Poly(dimethylsiloxane) hollow Abbe prism with microlenses for detection based on absorption and refractive index shift
A.
Llobera
,
R.
Wilke
and
S.
Büttgenbach
Institut für Mikrotechnik, Technische Universität Braunschweig, Alte Salzdahlumer Straße 203, 38124 Braunschweig, Germany. E-mail: a.llobera@tu-bs.de; Fax: +49 531/391-9751; Tel: +49 531/391-9752
First published on 5th January 2004
Abstract
In this paper we report on an optical detection method that utilizes two physical effects for signal transduction, namely absorption and shift of refractive index. The device consists of a hollow prism and was fabricated by means of soft-lithography. It exhibits a high degree of monolithic integration. In order to keep down the amount of external equipment that is necessary to run the device, we were able to integrate several functions, such as focussing of light and alignment of optical fibres. Since all components are fabricated in the same material and in the same process, compatibility with other microfluidic devices or components can be achieved easily. The functional efficiency and the performance of the detector were tested by investigating solutions containing fluorescein, with concentrations between 5 and 1000 µM. The results clearly show the two regions in which the two physical effects are effective.
Introduction
The successful miniaturization of fluidic components such as valves, pumps and mixers have proven the feasibility of the application of micromachining for this kind of device. In 1990 the area of microfluidics was extended by the introduction of a new concept, called miniaturized chemical analysis systems, also known as micro total analysis systems. The great benefit of physically scaling down analytical systems was not only a reduction in size but rather an improvement of the analytical performance.1 Since then several research groups have been spending efforts to pay tribute to this concept.2Chip-based capillary electrophoresis (CE) systems have been shown to be a powerful tool for many promising applications.3–6 They are at the focus of research for many groups working in this field. In most cases CE-chips are not only used for separating analytes of a sample but are interfaced with a detector.7–9 Due to its high sensitivity very often laser-induced fluorescence detection, a well-established detector in conventional CE-systems, is the method of choice. For efficient measurements the exciting light has to be focused into the channel and the intensity of the excited fluorescence has to be collected and focused onto a photodetector. This requires a considerable amount of optical components, such as lenses, collimators, mirrors, pinholes and filters that need to be aligned very carefully. In relation to the microfluidic device this assembly becomes fairly bulky. This stands in contrast to the incentive of microfabrication and miniaturization. The tackling of this drawback goes together with poly(dimethylsiloxane) (PDMS) finding its way into microfabrication. Recently, diverse groups reported on integrating optical fibres and lenses for fluorescence detection by taking advantage of the elastomer's extraordinary properties concerning the ability to form complex structures from a given mold.10,11 Other groups have reported on the performance enhancement of laser-induced fluorescence and absorbance measurements by integrating arrangements of collector and collimator lenses. In one case collimation was achieved by combining a lens with channels made in PDMS that can be filled with black ink.12,13 However, one problem that remains is the integration of filters. They are used to separate the wavelength of the exciting light from the one of the emitted fluorescence. This is essential when the output signal is generated by a photodetector. So far, filters are incorporated by either using conventional optical components or complex and expensive technological steps.14,15
In our work we tried to put emphasis on designing a transducer that is suitable for on-chip detection and requires as few additional components as possible. Exploiting the merits of PDMS in combination with a prism-based optical set-up, known from measuring refractive indices, we were able to design an optical system with a high degree of monolithic integration. It comprises a hollow prism that can be filled with the fluid under investigation via two fluidic ports and a pair of 2D biconvex lenses that modifies the optical path before and after propagating through the prism. All these components are fabricated in one single process step by replica molding with PDMS. For sealing the microfluidic device, the PDMS is bonded to a piece of conventional soda-lime glass. Light is coupled into the system through a multimode optical fibre inserted into a channel. This channel facilitates the correct positioning of the fibre with respect to the biconvex lens due to its geometrical shape. The same applies for the output fibre which enables the collected light to reach the photodetector. To demonstrate the utility of this detector we examined solutions with different concentrations of fluorescein. Nevertheless, the same hollow Abbe prism could be used for absorption detection of chemical species that do not have appreciable fluorescence, or transparent fluids with a variation of its refractive index. These outstanding properties confer the proposed device a huge versatility in μ-TAS.
