Syn-anti epimerization of aldols by aldolate dianions
Lizeng
Peng
a,
Tao
Zhang
b,
Tiansheng
Mei
b and
Yulin
Li
*b
aLunan Pharmaceutical Co. Ltd., Linyi, 276003, P. R. China
bNational Laboratory of Applied Organic Chemistry, Institute of Organic Chemistry, Lanzhou University, Lanzhou, 730000, P. R. China. E-mail: liyl@lzu.edu.cn
First published on 28th November 2003
Abstract
A proposed mechanism based on distal aldolate dianions is illustrated in this paper to explain the outcome of the directed aldol reaction when one uses more than 2 equivalents of base.
The aldol addition1 is a powerful and general method for stereocontrolled construction of carbon-carbon bonds. The most important stereochemical question in the directed aldol reaction concerns the formation of threo and/or erythro isomers of aldols or ketols.
One may use several mole equivalents of Li (or Na, Mg, etc.) enolates of the ketone and only 1 mole equivalent aldehyde to obtain the best yield of the desired condensation product. However, undesired products were usually obtained, along with the expected ones. One may explain the results through a rapid equilibration between the starting E- and Z-enolates based on the classic retro-aldol aldol process.1
During our studies on the total synthesis of brassinolide,2 we observed an interesting phenomenon, which we think can be well-explained through the formation of aldolated dianions, but not by the retro-aldol aldol process. A proposed mechanism is illustrated in Scheme 1 based on distal aldolate dianions3 when excess strong base was used during the aldol condensation.
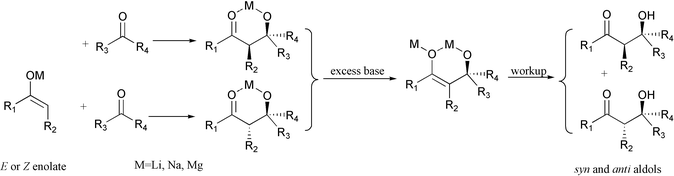 | ||
| Scheme 1 The proposed mechanism based on the distal aldolate dianions. | ||
In summary, in our experiments syn- and anti-aldols were produced at the same time, whether the starting enolate was the Z- or E-enolate, in the directed aldol condensation when using an excess amount of strong base, as explained by the above-proposed mechanism.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 20072012).References
- T. Mukaiyama, Org. React., 1982, 28, 203 CAS.
- L. Z. Peng, Y. L. Li and W. D. Z. Li, Tetrahedron Lett., 2003, 44, 3991 CrossRef CAS.
- For reports on the isomerization of aldols via an enol or enolate mechanism, see: (a) V. A. Martin, D. H. Murray, N. E. Pratt, Y. Zhao and K. F. Albizati, J. Am. Chem. Soc., 1990, 112, 6965 CrossRef CAS; (b) D. E. Ward, M. Sales and P. K. Sasmal, Org. Lett., 2001, 3, 3671 CrossRef CAS and references cited therein.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2004 |
