-step normalized EXAFS functions χ(k) were obtained for each data set.
1, where x is the effective sample thickness and Δμ is the jump in absorption coefficient at the Eu L3 absorption edge (6977 eV). The EXAFS measurements were taken in transmission mode at the X16C beamline of the National Synchrotron Light Source (Brookhaven National Laboratory, Upton, NY). X-Ray absorption spectra were collected using a double-crystal Si(111) monochromator. To reject higher order harmonics of the beam, the monochromator crystals were 30% detuned. The energy range was from the 6777 eV edge to 7618 eV (which corresponds to the Eu L2 edge). The incident beam intensity was measured in a 15 cm long ionization chamber filled with N2 while the transmitted beam intensity was measured in a 30 cm long chamber filled with a 1∶2 Ar–He mixture. After subtracting the smooth atomic background from the measured X-ray absorption coefficients, the edge-step normalized EXAFS functions χ(k) were obtained for each data set.
References
- L. F. Lindoy, The Chemistry of Macrocyclic Ligand Complexes, Cambridge University Press, Cambridge, 1989 Search PubMed.
- P. V. Bernhardt and G. A. Lawrance, Coord. Chem. Rev., 1990, 104, 297 CrossRef CAS.
- G. Dubois, R. J. P. Corriu, C. Reyé, S. Brandès, F. Denat and R. Guilard, Chem. Commun., 1999, 2283 RSC.
- R. J. P. Corriu, F. Embert, Y. Guari, C. Reyé and R. Guilard, Chem.-Eur. J., 2002, 8, 5732 CrossRef CAS.
- G. Dubois, C. Reyé, R. J. P. Corriu, S. Brandès, F. Denat and R. Guilard, Angew. Chem., Int. Ed., 2001, 40, 1087 CrossRef CAS.
- D. J. Macquarrie, D. B. Jackson, S. Tailland and K. A. Utting, J. Mater. Chem., 2001, 11, 1843 RSC.
- R. Richer and L. Mercier, Chem. Commun., 1998, 1775 RSC.
- R. J. P. Corriu, A. Mehdi and C. Reyé, C. R. Acad. Sci. Sér. IIc, 1999, 2(1), 35 Search PubMed.
- L. Mercier and T. Pinnavaia, Chem. Mater., 2000, 12, 188 CrossRef CAS.
- R. Richer and L. Mercier, Chem. Mater., 2001, 13, 2999 CrossRef CAS.
- D. Margolese, J. A. Melero, S. C. Christiansen, B. F. Chmelka and G. D. Stucky, Chem. Mater., 2000, 12, 2448 CrossRef CAS.
- R. J. P. Corriu, L. Datas, Y. Guari, A. Mehdi, C. Reyé and C. Thieuleux, Chem. Commun., 2001, 763 RSC.
- P. T. Tanev, M. Chibwe and T. J. Pinnavaia, Nature (London), 1994, 368, 321 CrossRef CAS.
- P. T. Tanev and T. J. Pinnavaia, Science, 1995, 267, 865 CAS.
- A. B. Sorokin and A. Tuel, New J. Chem., 1999, 23, 473 RSC.
- Y. Mori and T. J. Pinnavaia, Chem. Mater., 2001, 13, 2173 CrossRef CAS.
- F. S. Richardson, Chem. Rev., 1982, 82, 541 CrossRef CAS.
- E. A. Stern, Phys. Rev. B, 1993, 48, 9825 CrossRef CAS.
- S. Mossin, H. O. Sorensen and H. Wiehe, Acta Crystallogr., Sect. C, 2002, 58, m204–m206 CrossRef.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2004 |