Structures and fabrication
Design
One of the main applications of prisms is the measurement of the refractive index (RI). Concretely, for a given prism configuration, by measuring the incident angle and the so-called minimum deviation,16 the RI as a function of the wavelength can be obtained. Nowadays, this measurement method is one of the most exact techniques for determining the refractive index of transparent substances in a wide range of wavelengths and for a large variety of materials (including gases and liquids).Among the several prisms available in the literature, we have focused on the Abbe prism due to its simplicity. The basic structure of such a prism can be explained with the help of Fig. 1 and it can be understood as a combination of three prisms (ADE, AEB and BEC), where δ stands for the total deviation of a ray propagating through the Abbe prism.
 | ||
| Fig. 1 Configuration and ray-tracing in an Abbe prism. | ||
We assume that a monochromatic ray of a given wavelength λ1 is injected in one of the prism's facets. Using ray-tracing theory, it can be shown that with the appropriate incident tilt, this ray crosses symmetrically – that is orthogonal to the AE facet – the ADE prism and is reflected 30° from the AB facet. After crossing the BEC prism the ray exhibits a total deviation of 60°. Then, the overall system can be envisaged as a combination of a 60° prism (ADE combined with BEC) transmitted in the minimal deviation condition for a certain wavelength and a second prism that only does a pure reflection of 60°. If a small rotation is now applied on the Abbe prism, the former wavelength is no longer transmitted at 60°, but it is replaced by a second wavelength, named λ2, for which the conditions for minimal deviation are fullfiled. This is the reason why these types of prism are commonly called constant deviation prisms. We took advantage of this effect by using light of a constant wavelength and changing the RI of the prism.
On the basis of the previously defined behavior, a new compact optical detector (shown in Fig. 2) is presented. It consists of a hollow Abbe prism directly connected to fluidic input/output reservoirs. At the end of the two multimode optical fibres, two biconvex micro-lenses made of the same material as the walls of the prism (PDMS) are defined. According to the refractive indices of PDMS12 (n = 1.41) and air (n = 1.00), the distances between the lens and the optical fibre were chosen in order to have parallel beams on the surface of the prism. Finally, the relative position of the optical fibres and the lenses with respect to the Abbe prism is defined so as to have a maximum intensity for blue light (λ = 460 nm) when the prism is filled with buffer solution (phosphate buffer, pH 7.4, 10 mM, n = 1.334).
 | ||
| Fig. 2 System for fluorescence detection with a hollow Abbe prism (not to scale). | ||
According to this configuration, two different regimes can be predicted as the concentration of fluorescein, diluted in buffer solution, increases. For low concentrations, there will be no significant changes on the RI in the solution. Only a decrease of the maximum peak will be obtained due to the fluorescein absorption, without shifting the output wavelength. For high fluorescein concentrations, in addition to this absorption effect, the optical path will be changed due to a variation of the RI, resulting in a shift of the output peak. Since the prism is mainly designed for a wavelength of 460 nm, the high absorption of this wavelength, together with the shift of the RI, will result in a high increase of the sensitivity. For capillary electrophoresis purposes, the dimensions of the prism should be done as small as possible so as to avoid a reduction of the resolution due to deformation of the sample plug inside the prism.
The most crucial step to the design of the system is the adequate positioning of the input optical fibre in relation to the biconvex lens and the prism. As it can be observed in Fig. 3, stoppers at the channel fixes the distance between the optical fibre and the lens. In accordance with the refractive indices of air and PDMS, and selecting the radii to be R1 = 160 µm and R2 = −160 µm, the distance S0 = 200 µm should provide parallel beams at the output lens.
 | ||
| Fig. 3 Detailed scheme of the optical fibre channel, the lenses and the prism. Fibre optics are stopped at a distance S0 which, considering the RI of PDMS and air, together with the curvature of the lens (R1 and R2), allows having parallel beams at the biconvex lens output. Lens and channel are tilted an angle θ to have propagation with minimal deviation through the prism. An air gap, with a minimum distance d, separates the PDMS bulk region from the PDMS lens. L remains for the distance between the air gap and the prism in order to avoid leakage. | ||
It was previously mentioned that in the Abbe prism light propagates through the ADE and BEC prism with minimum deviation. To achieve this, it is required to study the relative position between the channel, the lenses and the prism wall. Concretely, considering that the prism is filled with buffer, tilting the lens–channel system at an angle θ = 54.8° with respect to the prism wall assures that the outcoming light from the biconvex lens propagates in minimal deviation angle at the prism. To separate the biconvex lens from the bulk PDMS region, an air gap, with a minimum distance d = 30 µm, is left just after the lens. No variation of the ray tilt is produced at the air–PDMS interface due to the 90° incidence. Finally, in order to assure that the liquid inside the prism will not fill, due to leakage, the air gap, a distance L = 140 µm is left between the lower corner of the air gap and the prism.
Fabrication
The microfluidic device was fabricated by casting of PDMS (Sylgard 184 elastomer kit, Dow Corning, Midland, MI, USA) against a master made of EPON SU8 (SU8-25, MicroChem Corporation, Newton, MA, USA). In order to obtain a sufficient height that allows a hassle-free insertion of the optical fibres we used a two-step spin-on process for SU8. After spin-coating and drying the negative photoresist was exposed to UV light through a mask. A post-exposure bake was followed by developing the SU8 in propylene glycol methyl ether acetate (PGMEA, MicroChem Corporation, Newton, MA, USA). The resulting master exhibited a height of 250 µm. A prepolymer of PDMS was prepared by mixing the curing agent and the elastomer base in a 1:10 ratio (v/v) and degassing in a vacuum chamber. After pouring the prepolymer over the master the PDMS was cured on a hotplate at 60°C for 1 h. Prior to bonding the PDMS slab to glass the elastomer was peeled off from the mold and holes were punched out with a stencil for access to the fluidic channels. We used a bonding procedure based on a surface treatment in an oxygen plasma.17 Both the PDMS slab and the glass were put in a barrel etcher (Surface Technology Systems, Newport, UK) and exposed to an oxygen plasma. Immediately after plasma oxidation the two surfaces were brought in contact and the fluidic system was irreversibly sealed. The total volume of the prism is 1.579 µl.Characterization
The fabricated prism can be seen in Fig. 4: light from an SLED (λ = 460 nm) is directed to a multimode optical fibre with a core of 200 µm. This fibre is placed at the input PDMS channel at the designed distance from the PDMS biconvex lens. The readout consists of another biconvex lens, that collects all the emerging light at 60° and focuses it on the multimode optical fibre. In turn, this optical fibre is connected to a spectrometer (P.117, STEAG microParts, Dortmund, Germany) with a spectral resolution of 12 nm and a time of integration of 2.5 s. | ||
| Fig. 4 Picture of the system used, displaying the input/output PDMS biconvex lens, the optical fibres and the Abbe prism filled with buffer solution + 50 µM fluorescein. | ||
To avoid air entrapment at the upper unstable meniscus of the Abbe prism, the liquid to investigate is injected at low speed.18 Measurements are done filling the prism with buffer solution and progressively increasing the fluorescein concentration.
Two different regions can be observed from the results shown in Fig. 5. For low fluorescein concentration, there is a progressive decrease of the output intensity as the concentration of fluorescein increases with the maximum remaining fixed at the excitation wavelength (460 nm). Light emitted from the fluorescein is mainly not collected by the output fibre optics since it is emitted in all directions and at a wavelength (510 nm) for which the Abbe prism has not been optimized. Hence, the emitted light that matches with the minimum dispersion angle is negligible. This behavior is in agreement with the previous discussion concerning the measurements with the Abbe prism: as the concentration of fluorescein increases there is an increase of the light at the excitation wavelength absorbed by the fluorescein and hence, the intensity collected by the input fibre decreases. However, since there is no shift on the measured wavelengths, it can be concluded that up to concentrations of 100 µM of fluorescein, no appreciable changes of the RI are produced in the buffer solution. For concentrations larger than 100 µM, a sharp variation of the behavior is observed, as shown in Fig. 5b. In this case, together with the decrease of the intensity, a shift of the peak at the excitation wavelength is observed, which can only be due to an RI variation of the liquid inside the Abbe prism.
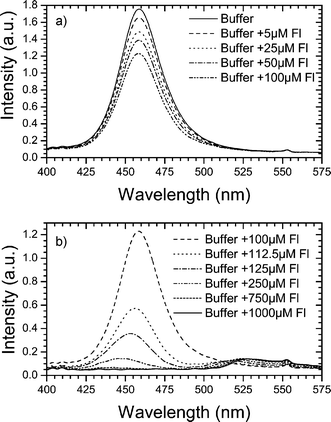 | ||
| Fig. 5 Output intensity as a function of the wavelength for different fluorescein concentrations in phosphate buffer (a) at the pure absorption region, (b) at the absorption–RI shift region. | ||
From the data of the previous figure, and considering the Lambert–Beer law,19 the limit of detection (LOD) of the hollow Abbe prism was estimated to be 6 µM, that corresponds to 2.34 mg l−1, with a linear region that fits the expression A = (1.07 × 10−3 ± 4 × 10−5)C + (0.046 ± 0.002), where A is the absorbance and C is the fluorescein concentration, with R2 = 0.99876. Comparing these values with an equivalent set-up11 (that is, only having microchannels, lenses, light source and spectrophotometer), it can be observed that the value of the LOD is lowered by two orders of magnitude using the hollow Abbe prism.
Treatment of the data provided by the spectrometer is shown in Fig. 6a, where the expected exponential decay of the intensity at the excitation wavelength (460 nm) can be observed, as the concentration of the fluorescein (i.e. the absorption of the excitation wavelength) increases. The abrupt deviation of the tendency for concentrations above 100 µM can be clearlier seen in Fig. 6a. As previously mentioned, the sharp decrease of the output intensity cannot be explained by only considering the fluorescein absorption, but the RI shift has to be taken into account. This assumption is confirmed with the help of the inset of Fig. 6a (intensity at the emission wavelength vs. fluorescein concentration) and Fig. 6b (output wavelength vs. fluorescein concentration). As can be observed, there is no shift of the output wavelengths (excitation and emission) for fluorescein concentrations below 100 µM (the intensity of emitted wavelength at this region is below the resolution of the measurement system and hence it is not shown in Fig. 6b). This result matches with the pure absorption regime, since a shift from the collected wavelengths will mean a variation of the RI of the liquid inside the Abbe prism. Conversely, a shift of the emission and excitation wavelengths collected at the output of the device (and hence, changes on the RI) are clearly observed for concentrations above 100 µM. As it is shown in the inset of Fig. 6a, the increase of the fluorescein concentration causes an increase of the intensity of the emission wavelength, tending to a stable value for high fluorescein concentrations. This point is in agreement with the spectra previously shown, in which it was noticeable that no light at the excitation wavelength reached the output optical fibre for fluorescein concentrations above 750 µM. Hence, it can be concluded that concentrations of fluorescein above this value will not cause a significant increase of the intensity at the emission wavelength, but the expected shift on the emission wavelength due to RI variation. At this region, due to the contributions of the absorption and RI shift, no linear range can be obtained by way of Beer's law and no LOD of detection can be established. Only the smallest fluorescein concentration (112.5 µM) that gives a significant signal of the emission wavelength can be determined.
 | ||
| Fig. 6 (a) Transmitted light intensity at the excitation wavelength as a function of the fluorescein concentration, showing the absoprtion (where an exponential fit has been applied) and the absorption + RI shift regimes. Inset: transmitted light intensity at the emission wavelength vs. fluorescein concentration. (b) Transmitted excitation and emission wavelength as a function of the fluorescein concentration. | ||
Conclusions
A compact system comprised of a PDMS hollow Abbe prism, biconvex lenses and self aligned channels for optical fibres has been presented. This set-up allows the simultaneous measurement of the absorption and the RI shift. The external parts of the set-up have been reduced to a connected SLED and a spectrometer. Results have shown that it is possible to distinguish two regions: For low fluorescein concentration, excitation light from the SLED is absorbed from the fluorescein, which emits at a wavelength that does not match the minimum deviation of the Abbe prism and hence is not detected by the sprectrometer. In this pure absoption region, where there is no shift of the excitation wavelength, it is possible to detect fluorescein concentration diluted in phosphate buffer, with an LOD of 2.34 mg l−1. As the fluorescein concentration increases, the RI variation cannot be neglected, resulting in a sharp variation of the measured intensity, together with a shift of both the excitation and emission wavelengths. Finally, at high concentrations, the intensity of the light at the emission wavelength tends to a constant value, which is in accordance with the complete absorption of the excitation wavelength. This behavior is in agreement with the Abbe prism working principle, with a variation of the wavelength collected by the output optical fibre as the refractive index of the liquid varies. Due to the contribution of the absorption and the RI shift, no LOD can be determined in this range, being the minimun fluorescein concentration detectable, by ways of measuring the emission wavelength, to be 112.5 µM.Acknowledgements
The authors thank the German Research Foundation (DFG) for support of this work. A. Llobera would also like to thank the AGAUR (Catalan council) for his grant Nanotec2002.References
- A. Manz, N. Graber and H. M. Widmer, Sens. Actuators, B, 1990, 1, 244–248 CrossRef.
- P.-A. Auroux, D. Iossifidis, D. R. Reyes and A. Manz, Anal. Chem., 2002, 74, 2637–2652 CrossRef CAS.
- C. S. Effenhauser, A. Manz and H. M. Widmer, Anal. Chem., 1993, 65, 2637–2642 CrossRef CAS.
- A. T. Wolley and R. A. Mathies, Anal. Chem., 1995, 67, 3676–3680 CrossRef CAS.
- D. C. Duffy, J. C. McDonald, O. J. A. Schueller and G. M. Whitesides, Anal. Chem., 1998, 70, 4974–4984 CrossRef CAS.
- J. Wang, M. P. Chatrathi, B. Tian and R. Polsky, Anal. Chem., 2000, 78, 2514–2518 CrossRef CAS.
- Z. Liang, N. Chiem, G. Ocvirk, T. Tang, K. Fluri and D. J. Harrison, Anal. Chem., 1996, 68, 1040–1046 CrossRef.
- A. T. Wolley, K. Lao, A. N. Glazer and R. A. Mathies, Anal. Chem., 1998, 70, 684–688 CrossRef CAS.
- J. Wang, M. P. Chatrathi and B. Tian, Anal. Chem., 2000, 72, 5774–5778 CrossRef CAS.
- M. L. Chabinyc, D. T. Chiu, J. C. McDonald, A. D. Stroock, J. F. Christian, A. M. Karger and G. M. Whitesides, Anal. Chem., 2001, 73, 4491–4498 CrossRef CAS.
- S. Camou, H. Fujita and T. Fujii, Lab Chip, 2003, 3, 40–45 RSC.
- J. Seo and L. P. Lee, Transducers '03, Boston 2003, June 8–12, 1136–1139 Search PubMed.
- K. W. Ro, B. C. Shim, K. Lim and J. H. Hahn, Micro Total Analysis Systems, 2001, 274–276 Search PubMed.
- J. R. Webster, M. A. Burns, D. T. Burke and C. H. Mastrangelo, Anal. Chem., 2001, 73, 1622–1626 CrossRef CAS.
- N. A. Lacher, N. F. de Rooij, E. Verpoorte and S. M. Lunte, J. Chromatogr., A, 2003, 1004, 225–235 CrossRef CAS.
- E. Hecht and A. Zajac, Optics. Addison-Wesley 1986, 139–141 Search PubMed.
- B.-H. Jo, L. M. Van Lerberghe, K. M. Motsegood and D. J. Beebe, J. Microelectromech. Syst., 2000, 9, 76–81 CrossRef CAS.
- F. Goldschmidtböing, R. Schlosser, S. Schonhart and P. Woias, Tranducers'03, Boston 2003, 8–12 June, 1883–1886 Search PubMed.
- W. Göpel, J. Hesse, J. N. Zemel, E. Wagner, R. Dändliker and K. Spenner, Sensors, a Comprehensive Survey, Volume 6, Optical Sensors, VCH 1991 Search PubMed.
| This journal is © The Royal Society of Chemistry 2004 |
